1 Chapter Seventeen Thermodynamics Spontaneity Entropy and Free










- Slides: 59

1 Chapter Seventeen Thermodynamics: Spontaneity, Entropy, and Free Energy Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

2 Introduction • Thermodynamics examines the relationship between heat and work. • Spontaneity is the notion of whether or not a process can take place unassisted. • Entropy is a mathematical concept describing the distribution of energy within a system. • Free energy is a thermodynamic function that relates enthalpy and entropy to spontaneity, and can also be related to equilibrium constants. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

3 Why Study Thermodynamics? • With a knowledge of thermodynamics and by making a few calculations before embarking on a new venture, scientists and engineers can save themselves a great deal of time, money, and frustration. – “To the manufacturing chemist thermodynamics gives information concerning the stability of his substances, the yield which he may hope to attain, the methods of avoiding undesirable substances, the optimum range of temperature and pressure, the proper choice of solvent. …” - from the introduction to Thermodynamics and the Free Energy of Chemical Substances by G. N. Lewis and M. Randall • Thermodynamics tells us what processes are possible. – (Kinetics tells us whether the process is practical. ) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

4 Spontaneous Change • A spontaneous process is one that can occur in a system left to itself; no action from outside the system is necessary to bring it about. • A nonspontaneous process is one that cannot take place in a system left to itself. • If a process is spontaneous, the reverse process is nonspontaneous, and vice versa. • Example: gasoline combines spontaneously with oxygen. • However, “spontaneous” signifies nothing about how fast a process occurs. • A mixture of gasoline and oxygen may remain unreacted for years, or may ignite instantly with a spark. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

5 Spontaneous Change (cont’d) • Thermodynamics determines the equilibrium state of a system. • Thermodynamics is used to predict the proportions of products and reactants at equilibrium. • Kinetics determines the pathway by which equilibrium is reached. • A high activation energy can effectively block a reaction that is thermodynamically favored. • Example: combustion reactions are thermodynamically favored, but (fortunately for life on Earth!) most such reactions also have a high activation energy. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

6 Example 17. 1 Indicate whether each of the following processes is spontaneous or nonspontaneous. Comment on cases where a clear determination cannot be made. (a) The action of toilet bowl cleaner, HCl(aq), on “lime” deposits, Ca. CO 3(s). (b) The boiling of water at normal atmospheric pressure and 65 °C. (c) The reaction of N 2(g) and O 2(g) to form NO(g) at room temperature. (d) The melting of an ice cube. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

7 Example 17. 1 Indicate whether each of the following processes is spontaneous or nonspontaneous. Comment on cases where a clear determination cannot be made. (a) The action of toilet bowl cleaner, HCl(aq), on “lime” deposits, Ca. CO 3(s). (b) The boiling of water at normal atmospheric pressure and 65 °C. (c) The reaction of N 2(g) and O 2(g) to form NO(g) at room temperature. (d) The melting of an ice cube. Solution (a) When we add the acid, the fizzing that occurs—the escape of a gas—indicates that the reaction occurs without any further action on our part. The net ionic equation is Ca. CO 3(s) + 2 H 3 O+(aq) Ca 2+(aq) + 3 H 2 O(l) + CO 2(g) The reaction is spontaneous. (b) We know that the normal boiling point of a liquid is the temperature at which the vapor pressure is equal to 1 atm. For water, this temperature is 100 °C. Thus, the boiling of water at 65 °C and 1 atm pressure is nonspontaneous and not possible. (c) Nitrogen and oxygen gases occur mixed in air, and there is no evidence of their reaction to form toxic NO at room temperature. This makes their reaction to form NO(g) appear to be nonspontaneous. However, this could be an example of a spontaneous reaction that occurs extremely slowly, like that of H 2 and O 2 to form H 2 O. We need additional criteria before we can answer this question. (d) We know that at 1 atm pressure ice melts spontaneously at temperatures above its normal melting point of 0 °C. Below this temperature, it does not. To answer this question, we would have to know the temperature. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

Example 17. 1 continued 8 Exercise 17. 1 A Indicate whether each of the following processes is spontaneous or nonspontaneous. Comment on cases where a clear determination cannot be made. (a) The decay of a piece of wood buried in soil. (b) The formation of sodium, Na(s), and chlorine, Cl 2(g), in a vigorously stirred aqueous solution of sodium chloride, Na. Cl(aq). (c) The ionization of hydrogen chloride when HCl(g) dissolves in liquid water. Exercise 17. 1 B Indicate whether each of the following processes is spontaneous or nonspontaneous. Comment on cases where a clear determination cannot be made. (a) The dissolution of 1. 00 m. L of liquid ethanol in 100 m. L of liquid water. (b) The formation of lime, Ca. O(s), and carbon dioxide gas at 1 atm pressure from limestone, Ca. CO 3(s), at 600 °C. (c) The condensation of CO 2(g) at 0. 50 atm to produce CO 2(s). (d) The displacement of H 2(g) at 1 atm from 1 M HCl(aq) by Cu(s). Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

9 Spontaneous Change (cont’d) • Early chemists proposed that spontaneous chemical reactions should occur in the direction of decreasing energy. • It is true that many exothermic processes are spontaneous and that many endothermic reactions are nonspontaneous. • However, enthalpy change is not a sufficient criterion for predicting spontaneous change … Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

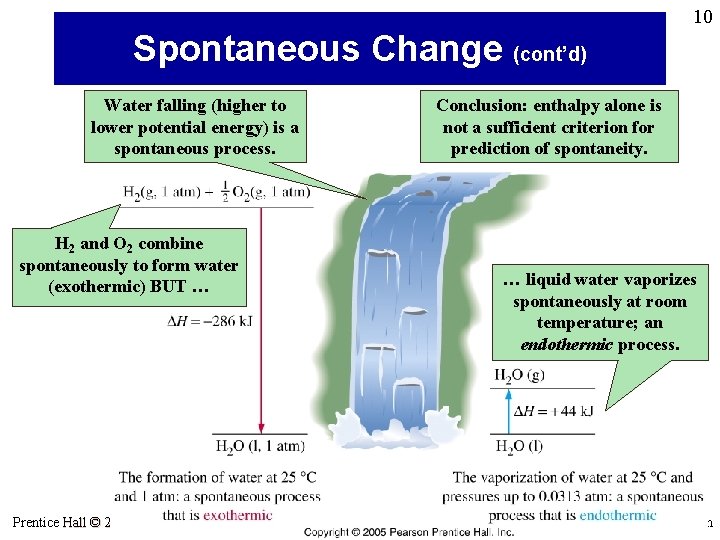
10 Spontaneous Change (cont’d) Water falling (higher to lower potential energy) is a spontaneous process. Conclusion: enthalpy alone is not a sufficient criterion for prediction of spontaneity. H 2 and O 2 combine spontaneously to form water (exothermic) BUT … Prentice Hall © 2005 … liquid water vaporizes spontaneously at room temperature; an endothermic process. General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

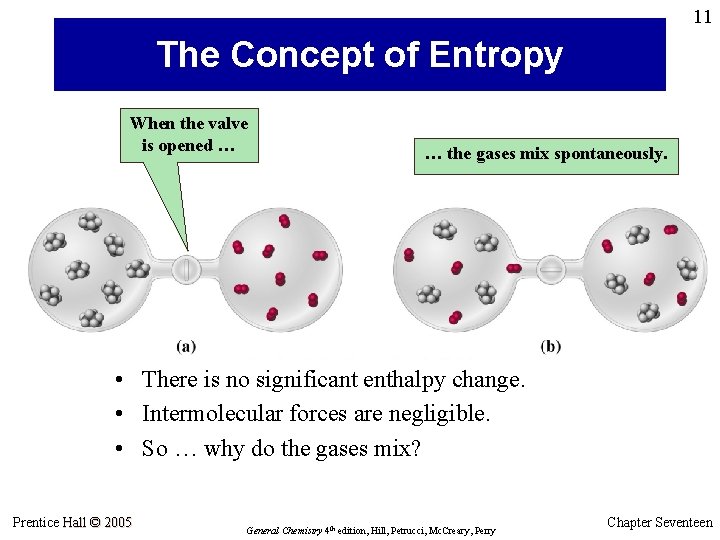
11 The Concept of Entropy When the valve is opened … … the gases mix spontaneously. • There is no significant enthalpy change. • Intermolecular forces are negligible. • So … why do the gases mix? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

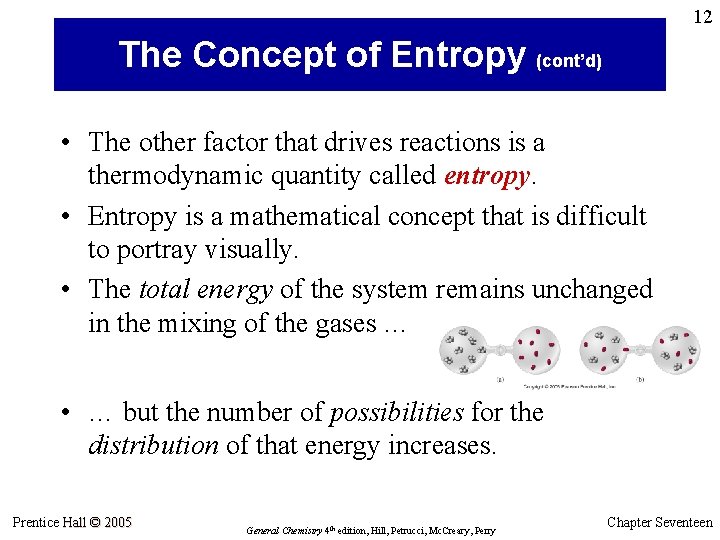
12 The Concept of Entropy (cont’d) • The other factor that drives reactions is a thermodynamic quantity called entropy. • Entropy is a mathematical concept that is difficult to portray visually. • The total energy of the system remains unchanged in the mixing of the gases … • … but the number of possibilities for the distribution of that energy increases. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

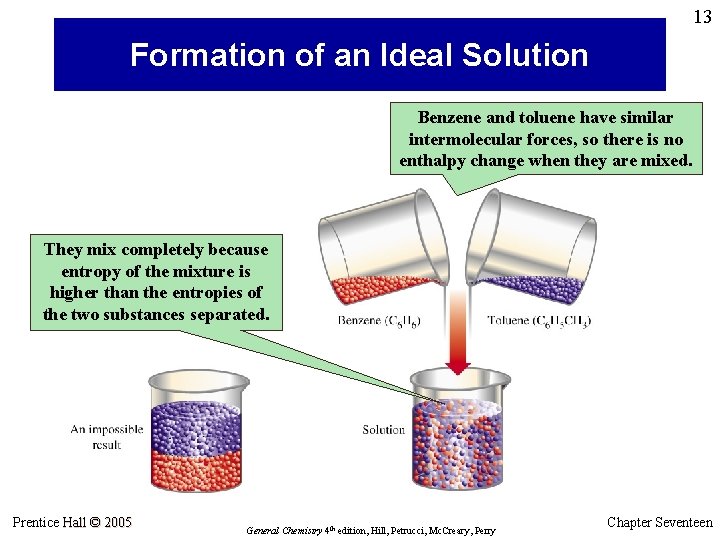
13 Formation of an Ideal Solution Benzene and toluene have similar intermolecular forces, so there is no enthalpy change when they are mixed. They mix completely because entropy of the mixture is higher than the entropies of the two substances separated. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

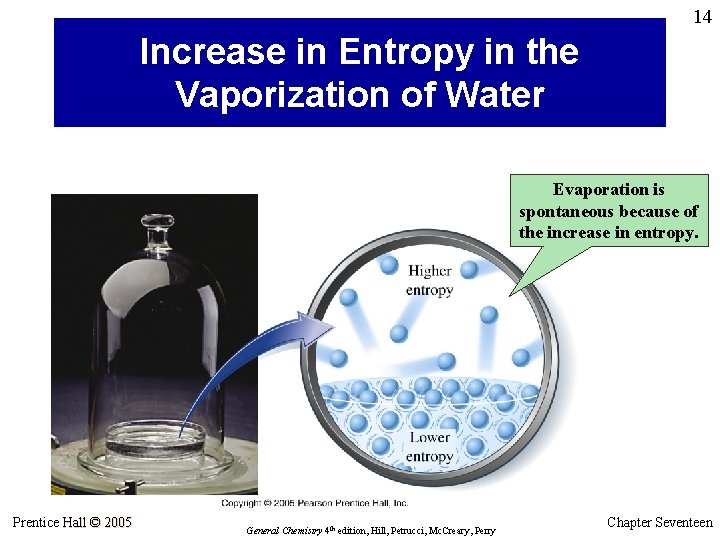
14 Increase in Entropy in the Vaporization of Water Evaporation is spontaneous because of the increase in entropy. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

15 The Concept of Entropy • The spreading of the energy among states, and increase of entropy, often correspond to a greater physical disorder at the microscopic level (however, entropy is not “disorder”). • There are two driving forces behind spontaneous processes: the tendency to achieve a lower energy state (enthalpy change) and the tendency for energy to be distributed among states (entropy). • In many cases, however, the two factors work in opposition. One may increase and the other decrease or vice versa. In these cases, we must determine which factor predominates. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

16 Assessing Entropy Change • The difference in entropy (S) between two states is the entropy change (DS). • The greater the number of configurations of the microscopic particles (atoms, ions, molecules) among the energy levels in a particular state of a system, the greater is the entropy of the system. • Entropy generally increases when: – Solids melt to form liquids. – Solids or liquids vaporize to form gases. – Solids or liquids dissolve in a solvent to form nonelectrolyte solutions. – A chemical reaction produces an increase in the number of molecules of gases. – A substance is heated. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

17 Example 17. 2 Predict whether each of the following leads to an increase or decrease in the entropy of a system. If in doubt, explain why. (a) The synthesis of ammonia: N 2(g) + 3 H 2(g) 2 NH 3(g) (b) Preparation of a sucrose solution: C 12 H 22 O 11(s) H 2 O(l) C 12 H 22 O 11(aq) (c) Evaporation to dryness of a solution of urea, CO(NH 2)2, in water: CO(NH 2)2(aq) CO(NH 2)2(s) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

18 Example 17. 2 Predict whether each of the following leads to an increase or decrease in the entropy of a system. If in doubt, explain why. (a) The synthesis of ammonia: (b) Preparation of a sucrose solution: (c) Evaporation to dryness of a solution of urea, CO(NH 2)2, in water: Solution (a) Four moles of gaseous reactants produce two moles of gaseous product. Because the reaction leads to a decrease in the number of gas molecules, we predict a decrease in entropy. (b) The sucrose molecules are confined in highly ordered crystals in the solid state, whereas they become widely distributed in the aqueous solution. The number of available energy levels for dispersal of the system’s energy increases, and we therefore predict an increase in entropy. (c) Crystallization of the urea from an aqueous solution is the reverse of the process described in part (b), thus indicating a decrease in entropy. At the same time, however, in the evaporation of water a liquid is converted to a gas, suggesting an increase in entropy (recall Figure 17. 4). Without further information, we cannot say whether the entropy of the system increases or decreases. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

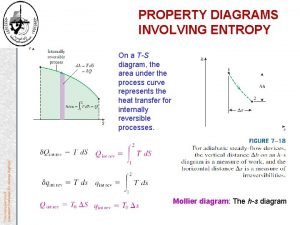
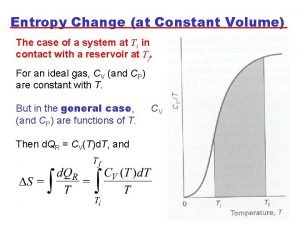
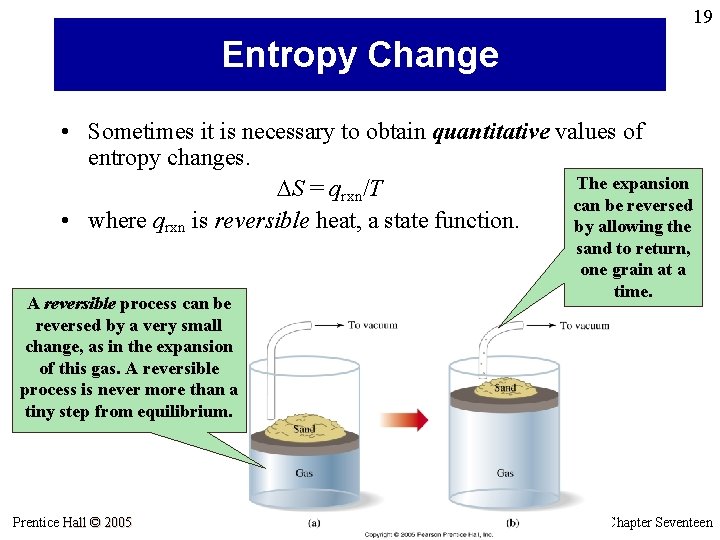
19 Entropy Change • Sometimes it is necessary to obtain quantitative values of entropy changes. The expansion S = qrxn/T can be reversed • where qrxn is reversible heat, a state function. by allowing the sand to return, one grain at a time. A reversible process can be reversed by a very small change, as in the expansion of this gas. A reversible process is never more than a tiny step from equilibrium. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

20 Entropy as a Function of Temperature Entropy always increases with temperature … Prentice Hall © 2005 … and it increases dramatically during a phase change. General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

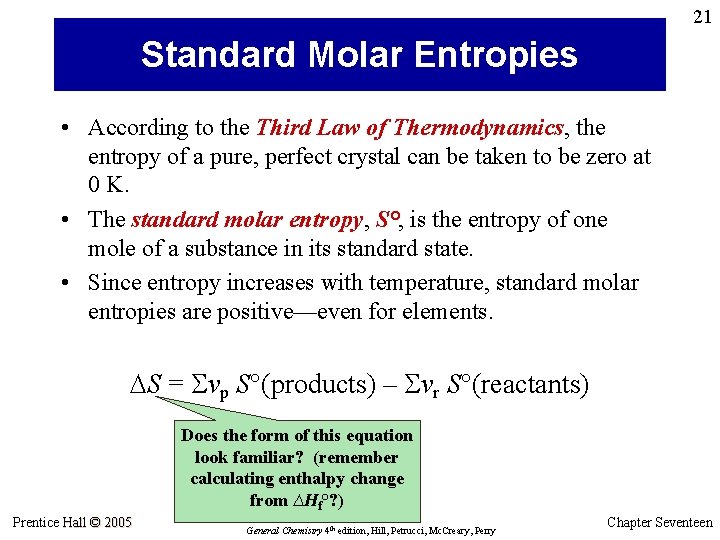
21 Standard Molar Entropies • According to the Third Law of Thermodynamics, the entropy of a pure, perfect crystal can be taken to be zero at 0 K. • The standard molar entropy, S°, is the entropy of one mole of a substance in its standard state. • Since entropy increases with temperature, standard molar entropies are positive—even for elements. S = Svp S°(products) – Svr S°(reactants) Does the form of this equation look familiar? (remember calculating enthalpy change from ∆Hf°? ) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

22 Example 17. 3 Use data from Appendix C to calculate the standard entropy change at 25 °C for the Deacon process, a high -temperature, catalyzed reaction used to convert hydrogen chloride (a by-product from organic chlorination reactions) into chlorine: 4 HCl(g) + O 2(g) 2 Cl 2(g) + 2 H 2 O(g) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

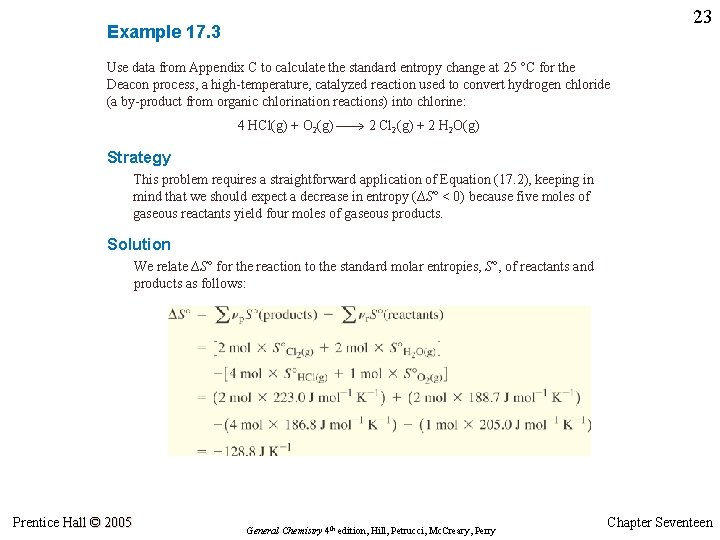
23 Example 17. 3 Use data from Appendix C to calculate the standard entropy change at 25 °C for the Deacon process, a high-temperature, catalyzed reaction used to convert hydrogen chloride (a by-product from organic chlorination reactions) into chlorine: 4 HCl(g) + O 2(g) 2 Cl 2(g) + 2 H 2 O(g) Strategy This problem requires a straightforward application of Equation (17. 2), keeping in mind that we should expect a decrease in entropy (∆S° < 0) because five moles of gaseous reactants yield four moles of gaseous products. Solution We relate ∆S° for the reaction to the standard molar entropies, S°, of reactants and products as follows: Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

24 Example 17. 3 continued Assessment Just as we expected, ∆S° is indeed negative; entropy decreases. Exercise 17. 3 A Use data from Appendix C to calculate the standard molar entropy change at 25 °C for the reaction CO(g) + H 2 O(g) CO 2(g) + H 2(g) Note that this is reaction (c) in Exercise 17. 2 A, for which your answer should have been that you could not predict whether entropy increases or decreases. Exercise 17. 3 B The following reaction may occur in the high-temperature, catalyzed oxidation of NH 3(g). Use data from Appendix C to calculate the standard molar entropy change for this reaction at 25 °C. NH 3(g) + O 2(g) N 2(g) + H 2 O(g) Prentice Hall © 2005 (not balanced) General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

25 The Second Law of Thermodynamics • Entropy can be used as a sole criterion for spontaneous change … • … but the entropy change of both the system and its surroundings must be considered. • The Second Law of Thermodynamics establishes that all spontaneous processes increase the entropy of the universe (system and surroundings). • If entropy increases in both the system and the surroundings, the process is spontaneous. – Is it possible for a spontaneous process to exhibit a decrease in entropy? Yes, if the surroundings __________. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

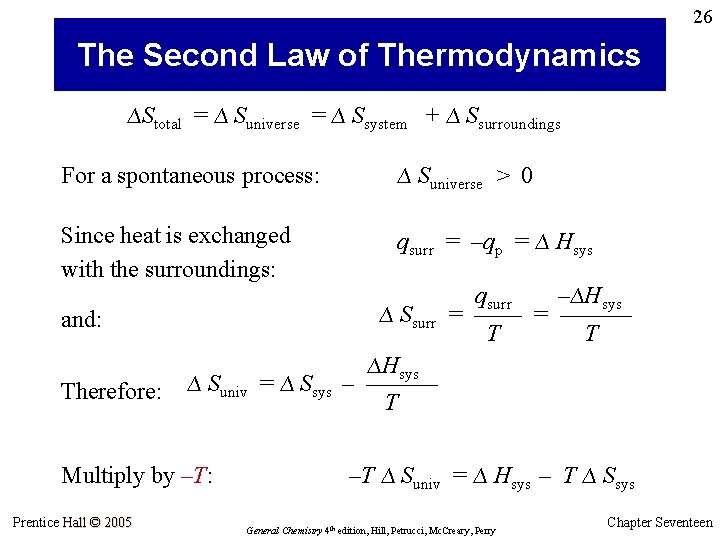
26 The Second Law of Thermodynamics ∆Stotal = ∆ Suniverse = ∆ Ssystem + ∆ Ssurroundings For a spontaneous process: ∆ Suniverse > 0 Since heat is exchanged with the surroundings: qsurr = –qp = ∆ Hsys ∆ Ssurr and: Therefore: ∆ Suniv = ∆ Ssys Multiply by –T: Prentice Hall © 2005 qsurr –∆Hsys = –––––– T T ∆Hsys – –––––– T –T ∆ Suniv = ∆ Hsys – T ∆ Ssys General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

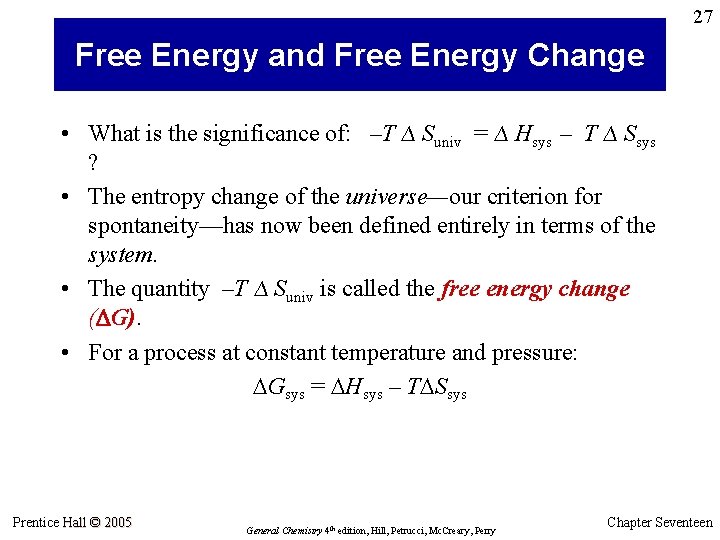
27 Free Energy and Free Energy Change • What is the significance of: –T ∆ Suniv = ∆ Hsys – T ∆ Ssys ? • The entropy change of the universe—our criterion for spontaneity—has now been defined entirely in terms of the system. • The quantity –T ∆ Suniv is called the free energy change (DG). • For a process at constant temperature and pressure: Gsys = Hsys – T Ssys Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

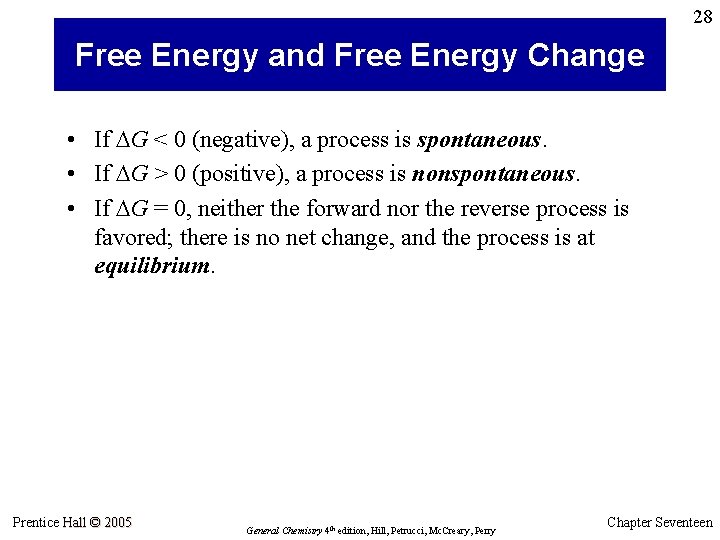
28 Free Energy and Free Energy Change • If G < 0 (negative), a process is spontaneous. • If G > 0 (positive), a process is nonspontaneous. • If G = 0, neither the forward nor the reverse process is favored; there is no net change, and the process is at equilibrium. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

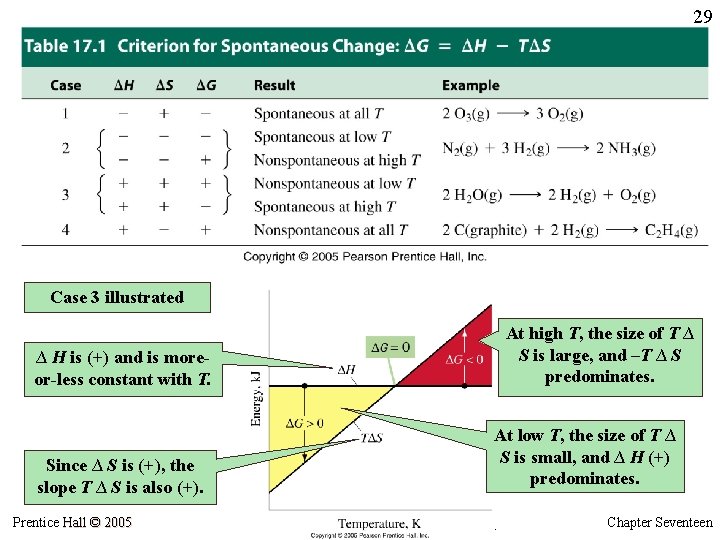
29 Case 3 illustrated At high T, the size of T ∆ S is large, and –T ∆ S predominates. ∆ H is (+) and is moreor-less constant with T. Since ∆ S is (+), the slope T ∆ S is also (+). Prentice Hall © 2005 At low T, the size of T ∆ S is small, and ∆ H (+) predominates. General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

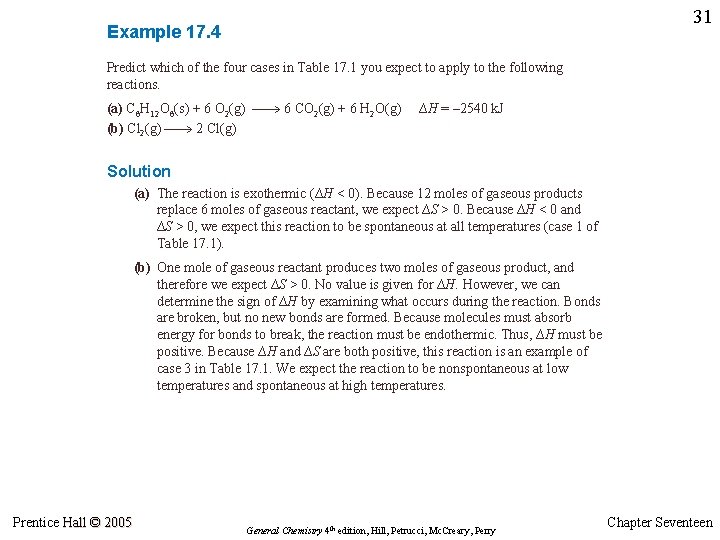
30 Example 17. 4 Predict which of the four cases in Table 17. 1 you expect to apply to the following reactions. (a) C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(g) H = -2540 k. J (b) Cl 2(g) 2 Cl(g) Example 17. 5 A Conceptual Example Molecules exist from 0 K to a few thousand kelvins. At elevated temperatures, they dissociate into atoms. Use the relationship between enthalpy and entropy to explain why this is to be expected. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

31 Example 17. 4 Predict which of the four cases in Table 17. 1 you expect to apply to the following reactions. (a) C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(g) (b) Cl 2(g) 2 Cl(g) ∆H = – 2540 k. J Solution (a) The reaction is exothermic (∆H < 0). Because 12 moles of gaseous products replace 6 moles of gaseous reactant, we expect ∆S > 0. Because ∆H < 0 and ∆S > 0, we expect this reaction to be spontaneous at all temperatures (case 1 of Table 17. 1). (b) One mole of gaseous reactant produces two moles of gaseous product, and therefore we expect ∆S > 0. No value is given for ∆H. However, we can determine the sign of ∆H by examining what occurs during the reaction. Bonds are broken, but no new bonds are formed. Because molecules must absorb energy for bonds to break, the reaction must be endothermic. Thus, ∆H must be positive. Because ∆H and ∆S are both positive, this reaction is an example of case 3 in Table 17. 1. We expect the reaction to be nonspontaneous at low temperatures and spontaneous at high temperatures. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

32 Example 17. 4 continued Exercise 17. 4 A Predict which of the four cases in Table 17. 1 is likely to apply to each of the following reactions. (a) N 2(g) + 2 F 2(g) N 2 F 4(g) (b) COCl 2(g) CO(g) + Cl 2(g) ∆H = – 7. 1 k. J ∆H = +110. 4 k. J Exercise 17. 4 B Predict which of the four cases in Table 17. 1 is likely to apply to the following reaction. for Br. F(g) and additional data from Appendix C Use the value – 93. 85 k. J/mol for as necessary. Br 2(g) + Br. F 3(g) 3 Br. F(g) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

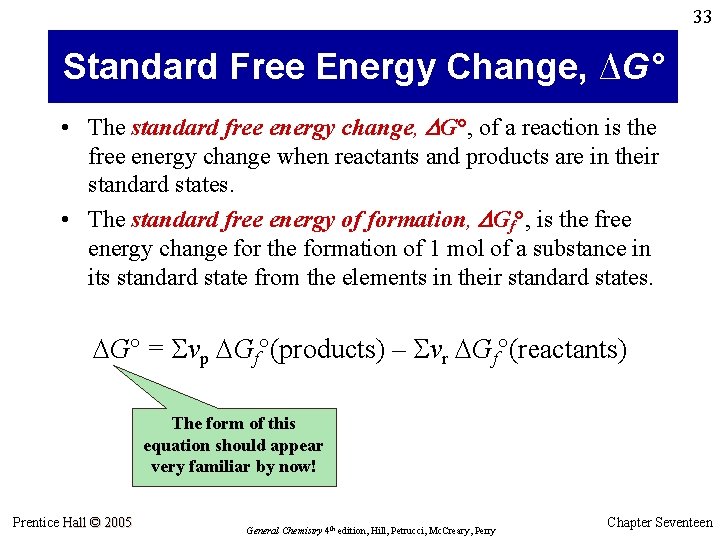
33 Standard Free Energy Change, ∆G° • The standard free energy change, DG°, of a reaction is the free energy change when reactants and products are in their standard states. • The standard free energy of formation, DGf°, is the free energy change for the formation of 1 mol of a substance in its standard state from the elements in their standard states. G° = Svp Gf°(products) – Svr Gf°(reactants) The form of this equation should appear very familiar by now! Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

34 Example 17. 6 Calculate G at 298 K for the reaction 4 HCl(g) + O 2(g) 2 Cl 2(g) + 2 H 2 O(g) ∆H° = -114. 4 k. J (a) using the Gibbs equation (17. 8) and (b) from standard free energies of formation. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

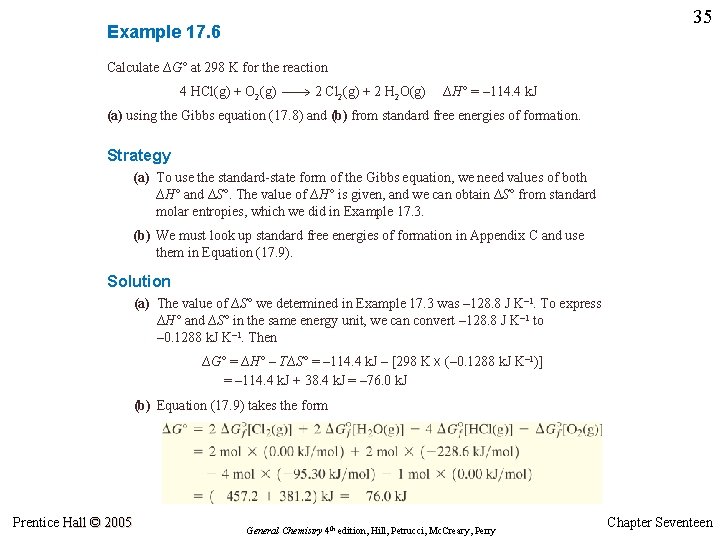
35 Example 17. 6 Calculate ∆G° at 298 K for the reaction 4 HCl(g) + O 2(g) 2 Cl 2(g) + 2 H 2 O(g) ∆H° = – 114. 4 k. J (a) using the Gibbs equation (17. 8) and (b) from standard free energies of formation. Strategy (a) To use the standard-state form of the Gibbs equation, we need values of both ∆H° and ∆S°. The value of ∆H° is given, and we can obtain ∆S° from standard molar entropies, which we did in Example 17. 3. (b) We must look up standard free energies of formation in Appendix C and use them in Equation (17. 9). Solution (a) The value of ∆S° we determined in Example 17. 3 was – 128. 8 J K– 1. To express ∆H° and ∆S° in the same energy unit, we can convert – 128. 8 J K– 1 to – 0. 1288 k. J K– 1. Then ∆G° = ∆H° – T∆S° = – 114. 4 k. J – [298 K x (– 0. 1288 k. J K– 1)] = – 114. 4 k. J + 38. 4 k. J = – 76. 0 k. J (b) Equation (17. 9) takes the form Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

Example 17. 6 continued 36 Assessment The principal check on part (a) is that the T∆S° product should be about 40 k. J (that is, 300 x 0. 13), which, when added to the value of ∆H°, should yield a value of ∆G° ≈ – 75 k. J. The principal check on part (b) is that it should give the same answer as part (a), and it does. Exercise 17. 6 A Use the standard-state form of the Gibbs equation to determine the standard free energy change at 25 °C for these reactions: (a) 2 NO(g) + O 2(g) 2 NO 2(g) ∆H° = – 114. 1 k. J ∆S° = – 146. 2 J K– 1 (b) 2 CO(g) + 2 NH 3(g) C 2 H 6(g) + 2 NO(g) ∆H° = +409. 0 k. J ∆S° = – 129. 1 J K– 1 Exercise 17. 6 B Use standard free energies of formation from Appendix C to determine the standard free energy change at 25 °C for these reactions: (a) CS 2(l) + 2 S 2 Cl 2(g) CCl 4(l) + 6 S(s) (b) NH 3(g) + O 2(g) N 2(g) + H 2 O(g) (not balanced) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

37 Free Energy Change and Equilibrium • At equilibrium, G = 0 (reaction is neither spontaneous nor nonspontaneous). • Therefore, at the equilibrium temperature, the free energy change expression becomes: H = T S and S = H/T • Trouton’s rule states that the entropy change is about the same when one mole of a substance is converted from liquid to vapor (at the normal boiling point). • S°vapn for many substances is about 87 J mol– 1 K– 1. • This rule works best with nonpolar substances. • It generally fails for liquids with a more ordered structure, such as those with extensive hydrogen bonding. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

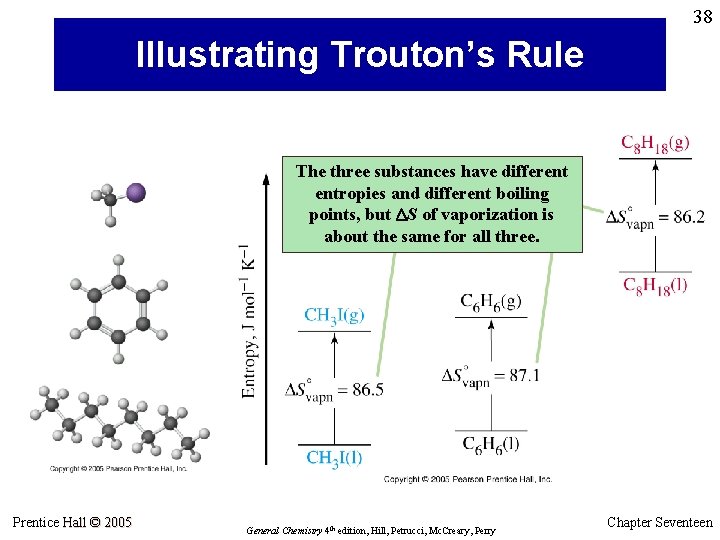
38 Illustrating Trouton’s Rule The three substances have different entropies and different boiling points, but DS of vaporization is about the same for all three. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

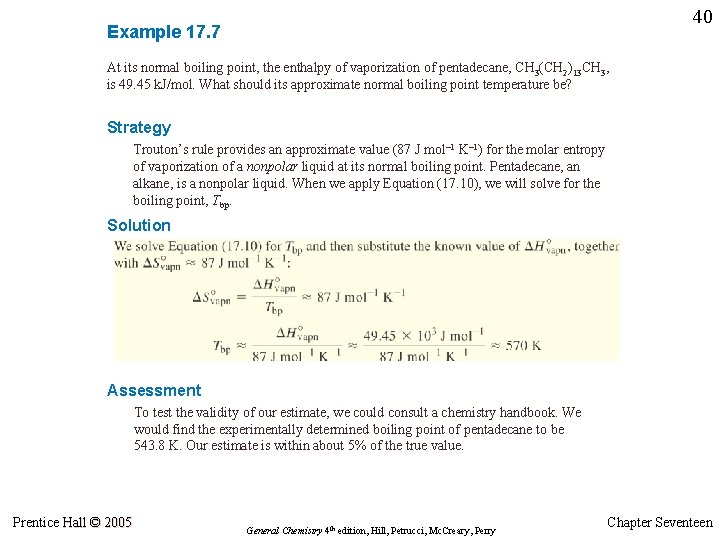
39 Example 17. 7 At its normal boiling point, the enthalpy of vaporization of pentadecane, CH 3(CH 2)13 CH 3, is 49. 45 k. J/mol. What should its approximate normal boiling point temperature be? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

40 Example 17. 7 At its normal boiling point, the enthalpy of vaporization of pentadecane, CH 3(CH 2)13 CH 3, is 49. 45 k. J/mol. What should its approximate normal boiling point temperature be? Strategy Trouton’s rule provides an approximate value (87 J mol– 1 K– 1) for the molar entropy of vaporization of a nonpolar liquid at its normal boiling point. Pentadecane, an alkane, is a nonpolar liquid. When we apply Equation (17. 10), we will solve for the boiling point, Tbp. Solution Assessment To test the validity of our estimate, we could consult a chemistry handbook. We would find the experimentally determined boiling point of pentadecane to be 543. 8 K. Our estimate is within about 5% of the true value. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

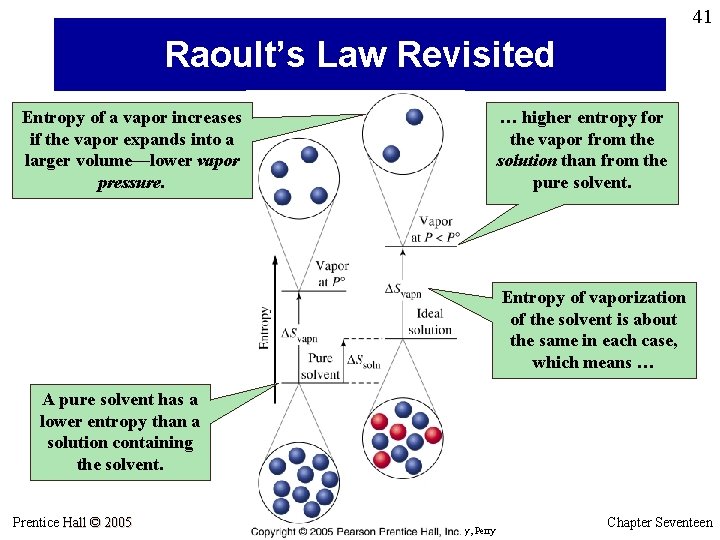
41 Raoult’s Law Revisited Entropy of a vapor increases if the vapor expands into a larger volume—lower vapor pressure. … higher entropy for the vapor from the solution than from the pure solvent. Entropy of vaporization of the solvent is about the same in each case, which means … A pure solvent has a lower entropy than a solution containing the solvent. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

Relationship of DG° to the Equilibrium Constant, Keq 42 • G = 0 is a criterion for equilibrium at any temperature. • G° = 0 is a criterion for equilibrium at a single temperature, that temperature at which the equilibrium state has all reactants and products in their standard states. G and Go are related through the reaction quotient, Q: G = G° + RT ln Q • When G = 0, then Q = Keq, and the equation becomes: G° = -RT ln Keq Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

43 The Equilibrium Constant, Keq • The concentrations and partial pressures we have used in Keq are approximations. • Activities (a) are the correct variables for Keq. But activities are very difficult to determine. That is why we use approximations. • For pure solid and liquid phases: a = 1. • For gases: Assume ideal gas behavior, and replace the activity by the numerical value of the gas partial pressure (in atm). • For solutes in aqueous solution: Replace solute activity by the numerical value of the solute molarity (M). Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

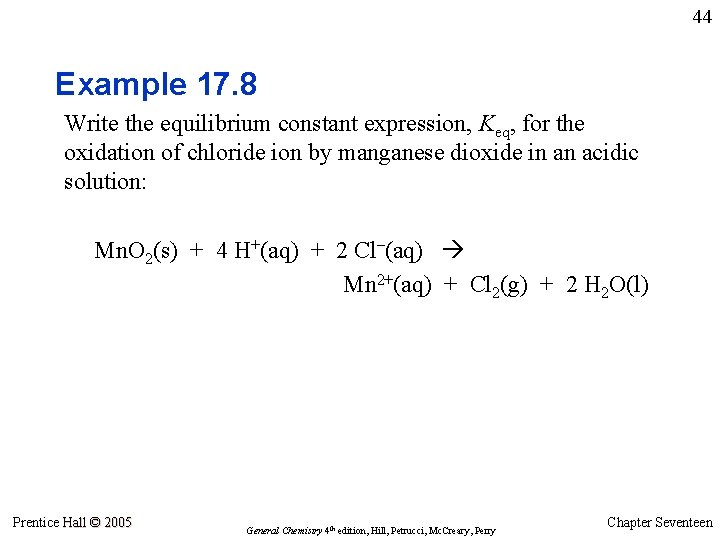
44 Example 17. 8 Write the equilibrium constant expression, Keq, for the oxidation of chloride ion by manganese dioxide in an acidic solution: Mn. O 2(s) + 4 H+(aq) + 2 Cl–(aq) Mn 2+(aq) + Cl 2(g) + 2 H 2 O(l) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

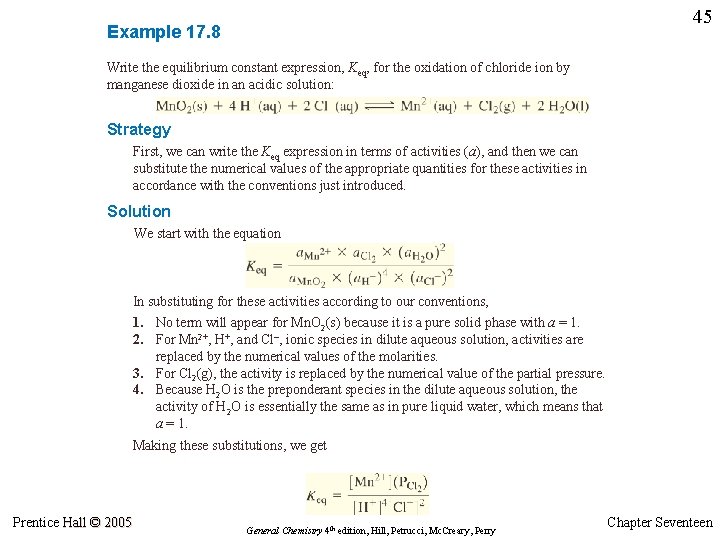
45 Example 17. 8 Write the equilibrium constant expression, Keq, for the oxidation of chloride ion by manganese dioxide in an acidic solution: Strategy First, we can write the Keq expression in terms of activities (a), and then we can substitute the numerical values of the appropriate quantities for these activities in accordance with the conventions just introduced. Solution We start with the equation In substituting for these activities according to our conventions, 1. No term will appear for Mn. O 2(s) because it is a pure solid phase with a = 1. 2. For Mn 2+, H+, and Cl–, ionic species in dilute aqueous solution, activities are replaced by the numerical values of the molarities. 3. For Cl 2(g), the activity is replaced by the numerical value of the partial pressure. 4. Because H 2 O is the preponderant species in the dilute aqueous solution, the activity of H 2 O is essentially the same as in pure liquid water, which means that a = 1. Making these substitutions, we get Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

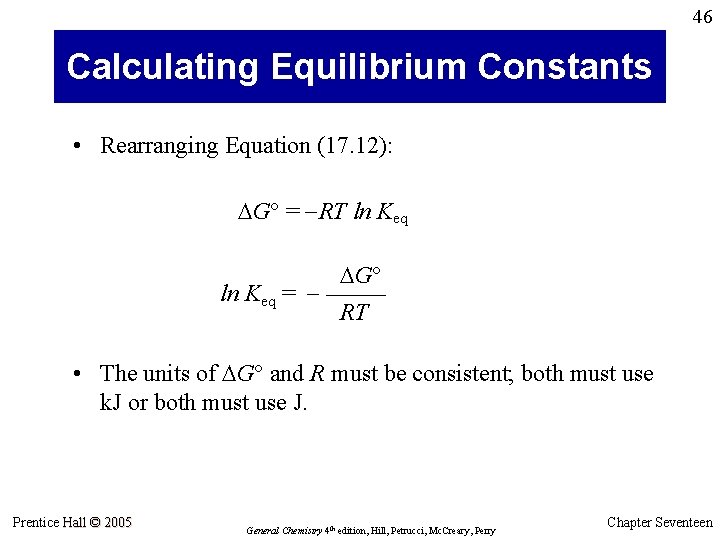
46 Calculating Equilibrium Constants • Rearranging Equation (17. 12): G° = -RT ln Keq G° ln Keq = - ––––– RT • The units of G° and R must be consistent; both must use k. J or both must use J. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

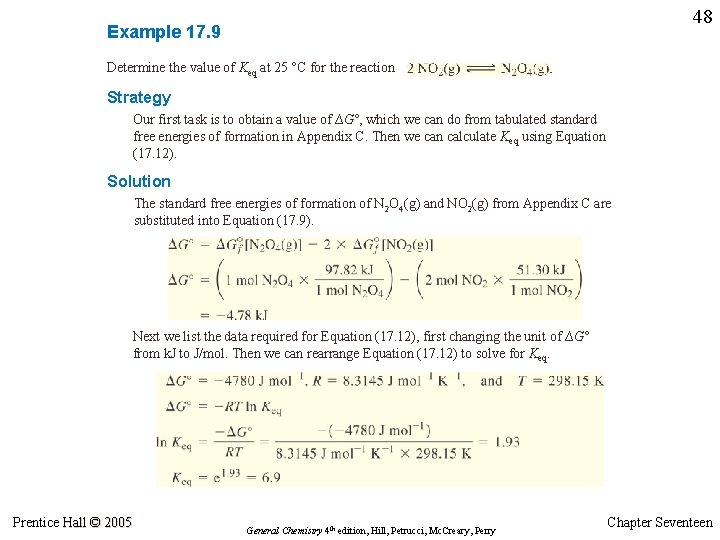
47 Example 17. 9 Determine the value of Keq at 25 °C for the reaction 2 NO 2(g) Prentice Hall © 2005 N 2 O 4(g) General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

48 Example 17. 9 Determine the value of Keq at 25 °C for the reaction Strategy Our first task is to obtain a value of ∆G°, which we can do from tabulated standard free energies of formation in Appendix C. Then we can calculate Keq using Equation (17. 12). Solution The standard free energies of formation of N 2 O 4(g) and NO 2(g) from Appendix C are substituted into Equation (17. 9). Next we list the data required for Equation (17. 12), first changing the unit of ∆G° from k. J to J/mol. Then we can rearrange Equation (17. 12) to solve for Keq. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

Example 17. 9 continued 49 Assessment In this problem, the value of ∆G° is negative, and so ln Keq must be greater than 1. If we had made a rather common error and forgotten about the minus sign on the right in Equation (17. 12), the value of Keq would have been less than 1 (that is, e– 1. 93 = 0. 145)—a tip-off that an error had been made. Exercise 17. 9 A Use data from Appendix C to determine Keq at 25 °C for the reaction Exercise 17. 9 B Use data from Appendix C to determine Keq at 25 °C for the reaction If NO(g) at 1 atm is allowed to react with an excess of Br 2(l), what will be the partial pressures of NO(g) and NOBr(g) at equilibrium? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

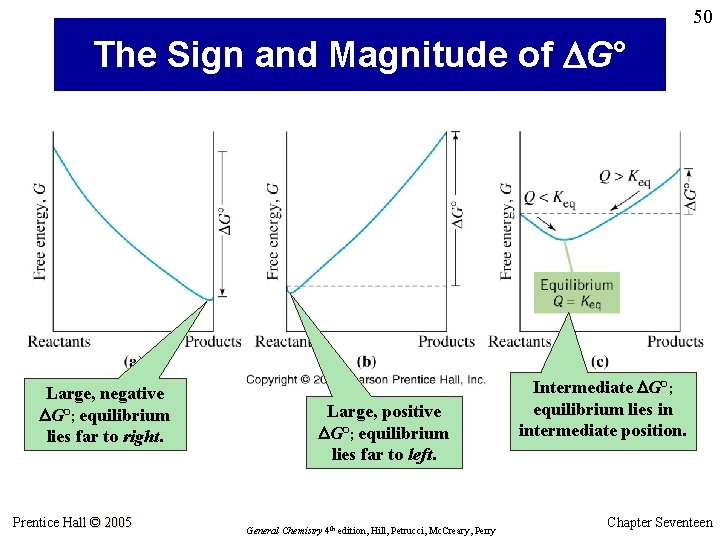
50 The Sign and Magnitude of DG° Large, negative DG°; equilibrium lies far to right. Prentice Hall © 2005 Large, positive DG°; equilibrium lies far to left. General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Intermediate DG°; equilibrium lies in intermediate position. Chapter Seventeen

51 The Dependence of DG° and Keq on Temperature • To obtain equilibrium constants at different temperatures, it will be assumed that H° does not change much with temperature. • To obtain Keq at the desired temperature, the van’t Hoff equation is used: - H° ln Keq = ––––– + constant or RT K 2 H° ln ––– = –––– K 1 R [ ] 1 1 ––– – ––– T 1 T 2 • The form used depends on whether we have a single value of Keq available, or multiple values. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

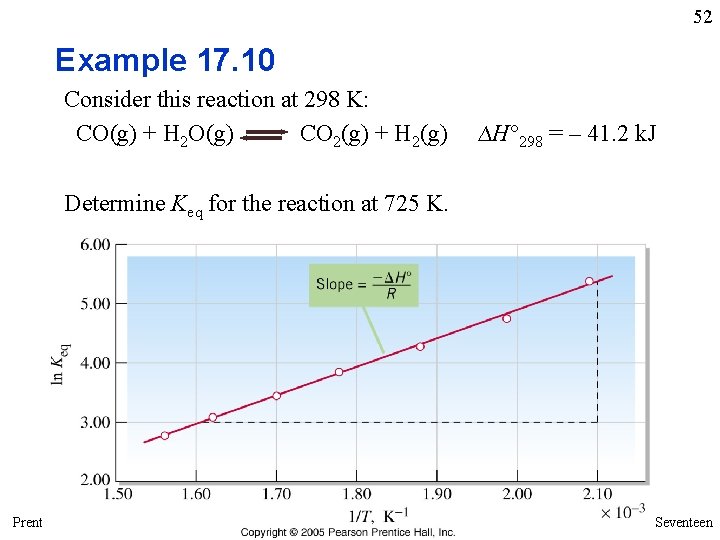
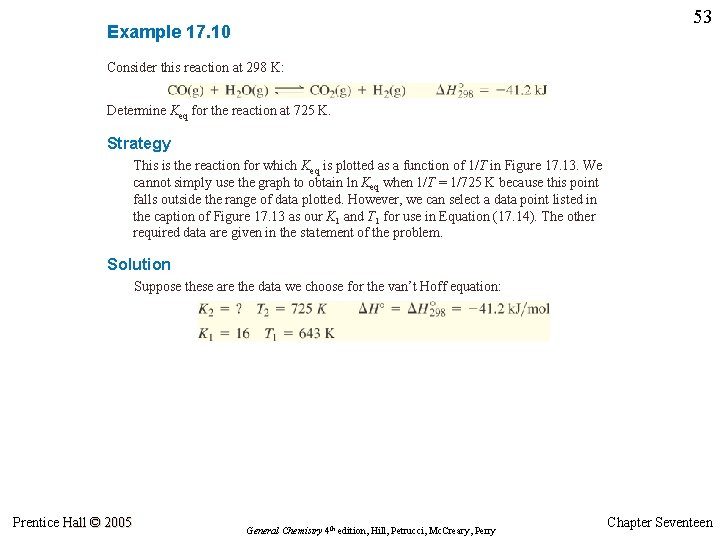
52 Example 17. 10 Consider this reaction at 298 K: CO(g) + H 2 O(g) CO 2(g) + H 2(g) ∆H° 298 = – 41. 2 k. J Determine Keq for the reaction at 725 K. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

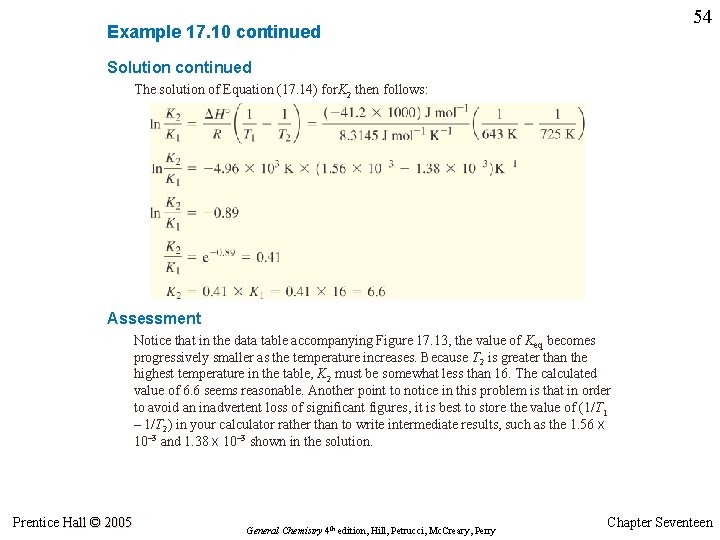
53 Example 17. 10 Consider this reaction at 298 K: Determine Keq for the reaction at 725 K. Strategy This is the reaction for which Keq is plotted as a function of 1/T in Figure 17. 13. We cannot simply use the graph to obtain ln Keq when 1/T = 1/725 K because this point falls outside the range of data plotted. However, we can select a data point listed in the caption of Figure 17. 13 as our K 1 and T 1 for use in Equation (17. 14). The other required data are given in the statement of the problem. Solution Suppose these are the data we choose for the van’t Hoff equation: Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

54 Example 17. 10 continued Solution continued The solution of Equation (17. 14) for. K 2 then follows: Assessment Notice that in the data table accompanying Figure 17. 13, the value of Keq becomes progressively smaller as the temperature increases. Because T 2 is greater than the highest temperature in the table, K 2 must be somewhat less than 16. The calculated value of 6. 6 seems reasonable. Another point to notice in this problem is that in order to avoid an inadvertent loss of significant figures, it is best to store the value of (1/T 1 – 1/T 2) in your calculator rather than to write intermediate results, such as the 1. 56 x 10– 3 and 1. 38 x 10– 3 shown in the solution. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

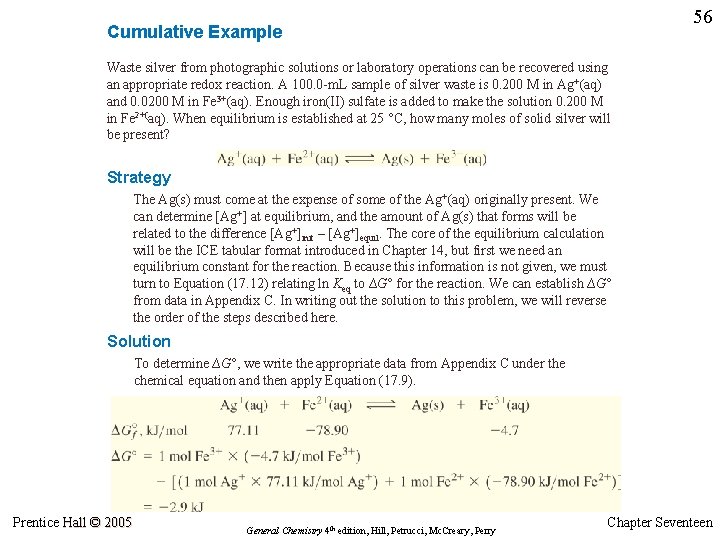
55 Cumulative Example Waste silver from photographic solutions or laboratory operations can be recovered using an appropriate redox reaction. A 100. 0 -m. L sample of silver waste is 0. 200 M in Ag+(aq) and 0. 0200 M in Fe 3+(aq). Enough iron(II) sulfate is added to make the solution 0. 200 M in Fe 2+(aq). When equilibrium is established at 25 °C, how many moles of solid silver will be present? Ag+(aq) + Fe 2+(aq) Ag(s) + Fe 3+(aq) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

56 Cumulative Example Waste silver from photographic solutions or laboratory operations can be recovered using an appropriate redox reaction. A 100. 0 -m. L sample of silver waste is 0. 200 M in Ag+(aq) and 0. 0200 M in Fe 3+(aq). Enough iron(II) sulfate is added to make the solution 0. 200 M in Fe 2+(aq). When equilibrium is established at 25 °C, how many moles of solid silver will be present? Strategy The Ag(s) must come at the expense of some of the Ag+(aq) originally present. We can determine [Ag+] at equilibrium, and the amount of Ag(s) that forms will be related to the difference [Ag+]init – [Ag+]equil. The core of the equilibrium calculation will be the ICE tabular format introduced in Chapter 14, but first we need an equilibrium constant for the reaction. Because this information is not given, we must turn to Equation (17. 12) relating ln Keq to ∆G° for the reaction. We can establish ∆G° from data in Appendix C. In writing out the solution to this problem, we will reverse the order of the steps described here. Solution To determine ∆G°, we write the appropriate data from Appendix C under the chemical equation and then apply Equation (17. 9). Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

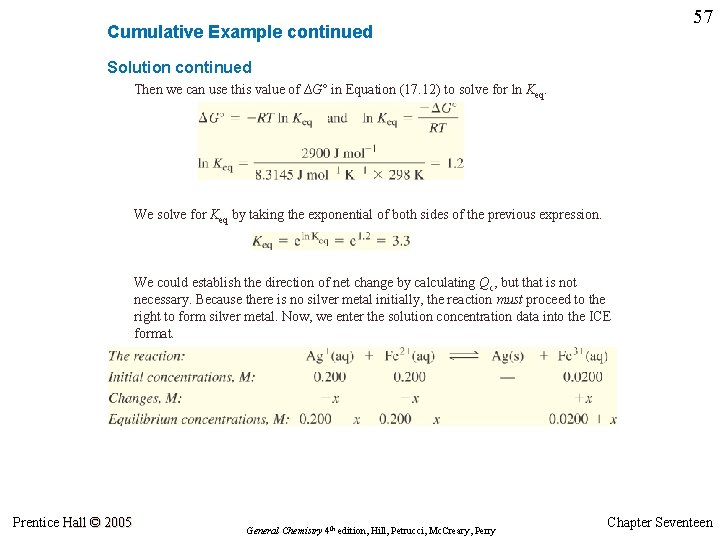
57 Cumulative Example continued Solution continued Then we can use this value of ∆G° in Equation (17. 12) to solve for ln Keq. We solve for Keq by taking the exponential of both sides of the previous expression. We could establish the direction of net change by calculating Qc, but that is not necessary. Because there is no silver metal initially, the reaction must proceed to the right to form silver metal. Now, we enter the solution concentration data into the ICE format. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

58 Cumulative Example continued Assessment In choosing the correct root of the quadratic equation, we were guided by the fact that x < 0. 200. That is, [Ag+] at equilibrium is (0. 200 – x) M, and this value must be a positive quantity. The rejected root had x = 0. 6. We can verify that the equilibrium concentrations are correct by showing that Qc based on equilibrium concentrations is equal to the value of Keq used in the calculation. Here, we obtain Qc = 0. 08/(0. 14)2 = 4. This matches Keq = 3. 3 quite well, given the rather limited precision of the calculation. The limitation in precision was introduced in calculating ∆G°, where we had to work with the difference between two numbers of nearly equal magnitude. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

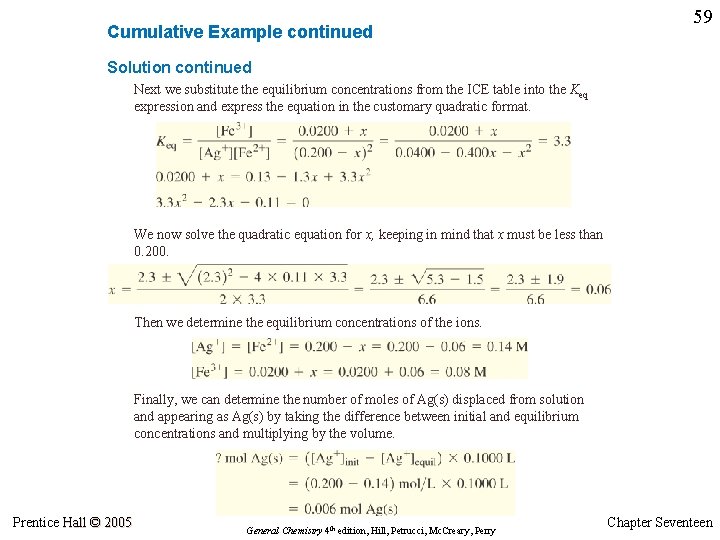
Cumulative Example continued 59 Solution continued Next we substitute the equilibrium concentrations from the ICE table into the Keq expression and express the equation in the customary quadratic format. We now solve the quadratic equation for x, keeping in mind that x must be less than 0. 200. Then we determine the equilibrium concentrations of the ions. Finally, we can determine the number of moles of Ag(s) displaced from solution and appearing as Ag(s) by taking the difference between initial and equilibrium concentrations and multiplying by the volume. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen
 Ap chem spontaneity entropy and free energy
Ap chem spontaneity entropy and free energy Chemistry microstates
Chemistry microstates Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Gibbs free energy spontaneous
Gibbs free energy spontaneous Thermodynamics
Thermodynamics Entropy in thermodynamics
Entropy in thermodynamics Thermodynamic second law
Thermodynamic second law Dance moms seventeen magazine
Dance moms seventeen magazine Seventeen master key
Seventeen master key Seventeen current manager
Seventeen current manager Seventeen table
Seventeen table Enthalpy vs entropy
Enthalpy vs entropy Gibbs free energy spontaneous
Gibbs free energy spontaneous Is delta g spontaneous
Is delta g spontaneous Enthalpy entropy free energy
Enthalpy entropy free energy Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity What is a half reaction
What is a half reaction Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Summary of the story of an hour
Summary of the story of an hour Free hearts free foreheads you and i are
Free hearts free foreheads you and i are Q system = -q surroundings
Q system = -q surroundings What is enthalpy and entropy
What is enthalpy and entropy Minimum enthalpy and maximum entropy
Minimum enthalpy and maximum entropy Entropy system and surroundings
Entropy system and surroundings Entropy order parameters and complexity
Entropy order parameters and complexity Chapter 6
Chapter 6 Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Thermodynamics chapter 2
Thermodynamics chapter 2 Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Thermodynamics chapter 4
Thermodynamics chapter 4 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Specific heat capacity
Specific heat capacity Thermodynamics chapter 3
Thermodynamics chapter 3 Chemical engineering thermodynamics 8th solution chapter 2
Chemical engineering thermodynamics 8th solution chapter 2 Negative free energy change
Negative free energy change Free energy
Free energy Kernel dynamic memory
Kernel dynamic memory Free-free absorption
Free-free absorption Entropy equation
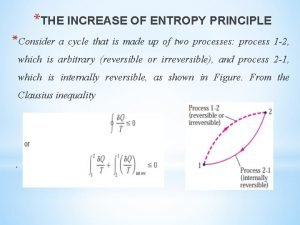
Entropy equation The increase of entropy principle
The increase of entropy principle Reverse entropy
Reverse entropy Non spontaneous process
Non spontaneous process Second law of thermodynamics
Second law of thermodynamics Entropy is scalar or vector
Entropy is scalar or vector δhsys
δhsys Entropy change formula
Entropy change formula Entropy in bits
Entropy in bits Huffman coding visualization
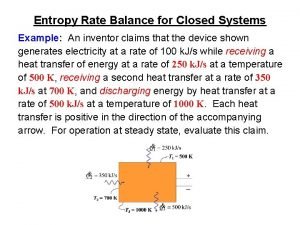
Huffman coding visualization Entropy balance equation for closed systems
Entropy balance equation for closed systems Dsuniverse
Dsuniverse Physical science chapter 6 review answers
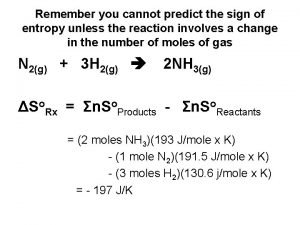
Physical science chapter 6 review answers Standard entropy change formula
Standard entropy change formula Refrigerator entropy
Refrigerator entropy Constant entropy
Constant entropy