Unit 7 Stoichiometry General Info Stoichiometry is the


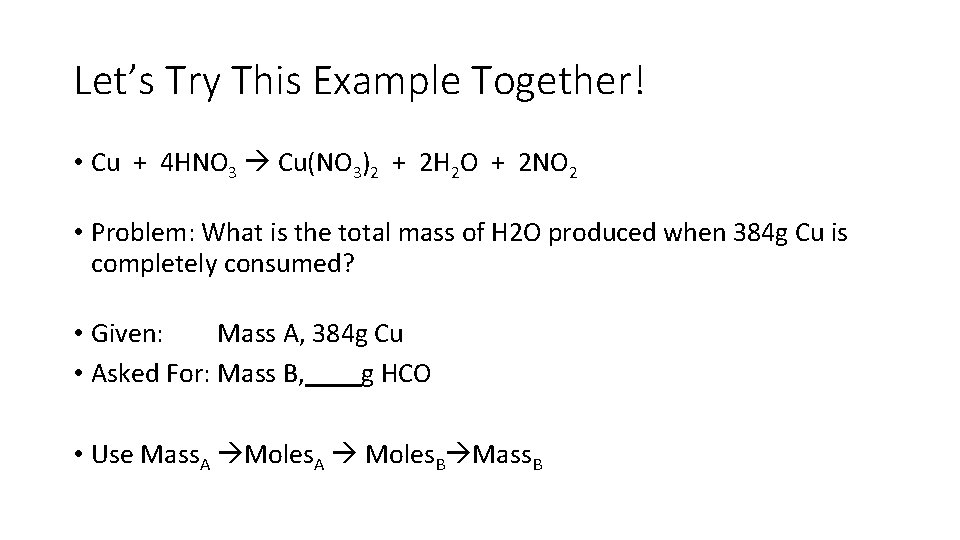











- Slides: 24

Unit 7 Stoichiometry

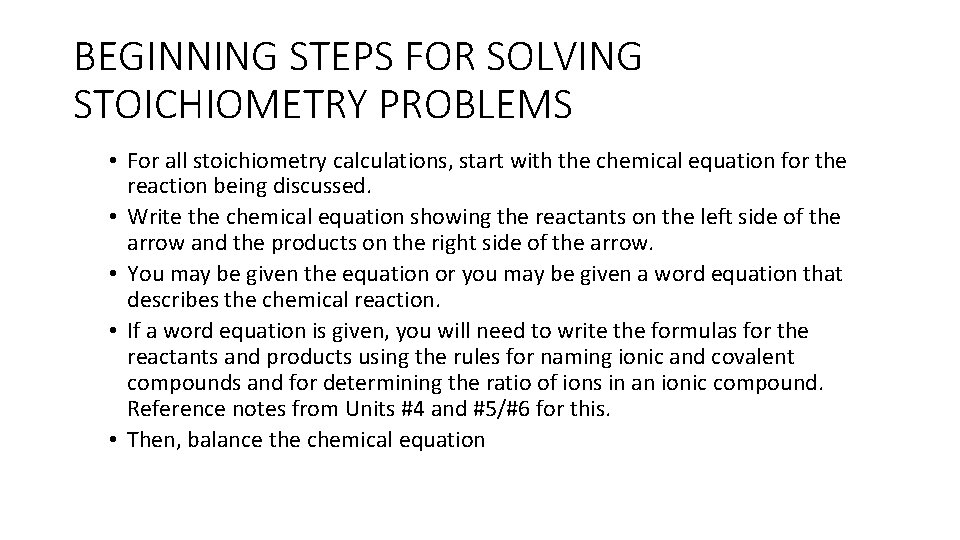
General Info • Stoichiometry is the quantitative relationships between the amounts of reactants used and the amounts of products formed by a chemical reaction. It is “Chemistry Accounting. ” • Starting quantities of a reactant or product may be given in moles, grams, number of particles/molecules or Liters of gas. These quantities are then used to calculate the amount of another substance involved in the chemical reaction. • Dimensional analysis (also known as the Factor Label Method) is most commonly used to solve stoichiometry problems and involves setting up a series of conversion factors dependent on units and known equalities to solve for an unknown. • It is very important that units are included with all values in a calculation to ensure a proper set-up.

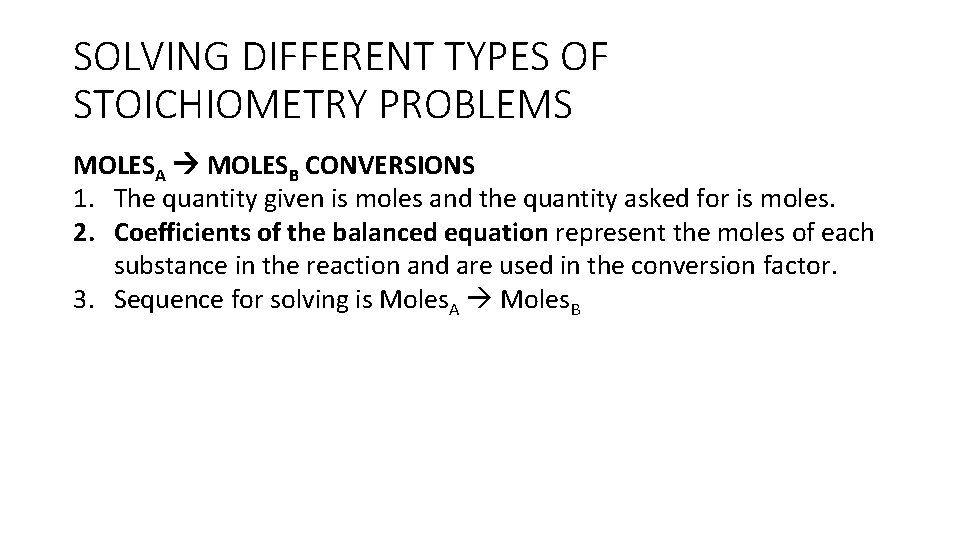
BEGINNING STEPS FOR SOLVING STOICHIOMETRY PROBLEMS • For all stoichiometry calculations, start with the chemical equation for the reaction being discussed. • Write the chemical equation showing the reactants on the left side of the arrow and the products on the right side of the arrow. • You may be given the equation or you may be given a word equation that describes the chemical reaction. • If a word equation is given, you will need to write the formulas for the reactants and products using the rules for naming ionic and covalent compounds and for determining the ratio of ions in an ionic compound. Reference notes from Units #4 and #5/#6 for this. • Then, balance the chemical equation

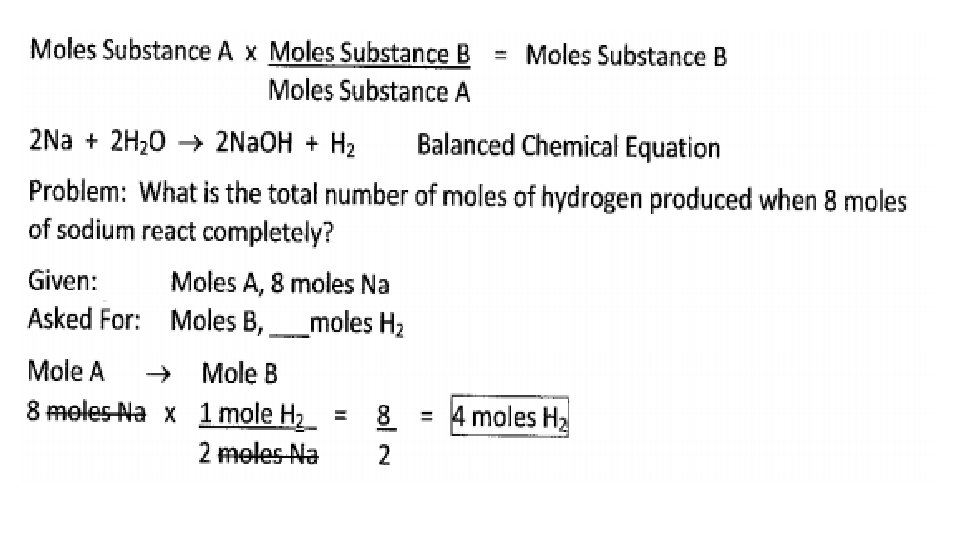
SOLVING DIFFERENT TYPES OF STOICHIOMETRY PROBLEMS MOLESA MOLESB CONVERSIONS 1. The quantity given is moles and the quantity asked for is moles. 2. Coefficients of the balanced equation represent the moles of each substance in the reaction and are used in the conversion factor. 3. Sequence for solving is Moles. A Moles. B


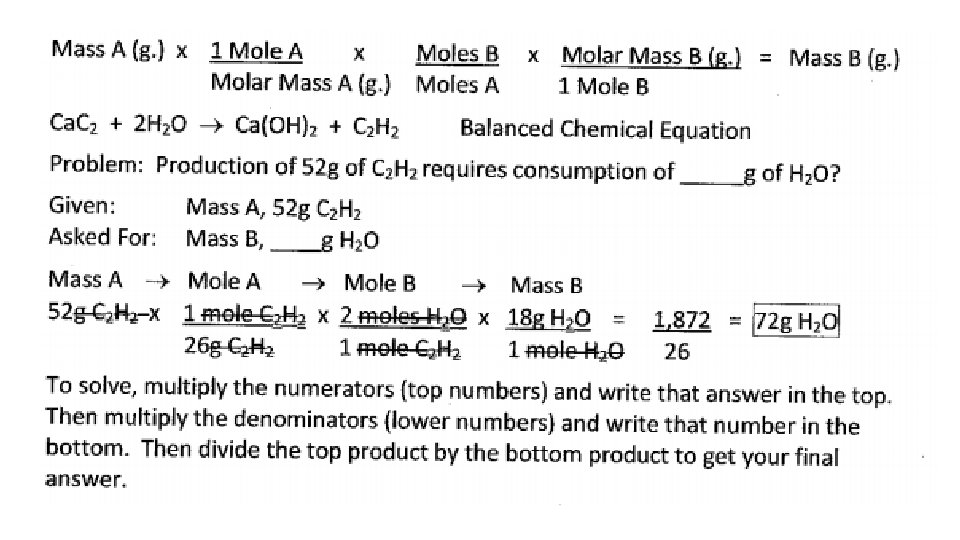
MASSA MASSB CONVERSIONS 1. The quantity given is mass in grams and the quantity asked for is mass in grams. 2. Coefficients of the balanced equation and molar masses of each substance involved are used in the conversion factors 3. Sequence for solving is Mass. A Moles. A Moles. B Mass. B

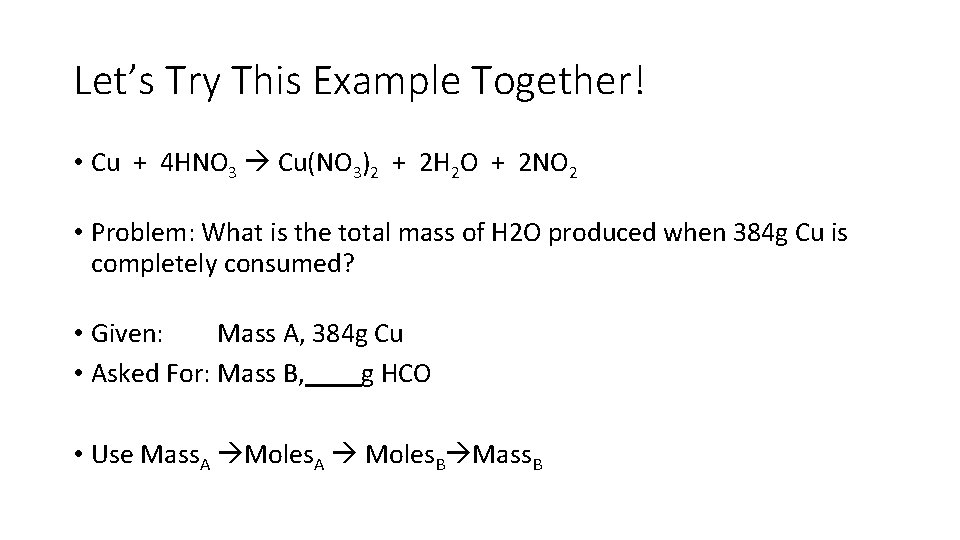
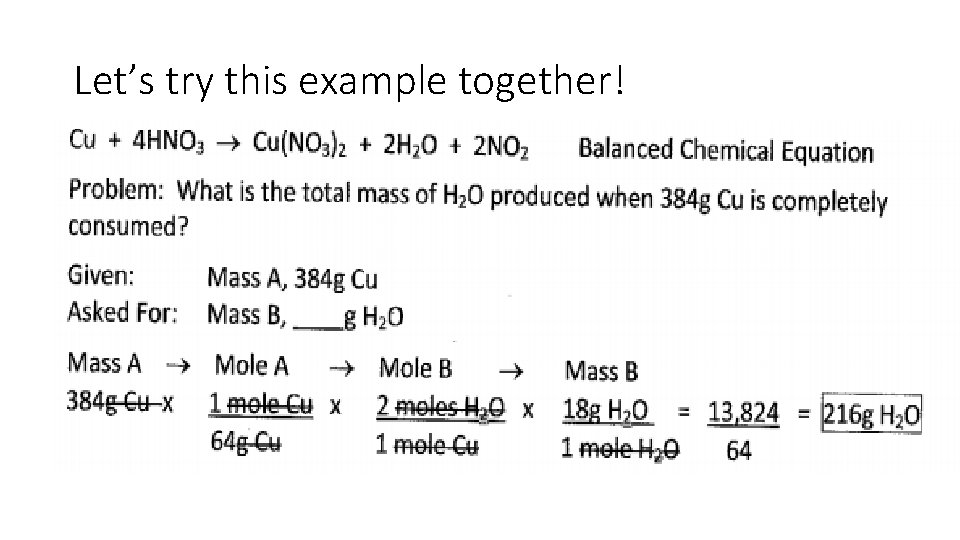
Let’s Try This Example Together! • Cu + 4 HNO 3 Cu(NO 3)2 + 2 H 2 O + 2 NO 2 • Problem: What is the total mass of H 2 O produced when 384 g Cu is completely consumed? • Given: Mass A, 384 g Cu • Asked For: Mass B, g HCO • Use Mass. A Moles. A Moles. B Mass. B

Let’s try this example together!

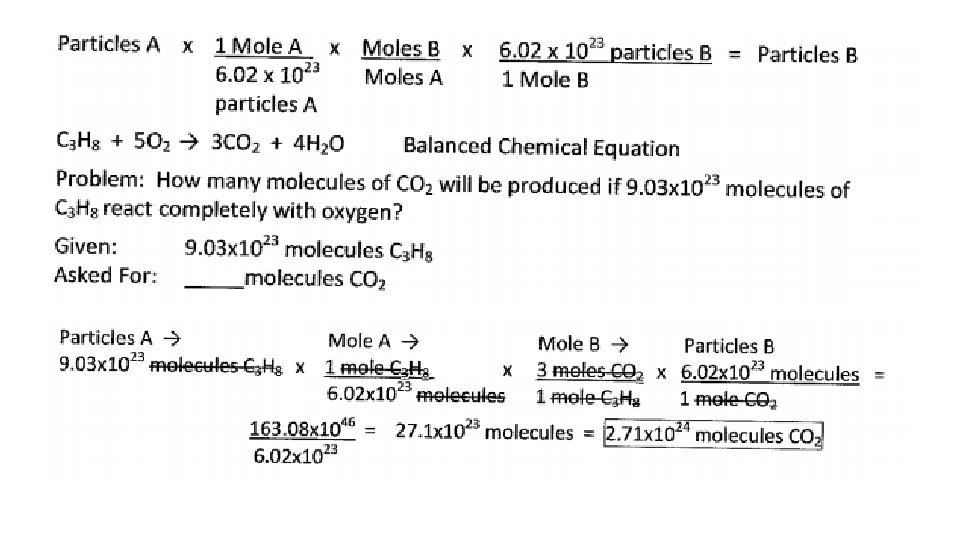
Particles. A Particles. B Conversion 1. The quantity given is particles/molecules and the quantity asked for is particles/molecules. 2. The coefficients of the balanced equation and Avogadro’s number (1 mo. Ie=6. 02 x 10 23 particles) are used in the conversion factors. 3. Sequence for solving is Particles. A Moles. A Moles. B Particles. B



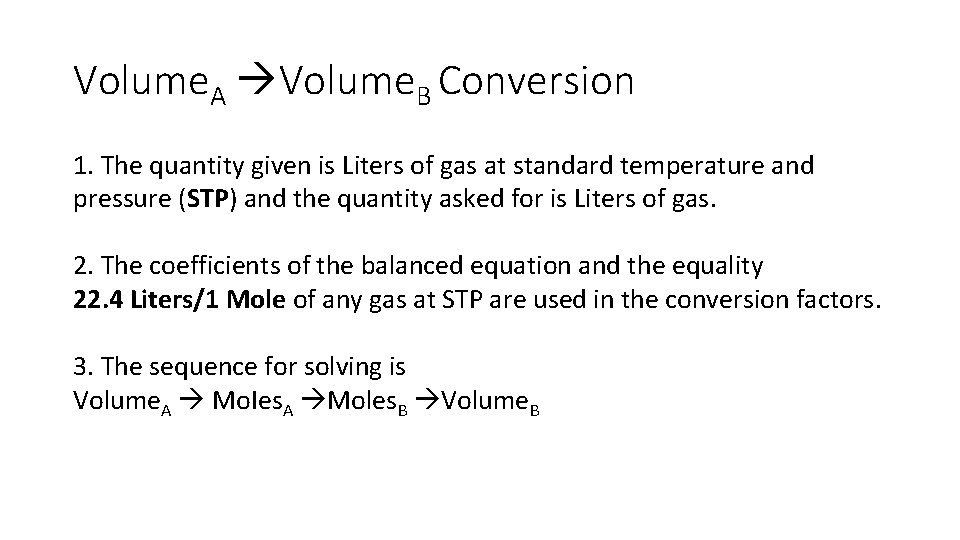
Volume. A Volume. B Conversion 1. The quantity given is Liters of gas at standard temperature and pressure (STP) and the quantity asked for is Liters of gas. 2. The coefficients of the balanced equation and the equality 22. 4 Liters/1 Mole of any gas at STP are used in the conversion factors. 3. The sequence for solving is Volume. A Mo. Ies. A Moles. B Volume. B



Mixed Problems: • Other Stoichiometric Problems used the same conversion factors as listed previously, but can include: • Mass. A Moles. B • Moles. A Mass. B

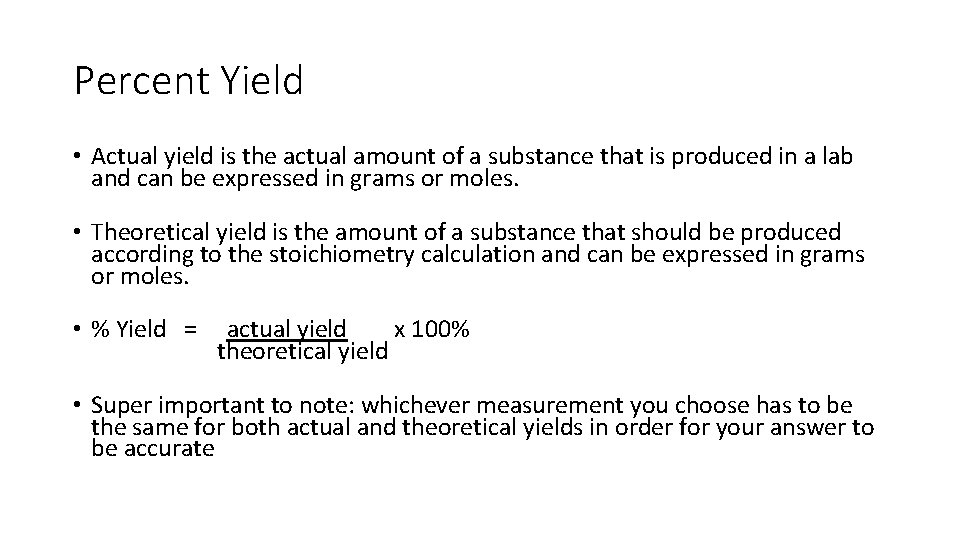
Percent Yield and Limiting Reactants Important Formula!! % Yield = actual yield x 100% theoretical yield

Percent Yield • Actual yield is the actual amount of a substance that is produced in a lab and can be expressed in grams or moles. • Theoretical yield is the amount of a substance that should be produced according to the stoichiometry calculation and can be expressed in grams or moles. • % Yield = actual yield x 100% theoretical yield • Super important to note: whichever measurement you choose has to be the same for both actual and theoretical yields in order for your answer to be accurate

Example:

Example:

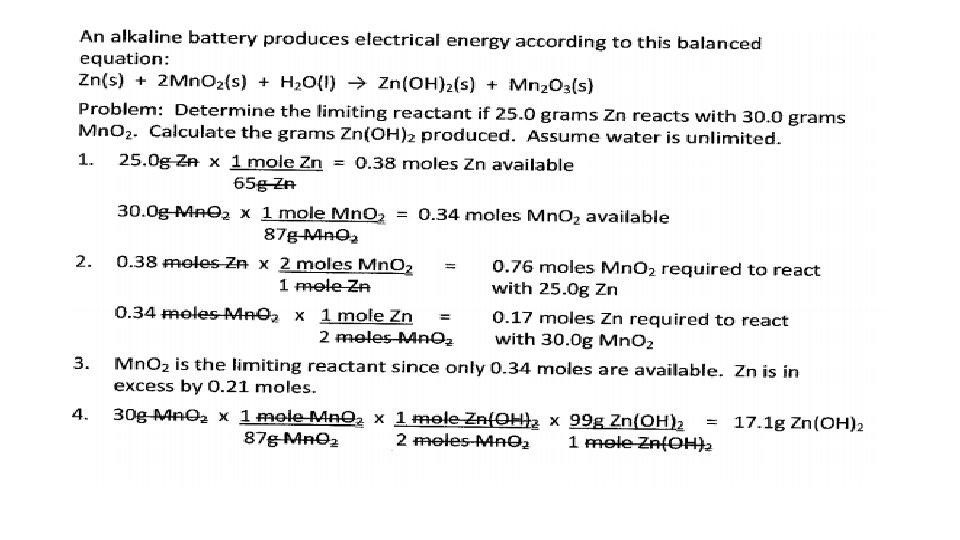
Limiting Reactants • Chemical reactions are limited by the amount of reactants available to produce product. • A limiting reactant is the reactant that is completely consumed during a chemical reaction. Reactants that remain after the reaction has stopped are called excess reactants. • To determine the limiting reactant, the moles of reactants available must be calculated (if not given). Then, using the coefficients of the balanced equation, the amount of each reactant required to react completely must be compared to what’s available. • The amount of product produced is calculated using the quantity of reactant determined to be limiting.


