To Start Isnt it unnerving that Doctors call











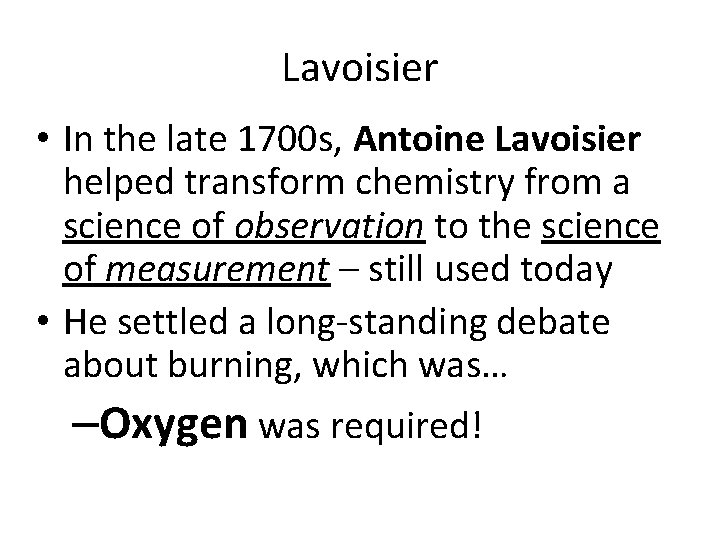































- Slides: 90

To Start Isn’t it unnerving that Doctors call they do “practice” Why do we park on a driveway and drive on a parkway If a turtle does not have a shell is it naked or homeless Why isn’t phonetic spelled the way it sounds

What is Chemistry? • Chemistry is the study of the composition of “matter” – (matter is anything with mass and occupies space), its composition, properties, and the changes it undergoes. • Has a definite affect on everyday life - taste of foods, grades of gasoline, etc. • Living and nonliving things are made of matter.

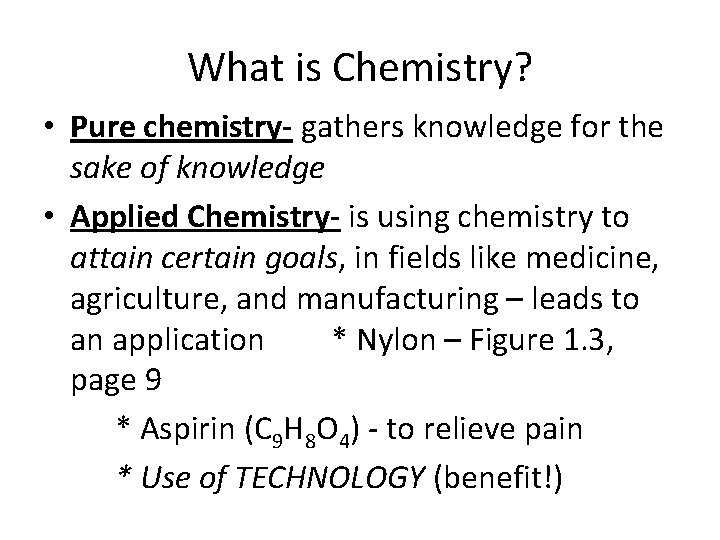
Chemistry is the study of the composition, structure, and properties of matter and the changes it undergoes – such as burning fuels. C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O + Energy Reactants Products

5 Major Areas of Chemistry 1) Analytical Chemistry- concerned with the composition of substances. 2) Inorganic Chemistry- primarily deals with substances without carbon 3) Organic Chemistry- essentially all substances containing carbon 4) Biochemistry- Chemistry of living things 5) Physical Chemistry- describes the behavior of chemicals (ex. stretching); involves lots of math! Boundaries not firm – they overlap and interact

What is Chemistry? • Pure chemistry- gathers knowledge for the sake of knowledge • Applied Chemistry- is using chemistry to attain certain goals, in fields like medicine, agriculture, and manufacturing – leads to an application * Nylon – Figure 1. 3, page 9 * Aspirin (C 9 H 8 O 4) - to relieve pain * Use of TECHNOLOGY (benefit!)

Why Study Chemistry? • Everyone and everything around us involves chemistry – explains our world • What in the world isn’t Chemistry? • Helps you make choices; helps make you a better informed citizen • A possible career for your future • Used to attain a specific goal • What did we describe as “pure” and “applied” chemistry?

Why Study Chemistry? –What benefits do each of the pictures represent in improving our lives? –Give examples in your daily life that involve use of chemistry, and things that do not?

Chemistry Far and Wide • Chemists design materials to fit specific needs – velcro (Patented in 1955) perfume, steel, ceramics, plastics, rubber, paints, nonstick cooking utensils, polyester fibers • Two different ways to look at the world: macroscopic and microscopic

Chemistry Far and Wide • Energy – we constantly have greater demands –We can conserve it; use wisely –We can try to produce more; oil from soybeans to make biodiesel –fossil fuels, solar, batteries (that store energy – rechargeable? ), nuclear (don’t forget pollution!)

Chemistry Far and Wide • Medicine and Biotechnology–Supply materials doctors use to treat patients –vitamin C, penicillin, aspirin (C H O ) –materials for artery transplants and hipbones –bacteria producing insulin 9 8 4

Chemistry Far and Wide • Agriculture –Produce the world’s food supply –Use chemistry for better productivity – soil, water, weeds –plant growth hormones –ways to protect crops; insecticides –disease resistant plants

Chemistry Far and Wide • The Environment – both risks and benefits involved in discoveries – Pollutants need to be 1) identified and 2) prevented – Lead paint was prohibited in 1978; Leaded gasoline? Drinking water? – carbon dioxide, ozone, global warming

Chemistry Far and Wide • The Universe –Need to gather data from afar, and analyze matter brought back to Earth –composition of the planets –analyze moon rocks –planet atmospheres –life on other planets?

Alchemy – developed the tools and techniques for working with chemicals • The word chemistry comes from alchemy – practiced in China and India since 400 B. C. • Alchemy has two sides: – Practical: techniques for working with metals, glass, dyes, etc. – Mystical: concepts like perfection – gold was a perfect metal

An Experimental Approach • In the 1500 s, a shift started from alchemy to science – King Charles II was a supporter of the sciences • “Royal Society of London for the Promotion of Natural Knowledge” • Encouraged scientists to use more experimental evidence, and not philosophical debates
Lavoisier • In the late 1700 s, Antoine Lavoisier helped transform chemistry from a science of observation to the science of measurement – still used today • He settled a long-standing debate about burning, which was… –Oxygen was required!

What? • Why don't sheep shrink when it rains? • Where do forest rangers go to get away from it all? • Can vegetarians eat animal crackers?

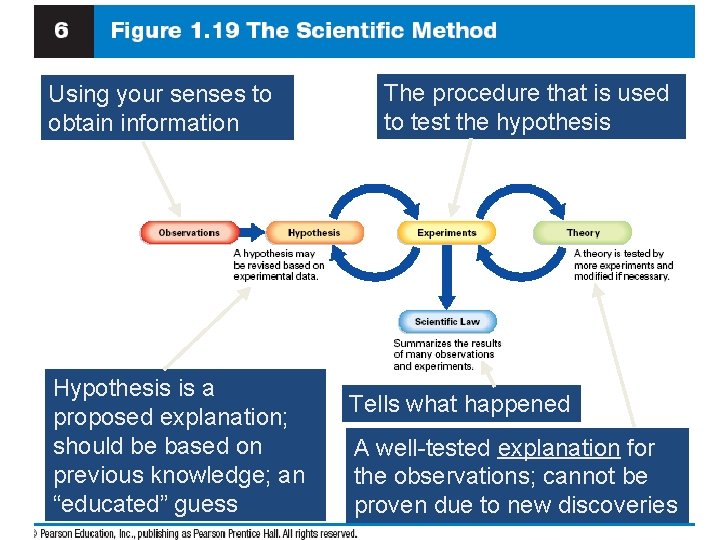
The Scientific Method • A logical approach to solving problems or answering questions. • Starts with observation- noting and recording information and facts • hypothesis- a proposed explanation for the observation; must be tested by an experiment

Steps in the Scientific Method 1. Observations (uses your senses) a) quantitative involves numbers = 95 o. F b) qualitative is word description = hot 2. Formulating hypotheses (ideas) - possible explanation for the observation, or “educated” guess 3. Performing experiments (the test) - gathers new information to help decide whether the hypothesis is valid

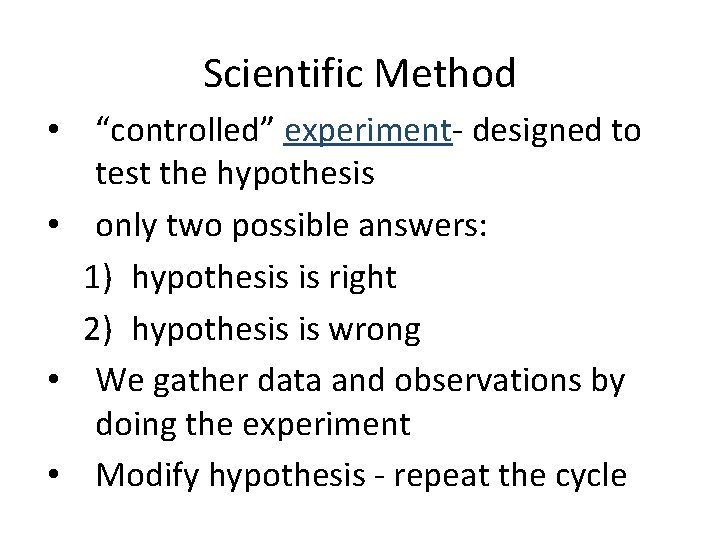
Scientific Method • “controlled” experiment- designed to test the hypothesis • only two possible answers: 1) hypothesis is right 2) hypothesis is wrong • We gather data and observations by doing the experiment • Modify hypothesis - repeat the cycle

Scientific Method • We deal with variables, or factors that can change. Two types: 1) Manipulated variable (or independent variable) is the one that we change 2) Responding variable (or dependent variable) is the one observed during the experiment • For results to be accepted, the experiment needs to always produce the same result

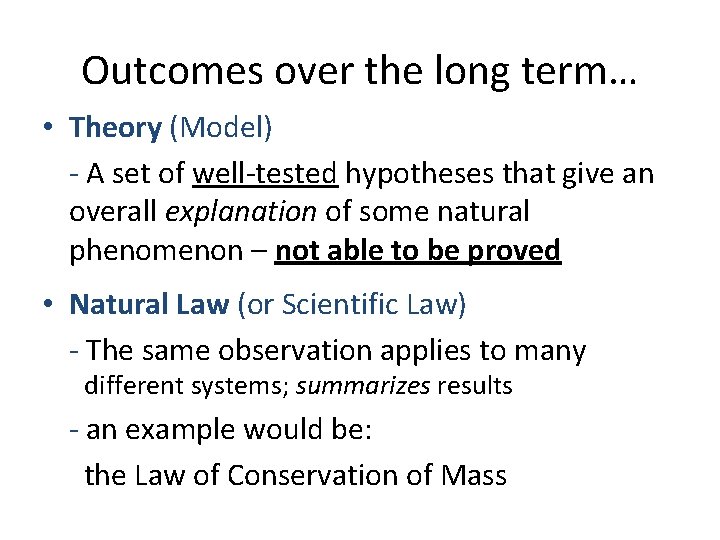
Outcomes over the long term… • Theory (Model) - A set of well-tested hypotheses that give an overall explanation of some natural phenomenon – not able to be proved • Natural Law (or Scientific Law) - The same observation applies to many different systems; summarizes results - an example would be: the Law of Conservation of Mass

Law vs. Theory v. A law summarizes what has happened. v. A theory (model) is an attempt to explain why it happened – this changes as new information is gathered.

Using your senses to obtain information Hypothesis is a proposed explanation; should be based on previous knowledge; an “educated” guess The procedure that is used to test the hypothesis Tells what happened A well-tested explanation for the observations; cannot be proven due to new discoveries

Collaboration / Communication • When scientists share ideas by collaboration and communication, they increase the likelihood of a successful outcome • Collaboration How is communication done? • Is the Internet reliable information? – http: //www. dhmo. org

Problem Solving in Chemistry • We are faced with problems each day, and not just in chemistry • A solution (answer) needs to be found • Trial and Error may work sometimes? • But, there is a method to problem solving that works better, and these are skills that no one is born knowing – they need to be learned.

Problem Solving in Chemistry • Effective problem solving usually involves two general steps: 1) Developing a plan 2) Implementing that plan • The skills you use to solve a word problem in chemistry are NOT different from those techniques used in shopping, cooking, or planning a party.

Solving Numeric Problems • Measurements are an important part of chemistry; thus many of our word problems involve use of mathmatics – Word problems are real life problems, and sometimes more information is presented than needed for a solution • Following skills presented will help you become more successful

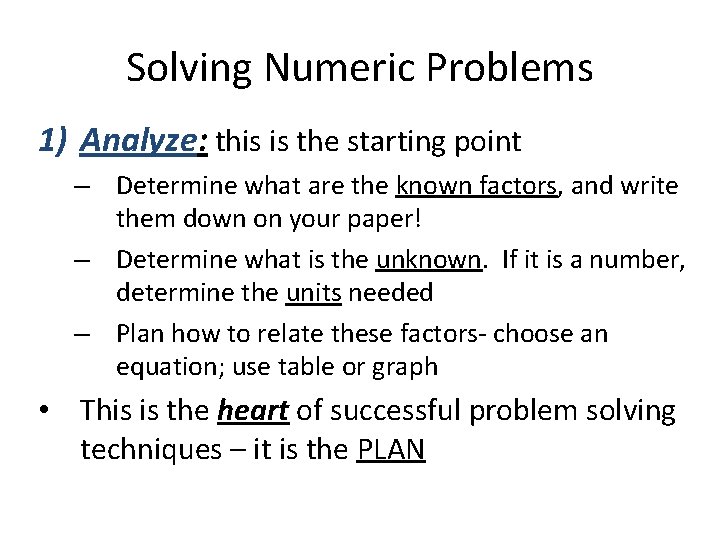
Solving Numeric Problems • The three steps we will use for solving a numeric word problem are: 1) Analyze 2) Calculate 3) Evaluate Let’s learn how to ACE these numeric word problems! • The following slides tell the meaning of these three steps in detail.

Solving Numeric Problems 1) Analyze: this is the starting point – Determine what are the known factors, and write them down on your paper! – Determine what is the unknown. If it is a number, determine the units needed – Plan how to relate these factors- choose an equation; use table or graph • This is the heart of successful problem solving techniques – it is the PLAN

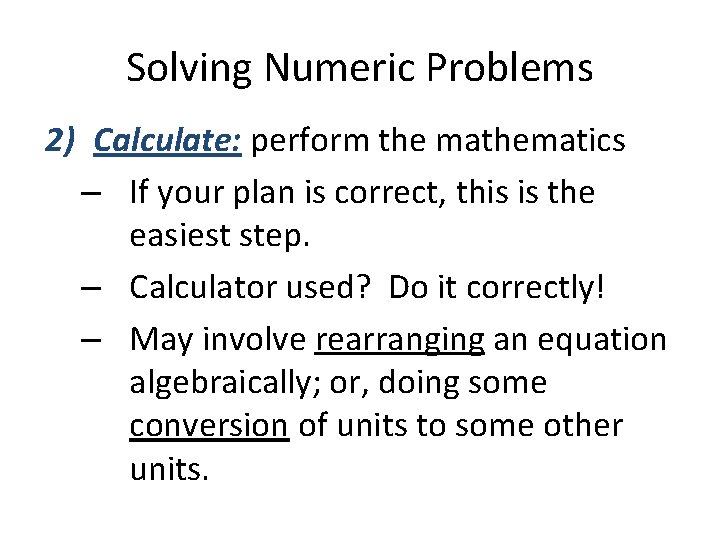
Solving Numeric Problems 2) Calculate: perform the mathematics – If your plan is correct, this is the easiest step. – Calculator used? Do it correctly! – May involve rearranging an equation algebraically; or, doing some conversion of units to some other units.

Solving Numeric Problems 3) Evaluate: – the finishing step – Is it reasonable? Make sense? Do an estimate for the answer, and check your calculations. – Need to round off the answer? – Do you need scientific notation? – Do you have the correct units? – Did you answer the question?

Solving Conceptual Problems • • • Not all word problems in chemistry involve doing calculations Nonnumeric problems are called conceptual problems – ask you to apply concepts to a new situation Steps are: 1) Analyze and 2) Solve • • Plan needed to link known to unknown, but no checking units or calculations Do Conceptual Problem 2. 1 on page 46

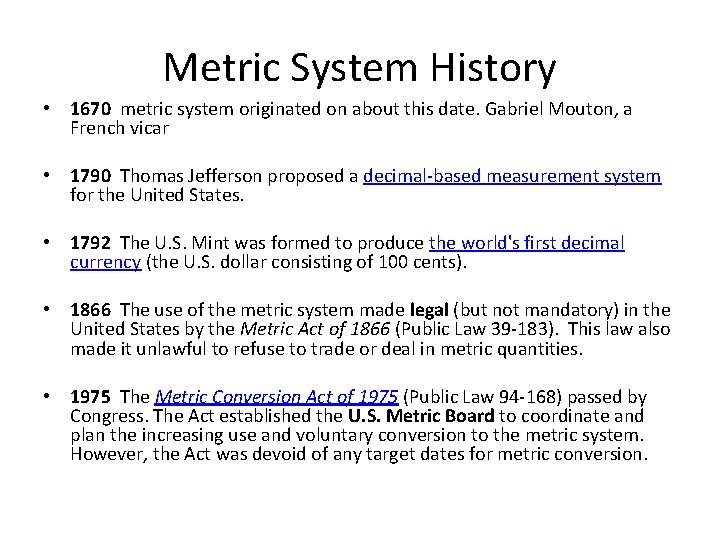
Metric System History • 1670 metric system originated on about this date. Gabriel Mouton, a French vicar • 1790 Thomas Jefferson proposed a decimal-based measurement system for the United States. • 1792 The U. S. Mint was formed to produce the world's first decimal currency (the U. S. dollar consisting of 100 cents). • 1866 The use of the metric system made legal (but not mandatory) in the United States by the Metric Act of 1866 (Public Law 39 -183). This law also made it unlawful to refuse to trade or deal in metric quantities. • 1975 The Metric Conversion Act of 1975 (Public Law 94 -168) passed by Congress. The Act established the U. S. Metric Board to coordinate and plan the increasing use and voluntary conversion to the metric system. However, the Act was devoid of any target dates for metric conversion.

• 1979 BATF requires wine producers and importers to switch to metric bottles in seven standard [liter and milliliter] sizes. • 1983 The meter is redefined in terms of the speed of light by the 17 th CGPM, resulting in better precision but keeping its length the same. • 1988 The Omnibus Trade and Competitiveness Act of 1988 amended and strengthened the Metric Conversion Act of 1975, designating the SI metric system as the preferred measurement system, and requiring each federal agency to be metric by the end of fiscal year 1992. • 1991 President George Bush signed Executive Order 12770, Metric Usage in Federal Government Programs directing all executive departments and federal agencies implement the use of the metric system. The Executive Order is also available as an appendix to: Interpretation of the SI for the United States and Federal Government Metric Conversion Policy

• 1994 The Fair Packaging and Labeling Act (FPLA) was amended by the Food and Drug and Administration (FDA) to require the use of dual units (inch-pound AND metric) on all consumer products. • 1996 As of July 1996 all surface temperature observations in National Weather Service METAR/TAF reports are now transmitted in degrees Celsius. • 2001 April 09 U. S. Stock Exchanges changed to decimal trading. The Securities and Exchange Commission has ordered that all stocks must be quoted in dollars and cents rather than fractions by this date. The switch to decimal trading brought the U. S. in line with the rest of the world's major exchanges. This follows the change of the Canadian Stock Exchanges to decimal trading in 1996.

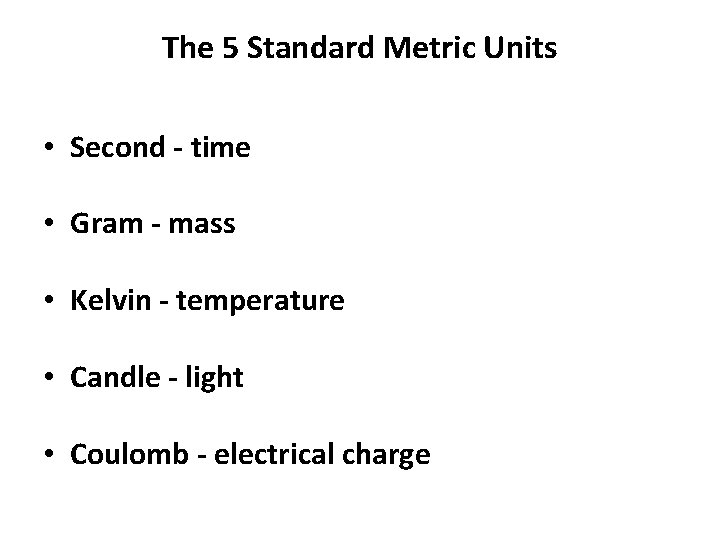
The 5 Standard Metric Units • Second - time • Gram - mass • Kelvin - temperature • Candle - light • Coulomb - electrical charge

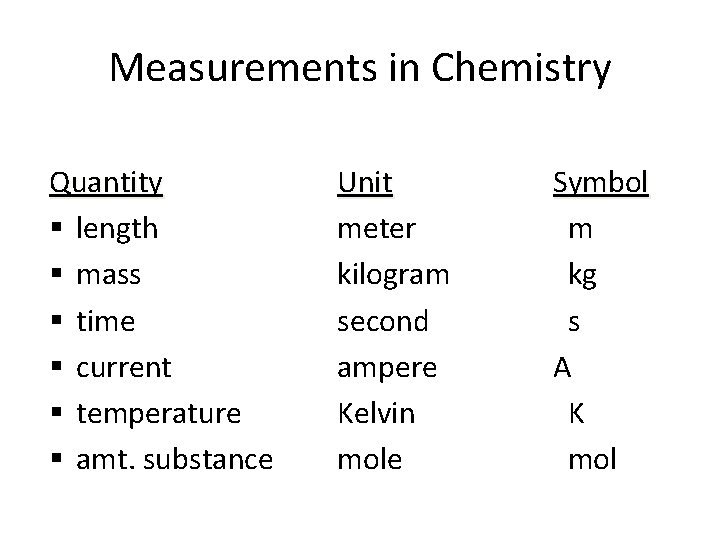
Measurements in Chemistry Quantity § length § mass § time § current § temperature § amt. substance Unit Symbol meter m kilogram kg second s ampere A Kelvin K mole mol

Staircase Method-we do not use!

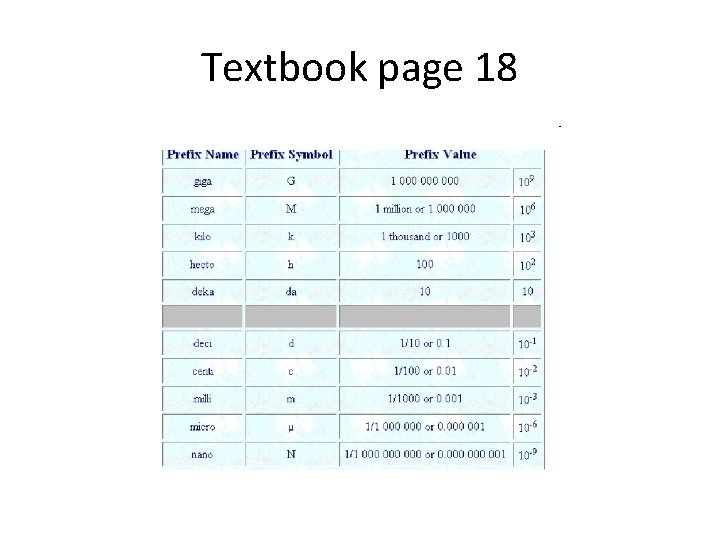
Textbook page 18


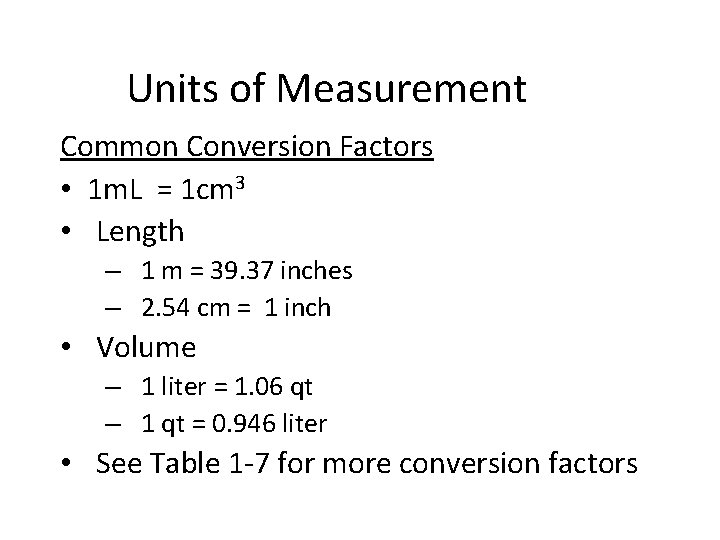
Units of Measurement Definitions • Mass – measure of the quantity of matter in a body • Weight – measure of the gravitational attraction for a body

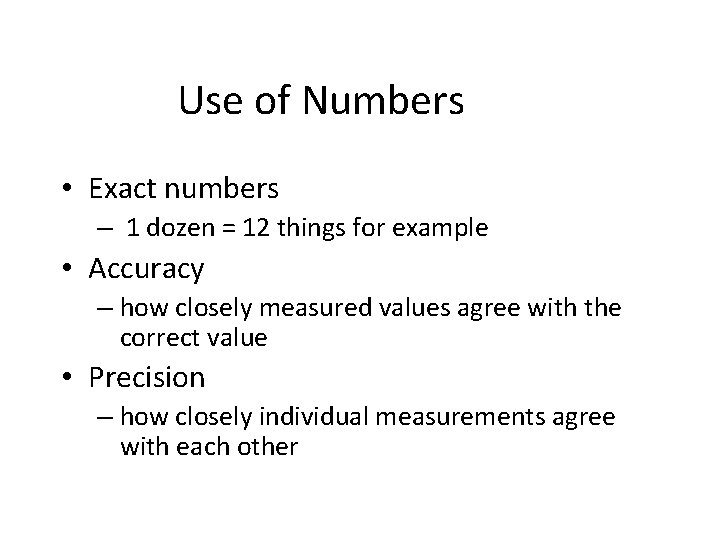
Units of Measurement Common Conversion Factors • 1 m. L = 1 cm 3 • Length – 1 m = 39. 37 inches – 2. 54 cm = 1 inch • Volume – 1 liter = 1. 06 qt – 1 qt = 0. 946 liter • See Table 1 -7 for more conversion factors


Use of Numbers • Exact numbers – 1 dozen = 12 things for example • Accuracy – how closely measured values agree with the correct value • Precision – how closely individual measurements agree with each other

When significant figures are used as way of indicating uncertainty, the last digit is considered uncertain. • For example, a result reported as 1. 23 implies a minimum uncertainty of ± 0. 01 and a range of 1. 22 to 1. 24.

Use of Numbers • Significant figures – digits believed to be correct by the person making the measurement • Measure a mile with a 6 inch ruler vs. surveying equipment • Exact numbers have an infinite number of significant figures 12. 00000000 = 1 dozen because it is an exact number

Use of Numbers Significant Figures - Rules • Leading zeroes are never significant 0. 000357 has three significant figures • Trailing zeroes may be significant must specify significance by how the number is written 1300 nails - counted or weighed? • Use scientific notation to remove doubt 2. 40 x 103 has ? significant figures

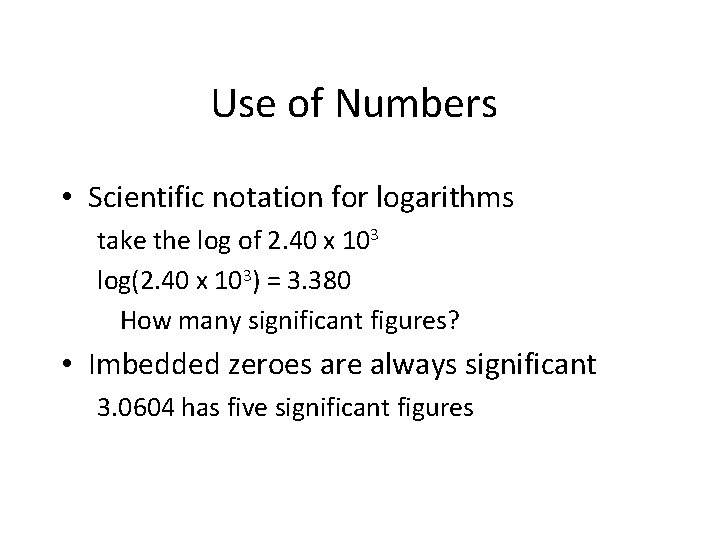
Use of Numbers • Scientific notation for logarithms take the log of 2. 40 x 103 log(2. 40 x 103) = 3. 380 How many significant figures? • Imbedded zeroes are always significant 3. 0604 has five significant figures

Use of Numbers • Piece of Black Paper – with rulers beside the edges

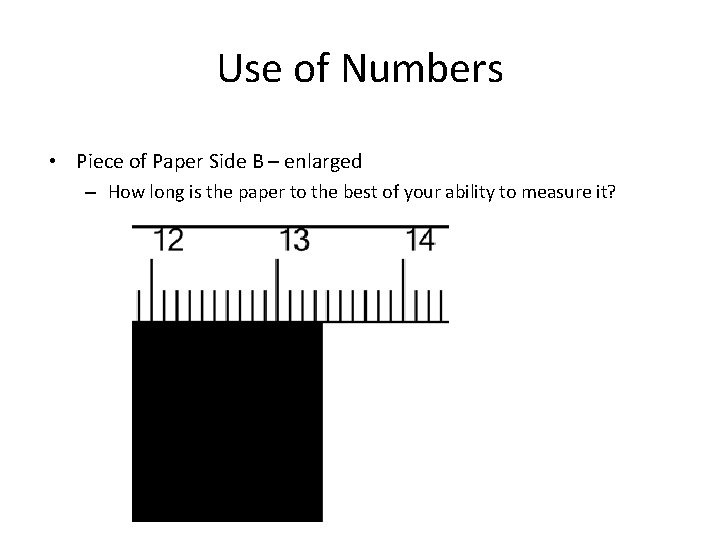
Use of Numbers • Piece of Paper Side B – enlarged – How long is the paper to the best of your ability to measure it?

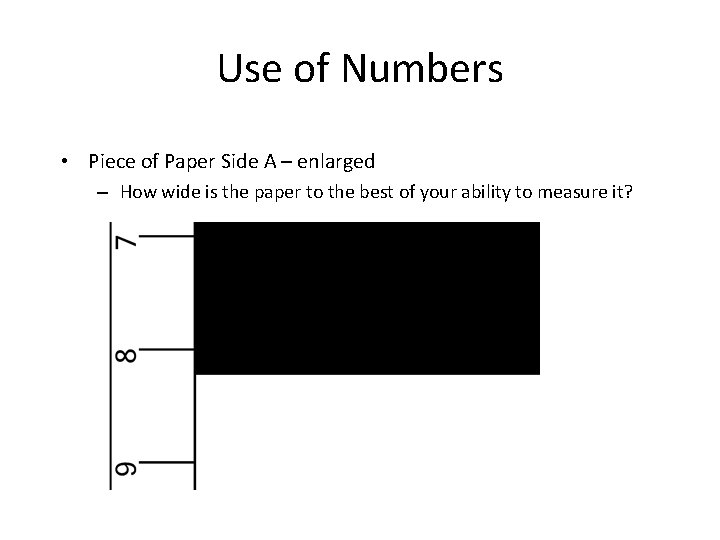
Use of Numbers • Piece of Paper Side A – enlarged – How wide is the paper to the best of your ability to measure it?

Use of Numbers • Determine the area of the piece of black paper using your measured values. • Compare your answer with your classmates. – Where do your answers differ in the numbers? • Significant figures rules for multiplication and division must help us determine where answers would differ.

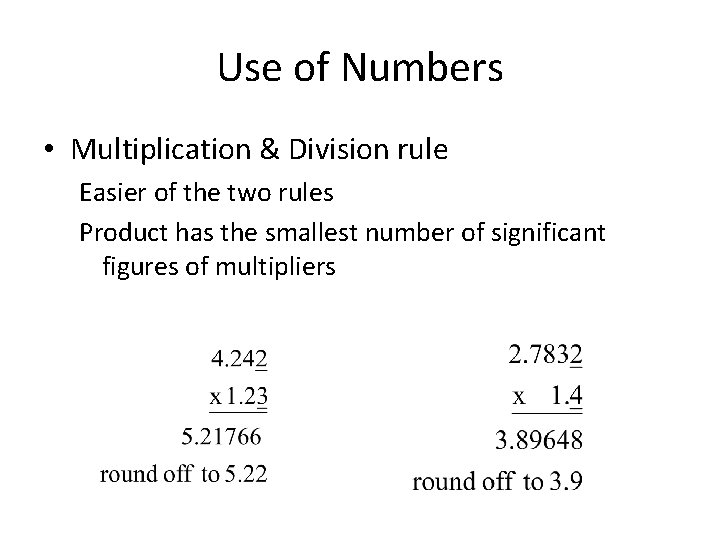
Use of Numbers • Multiplication & Division rule Easier of the two rules Product has the smallest number of significant figures of multipliers

Use of Numbers • Multiplication & Division rule Easier of the two rules Product has the smallest number of significant figures of multipliers

Use of Numbers • Multiplication & Division rule Easier of the two rules Product has the smallest number of significant figures of multipliers

Use of Numbers • Determine the perimeter of the piece of black paper using your measured values. • Compare your answer with your classmates. – Where do your answers differ in the numbers? • Significant figures rules for addition and subtraction must help us determine where answers would differ.

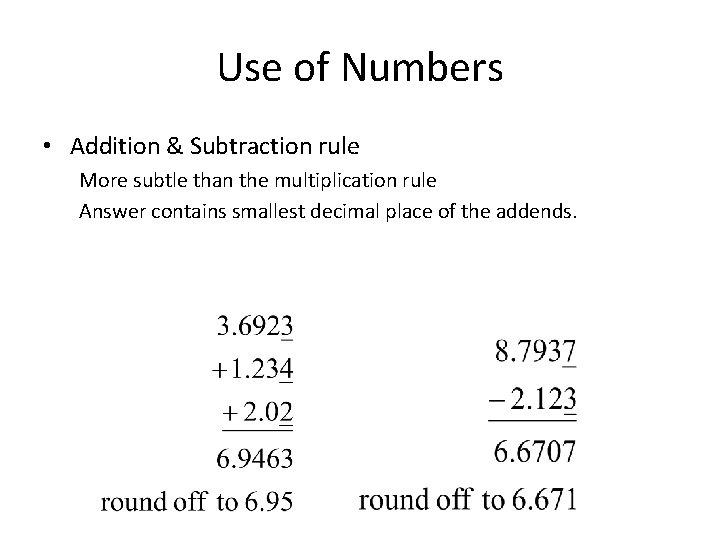
Use of Numbers • Addition & Subtraction rule More subtle than the multiplication rule Answer contains smallest decimal place of the addends.

Use of Numbers • Addition & Subtraction rule More subtle than the multiplication rule Answer contains smallest decimal place of the addends.

Use of Numbers • Addition & Subtraction rule More subtle than the multiplication rule Answer contains smallest decimal place of the addends.

The Unit Factor Method • Simple but important method to get correct answers in word problems. • Method to change from one set of units to another. • Visual illustration of the idea.

The Unit Factor Method • Change from a to a by obeying the following rules.

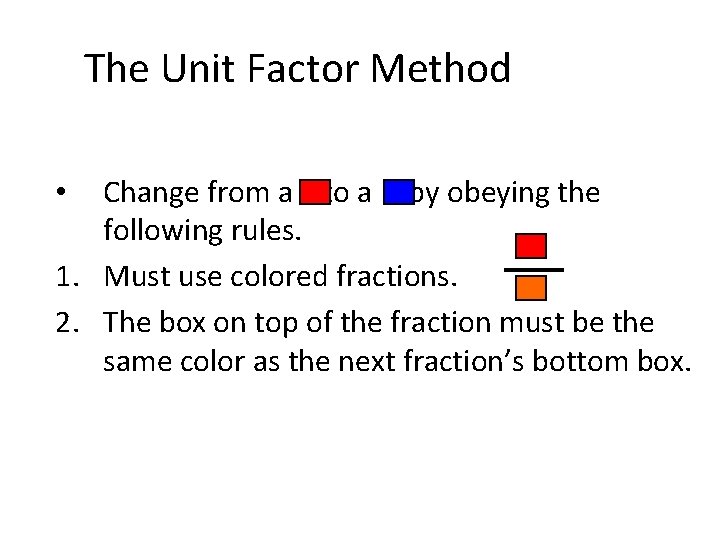
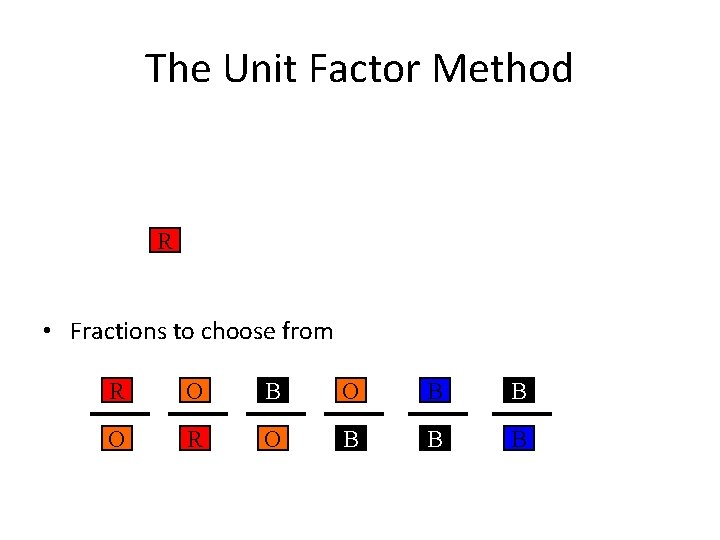
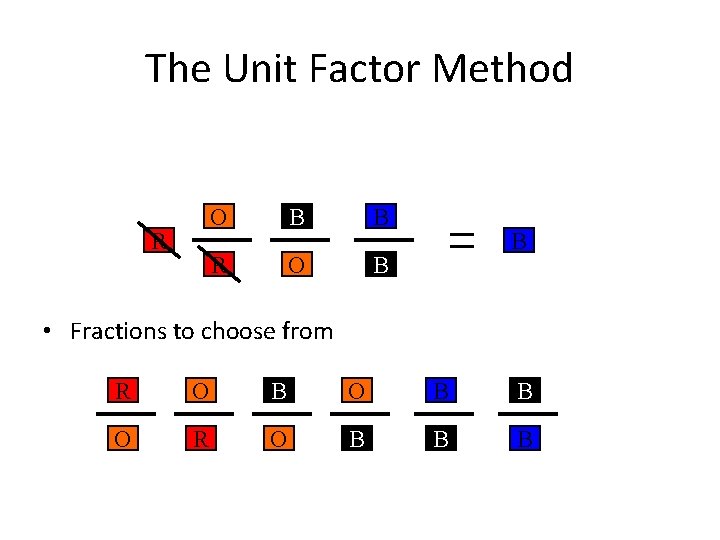
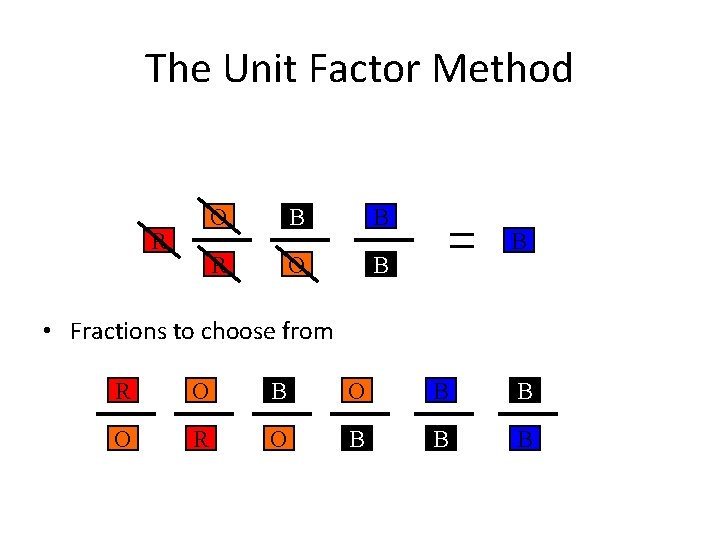
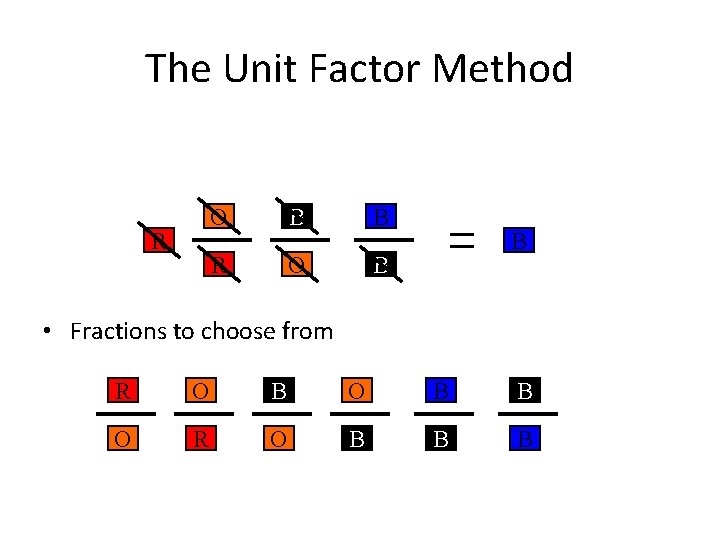
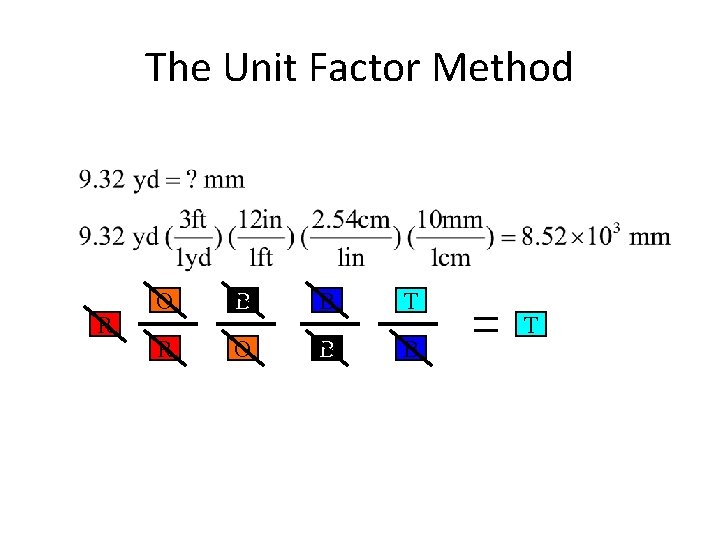
The Unit Factor Method Change from a to a by obeying the following rules. 1. Must use colored fractions. •

The Unit Factor Method Change from a to a by obeying the following rules. 1. Must use colored fractions. 2. The box on top of the fraction must be the same color as the next fraction’s bottom box. •

The Unit Factor Method R • Fractions to choose from R O B B O R O B B B

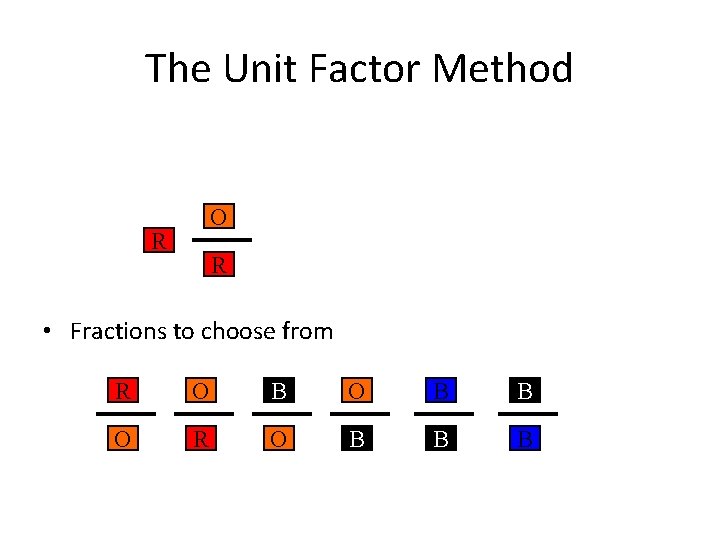
The Unit Factor Method O R R • Fractions to choose from R O B B O R O B B B

The Unit Factor Method R O B R O • Fractions to choose from R O B B O R O B B B

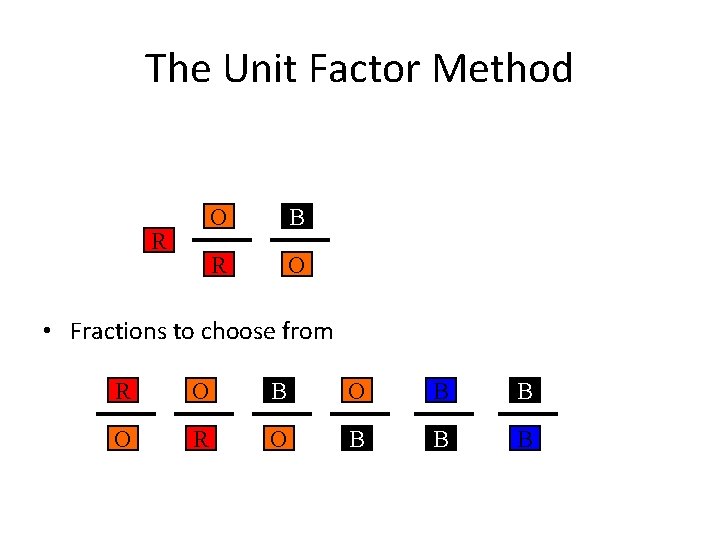
The Unit Factor Method R O B B • Fractions to choose from R O B B O R O B B B

The Unit Factor Method R O B B • Fractions to choose from R O B B O R O B B B

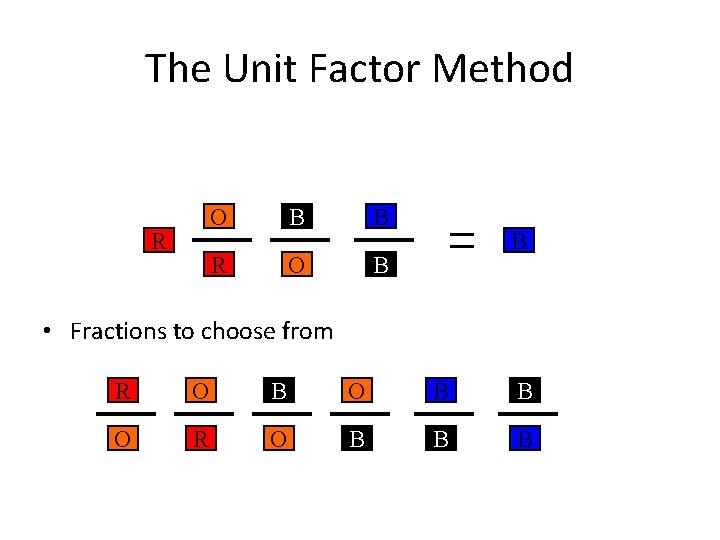
The Unit Factor Method R O B B • Fractions to choose from R O B B O R O B B B

The Unit Factor Method R O B B • Fractions to choose from R O B B O R O B B B

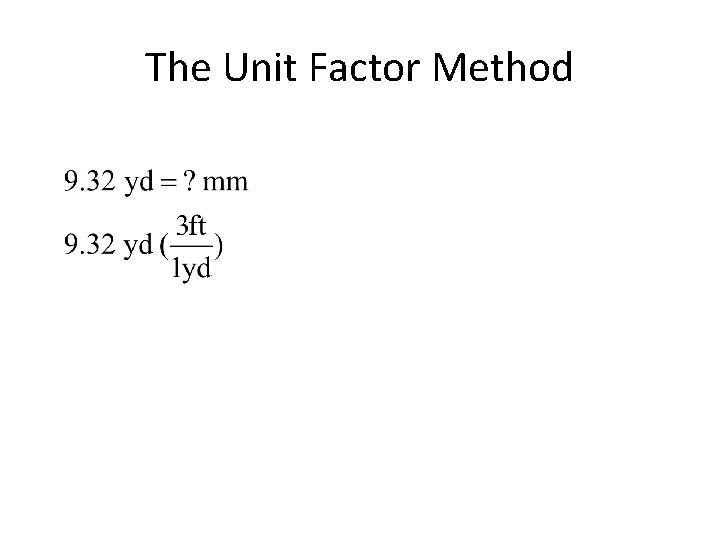
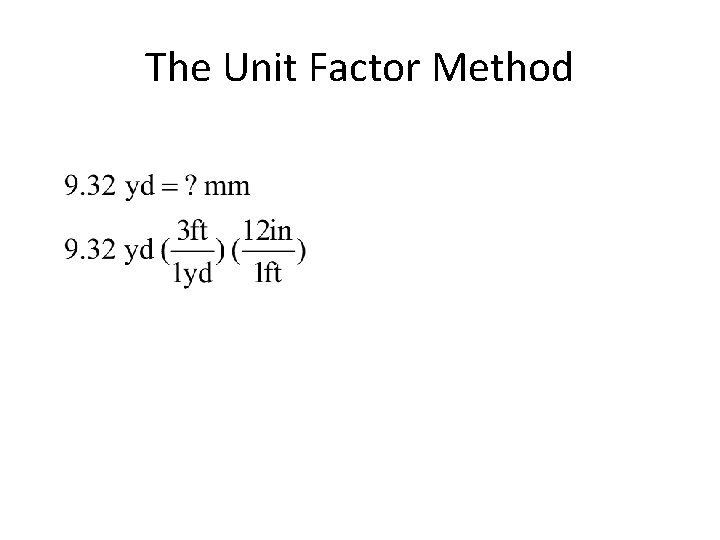
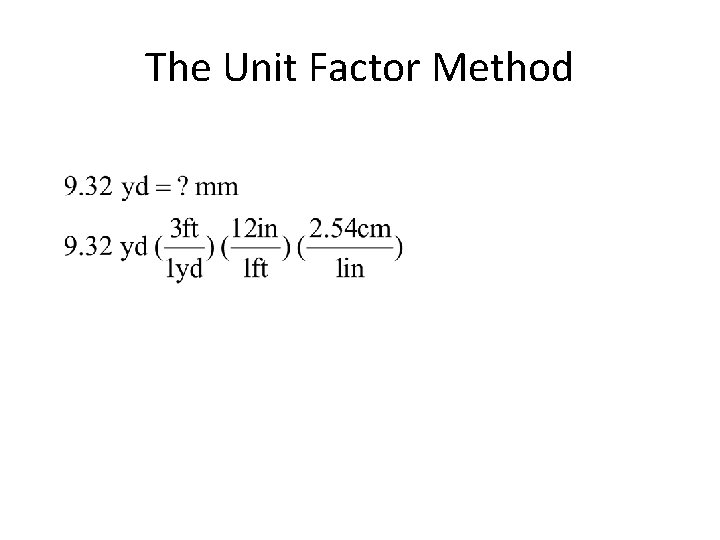
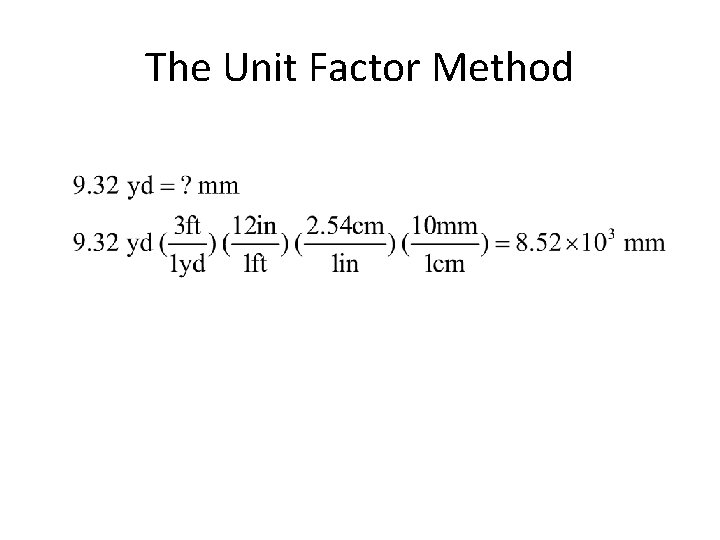
The Unit Factor Method • colored fractions represent unit factors 1 ft = 12 in becomes or • Example 1 -1: Express 9. 32 yards in millimeters.

The Unit Factor Method

The Unit Factor Method

The Unit Factor Method

The Unit Factor Method

The Unit Factor Method R O B B T

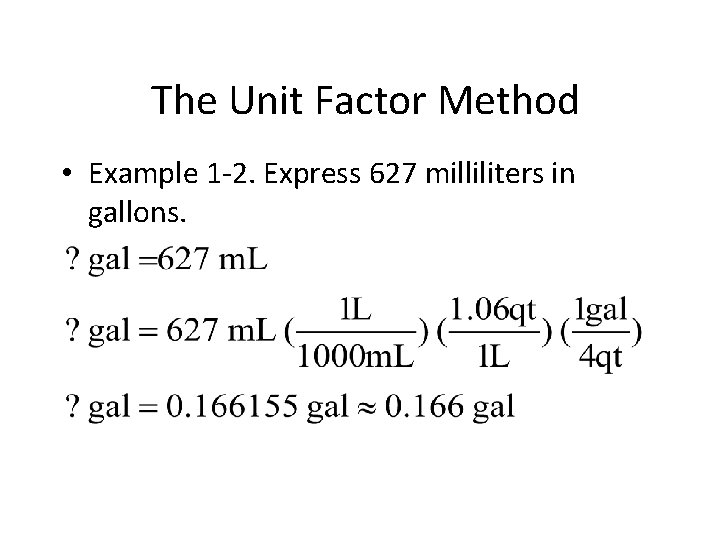
The Unit Factor Method • Example 1 -2: Express 627 milliliters in gallons. You do it!

The Unit Factor Method • Example 1 -2. Express 627 milliliters in gallons.

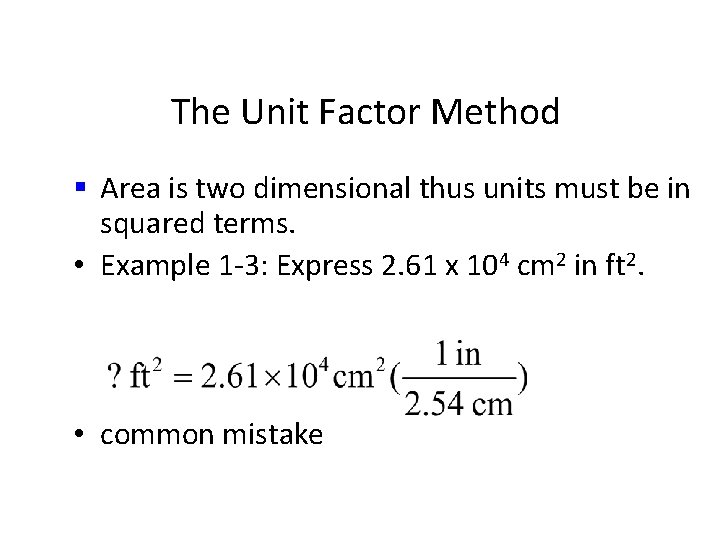
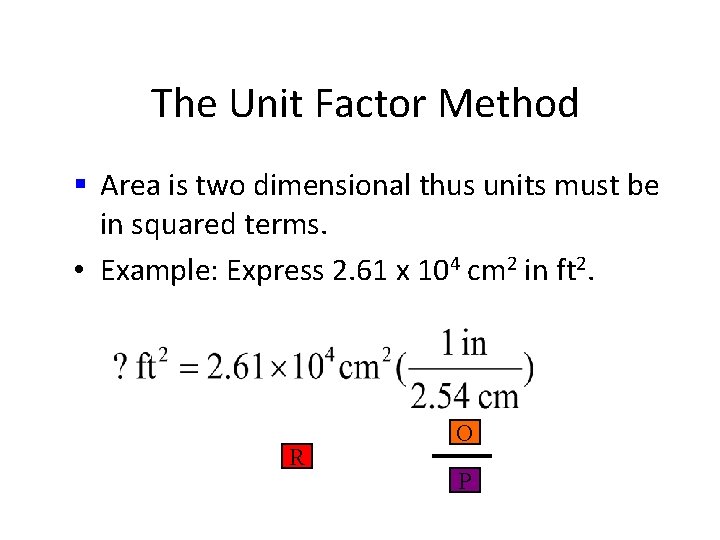
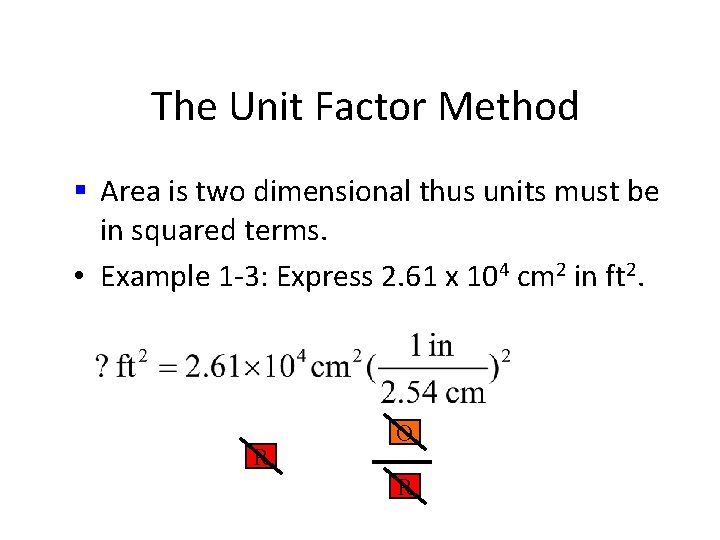
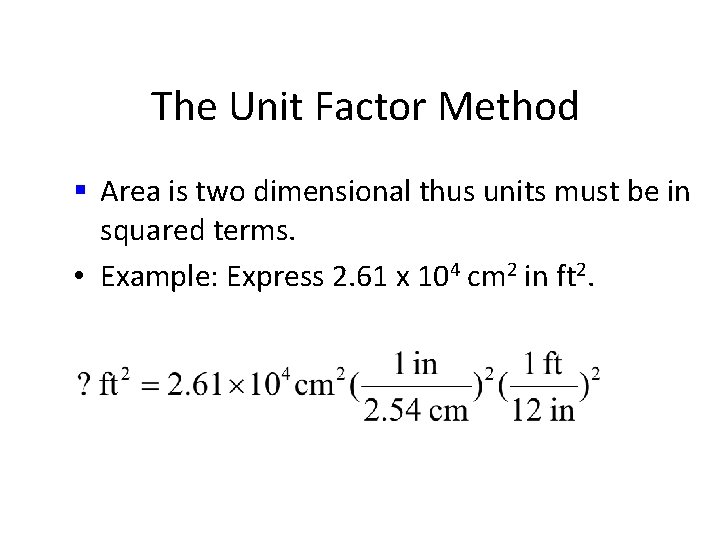
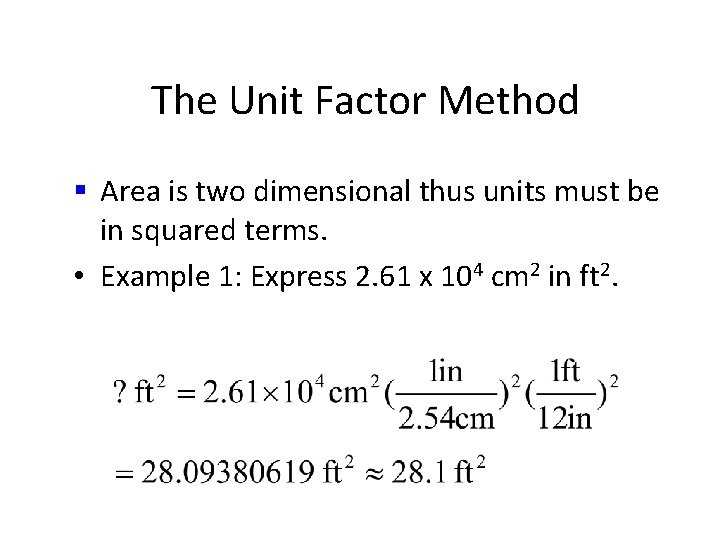
The Unit Factor Method • Area is two dimensional thus units must be in squared terms. • Example 1 -3: Express 2. 61 x 104 cm 2 in ft 2.

The Unit Factor Method § Area is two dimensional thus units must be in squared terms. • Example 1 -3: Express 2. 61 x 104 cm 2 in ft 2. • common mistake

The Unit Factor Method § Area is two dimensional thus units must be in squared terms. • Example: Express 2. 61 x 104 cm 2 in ft 2. R O P

The Unit Factor Method § Area is two dimensional thus units must be in squared terms. • Example 1 -3: Express 2. 61 x 104 cm 2 in ft 2. R O R

The Unit Factor Method § Area is two dimensional thus units must be in squared terms. • Example: Express 2. 61 x 104 cm 2 in ft 2.

The Unit Factor Method § Area is two dimensional thus units must be in squared terms. • Example 1: Express 2. 61 x 104 cm 2 in ft 2.

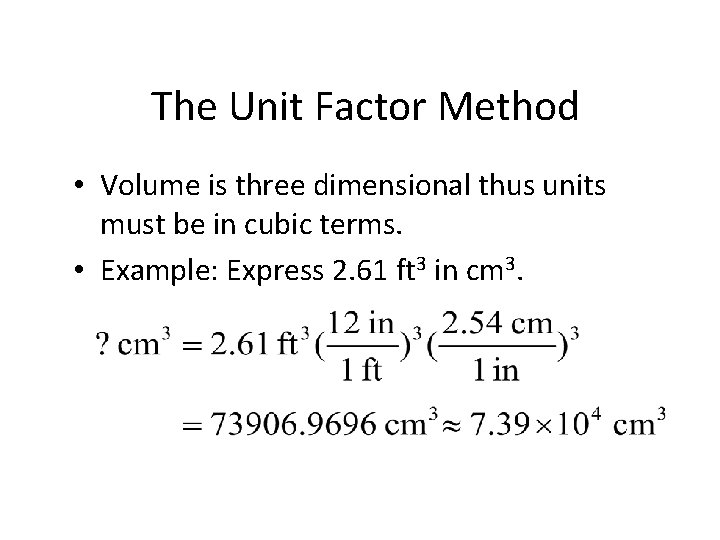
The Unit Factor Method • Volume is three dimensional thus units must be in cubic terms. • Example 1 -4: Express 2. 61 ft 3 in cm 3. You do it!

The Unit Factor Method • Volume is three dimensional thus units must be in cubic terms. • Example: Express 2. 61 ft 3 in cm 3.

Dual Units • mph actually means • Easy to do just do one unit at a time

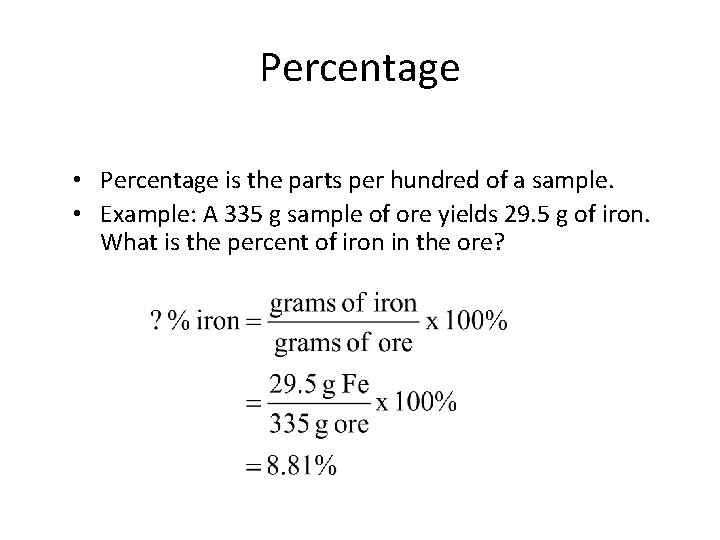
Percentage • Percentage is the parts per hundred of a sample. • Example: A 335 g sample of ore yields 29. 5 g of iron. What is the percent of iron in the ore? You do it!

Percentage • Percentage is the parts per hundred of a sample. • Example: A 335 g sample of ore yields 29. 5 g of iron. What is the percent of iron in the ore?