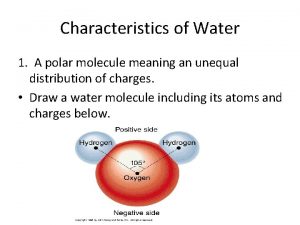
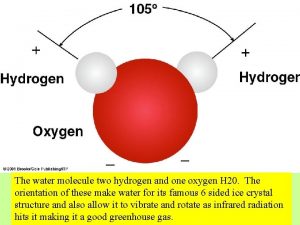
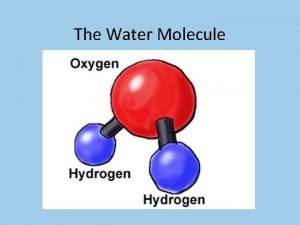
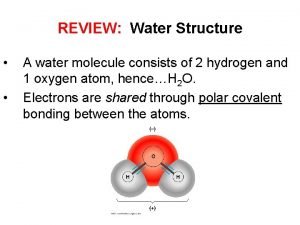
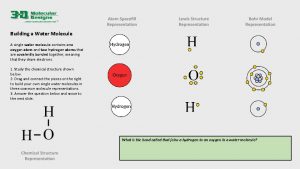
Water Chemistry I Water A The water molecule

















- Slides: 24

Water Chemistry

I. Water A. The water molecule is composed of 2 Hydrogen atoms & 1 oxygen atom 1. Due to the electronic structure the oxygen atom is slightly negative while the hydrogen atoms are slightly positive. B. The charge causes water molecules to be attracted to one another forming hydrogen bonds (cohesion & adhesion) C. Water is the only compound that is found in all 3 states in nature.


II. Special Properties of Water A. Due to hydrogen bonds water is the most dense in its liquid form. Adding salt increases liquid water density 1. Explains why ice floats B. Water has a very high specific heat 1. A lot of energy is needed to increase its temperature by 1°C C. Water also has a high heat of vaporization. 1. As water vapor moves from warm areas to cooler regions it changes back to a liquid and may form rain. Releasing heat which warms the air. The enormous amount of energy involved powers the storms and winds on Earth

II. Special Properties of Water D. Water is a universal solvent 1. Allows the transport of oxygen, carbon dioxide, nutrients and waste materials in water

Now we know a little about water. But where did it all come from? Possible explanations 1. Comets colliding into Earth 2. Volcanism

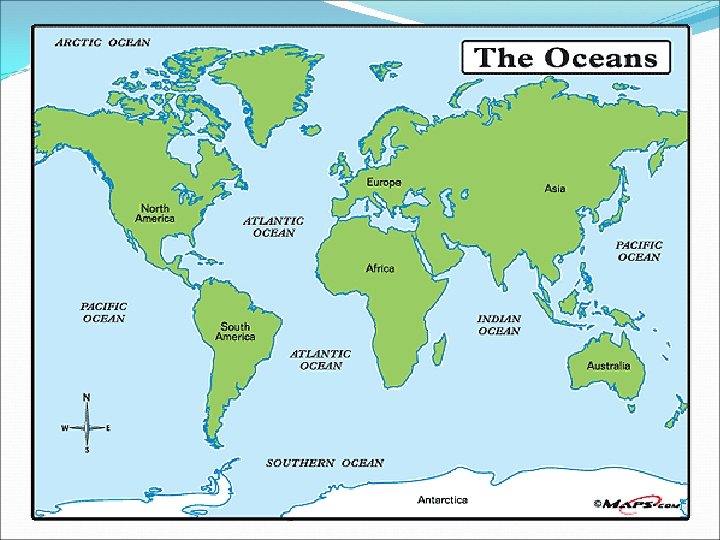
III. Earth’s Oceans A. The 3 major oceans (from largest to smallest) are: 1. Pacific (contains half of Earth’s sea water) 2. Atlantic 3. Indian B. The 2 other oceans mostly composed of ice are: 1. Arctic 2. Antarctic


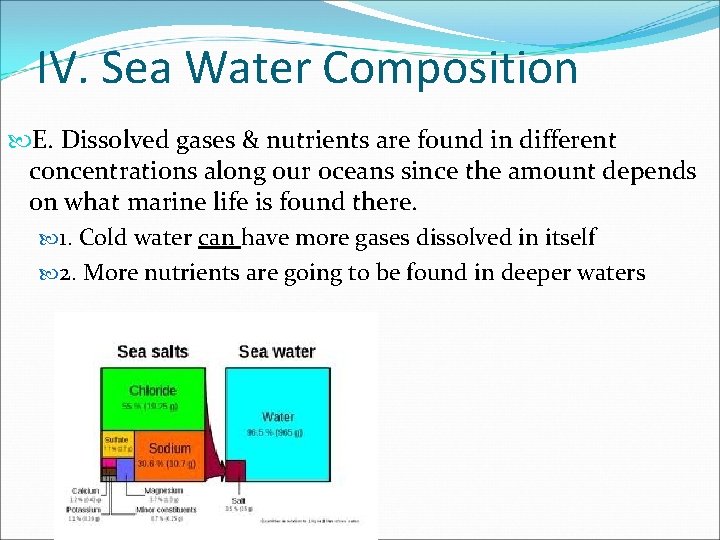
IV. Sea Water Composition A. 71% of our Earth is covered by water & 97% of that water is found in our oceans. B. The salinity of our oceans is usually 35 ppt (parts per thousand) and are usually composed of the same type of salts (mostly sodium chloride) 1. Evaporation/Precipitation can effect the salinity level slightly…WHY?

Osmotic Regulators: Artci Charr Fish & Humpback whales

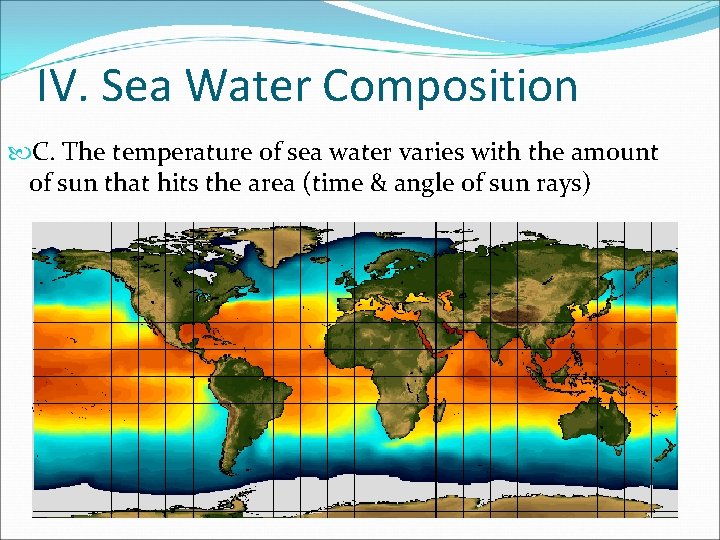
IV. Sea Water Composition C. The temperature of sea water varies with the amount of sun that hits the area (time & angle of sun rays)

Cold blooded: poikilothermic Warm blooded: homeothermic

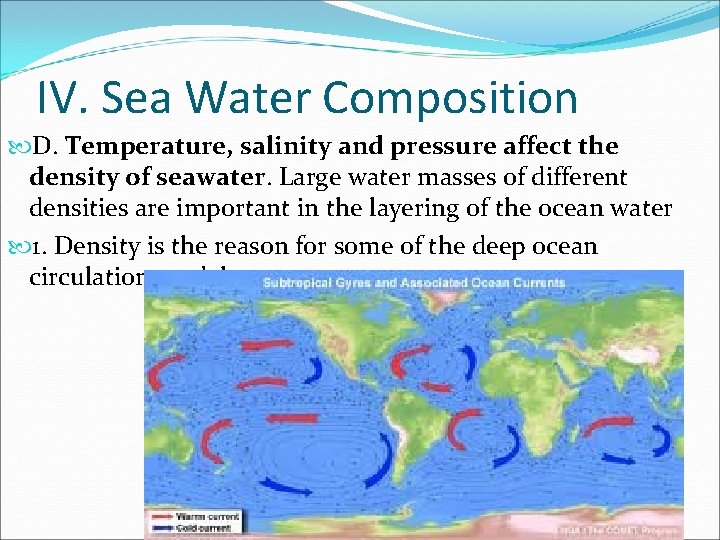
IV. Sea Water Composition D. Temperature, salinity and pressure affect the density of seawater. Large water masses of different densities are important in the layering of the ocean water 1. Density is the reason for some of the deep ocean circulation models

IV. Sea Water Composition E. Dissolved gases & nutrients are found in different concentrations along our oceans since the amount depends on what marine life is found there. 1. Cold water can have more gases dissolved in itself 2. More nutrients are going to be found in deeper waters

V. Sea Water Movement A. Oceans are never motionless 1. Waves – the most obvious movement, is a rhythmic movement that carries energy through space & matter 2. Wave height depends on 3 things: 1. Wind Speed 2. Wind Duration 3. Fetch (how much water wind has blown across)

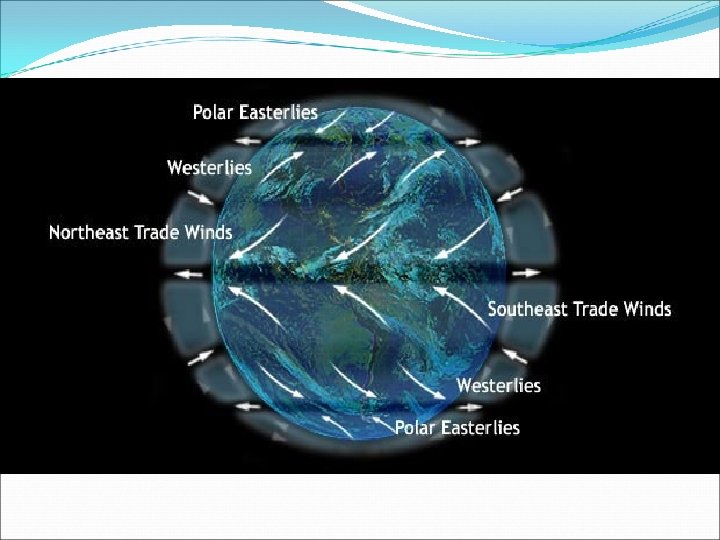
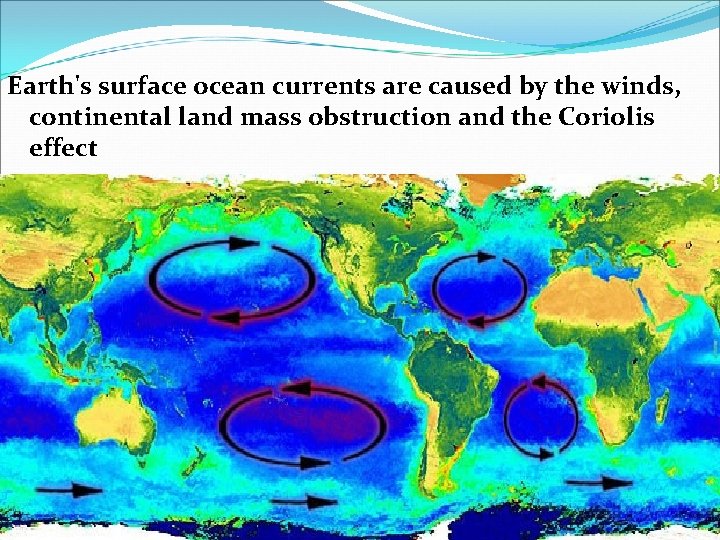
V. Sea Water Movement B. Ocean currents (large mass of moving water)can be caused by 3 different things: 1. Density currents (subsurface)– caused by differences in temperature & salinity, these move very slowly in deep waters 2. Upwelling – upward motion of cold water to replace misplaced warm surface water 3. Surface currents – caused by global wind systems a. Move with the major global wind systems


Earth's surface ocean currents are caused by the winds, continental land mass obstruction and the Coriolis effect

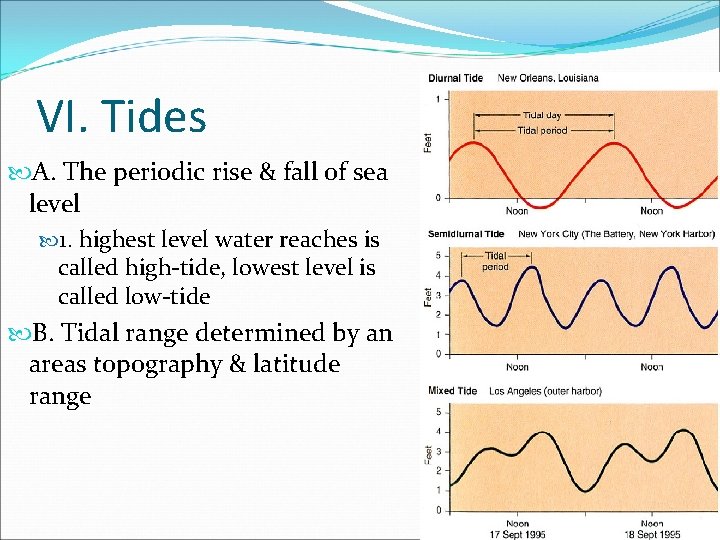
VI. Tides A. The periodic rise & fall of sea level 1. highest level water reaches is called high-tide, lowest level is called low-tide B. Tidal range determined by an areas topography & latitude range

VI. Tides C. The gravitational attraction between Earth, Moon & Sun and the fact that gravitational attraction decreases with distance are the basic causes of tides D. The fact that Earth & the Moon orbit around a common center of gravity causes tidal bulges since they both experience differing gravitational forces due to their size Think of a coffee cup in a car when you turn


VI. Tides E. The gravitational attraction of the Sun also pulls on the tides but since the Sun is not as close to Earth as the moon, Lunar tides are twice as high. F. Depending on the phase of the moon solar tides can either diminish or enhance lunar tides 1. Spring tide – during new moon/full moon, highest high tides and lowest low tides 2. Neap tide – during quarter moons/ high tides are lower & low tides are higher


Homework: Draw a picture of a water molecule. Label the oxygen and hydrogens. Create a graph density vs. temperature graph for water. Explain why water is not most dense when it is frozen. The picture of the iceberg demonstrates the fact that liquid water is more dense than solid water (Ice floats. ) Explain how this property makes life in the arctic possible for marine organisms like polar bears or ring seals. Explain what is the average salinity of sea water. How does the addition of salt changes the density of water. Why and how does changes in salinity affect living organisms? Write down the following sentence, adding the words adhesion and cohesion in the correct places. "________ is the property of water that allows plankton to float near the water's surface. ________ is the property of water that allows trees to easily draw water up to their leaves. "
 Water molecule evaporation
Water molecule evaporation Describe the polar characteristics of a water molecule.
Describe the polar characteristics of a water molecule. Water molecule
Water molecule Hydrogen bonding
Hydrogen bonding Polar covalent bond in water molecule
Polar covalent bond in water molecule Water molecule
Water molecule Water molecule
Water molecule Water molecule
Water molecule Why is water polar
Why is water polar Ice
Ice Water and water and water water
Water and water and water water Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ