The Periodic Law Electron Configuration and the Periodic
















- Slides: 37

The Periodic Law Electron Configuration and the Periodic Table

Tuesday, September 25 Create new page of notes, “Electron Configuration of Periodic Table” Take out your Chapter 5 vocab words- leave out for me to check Without looking at the periodic table, identify the group, period, and block in which the element that has the electron configuration [Ne]3 s 1 is located

Wednesday, September 26 Take out Ch. 5 Vocab Words Solve the Riddle(s): At night they come without being fetched. By day they are lost without being stolen. What are they? What is in seasons, seconds, centuries and minutes but not in decades, years or days?

Thursday, September 27 Take out your Mystery Element Card and finish any additional work needed (10 minutes) Take out one sheet of paper, number it 1 -35 (skipping one line between each #), draw two columns, label them element and electron configuration

Friday, September 28 You will have a Periodic Trend quiz. You will need your Periodic Table. Once finished, we will finish the Mystery Element rotation.

A Star The letter “n”

Riddle Me This? It walks on four legs in the morning, two legs at noon and three legs in the evening. What is it?

Man (or woman). Crawls on all fours as a baby, walks on two legs as an adult and uses two legs and a cane when they're old.

Riddle Me This? What always runs but never walks, often murmurs, never talks, has a bed but never sleeps, has a mouth but never eats?

A river.

Mystery Element Card Fold a sheet of paper in half (hamburger) On front cover, create a riddle or poem for element On inside page, include: Physical properties Chemical properties Common Uses Principal, Angular, Magnetic Quantum Number Group and Period Ionization Energy (p 153) Atomic Radii (p 151) Electronegativity (p 161) DO NOT INCLUDE NAME OF ELEMENT

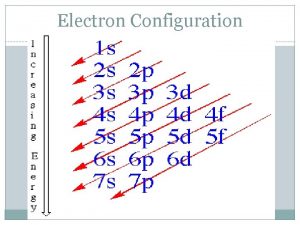
Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements are arranged vertically in the periodic table in groups that share similar chemical properties Elements are also organized horizontally in rows, or periods The length of each period is determined by the number of electrons that can occupy the sublevels being filled in that period The periodic table is divided into four blocks, the s, p, d, and f blocks. The name of each block is determined by the electron sublevel being filled in that block

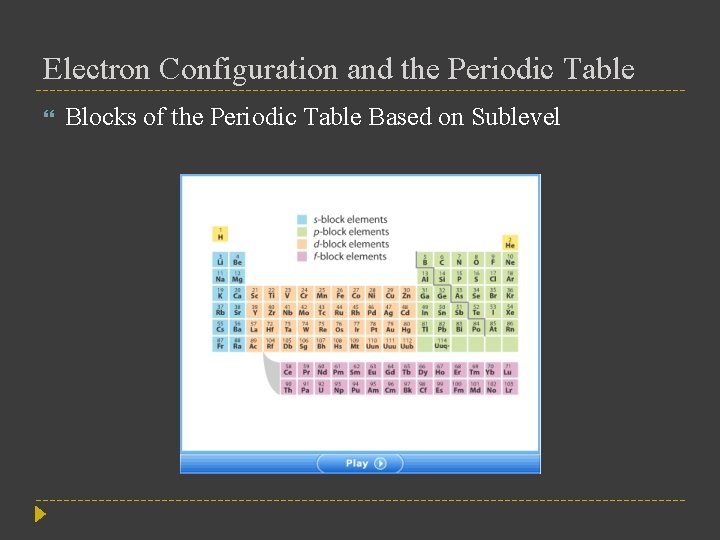
Electron Configuration and the Periodic Table Blocks of the Periodic Table Based on Sublevel

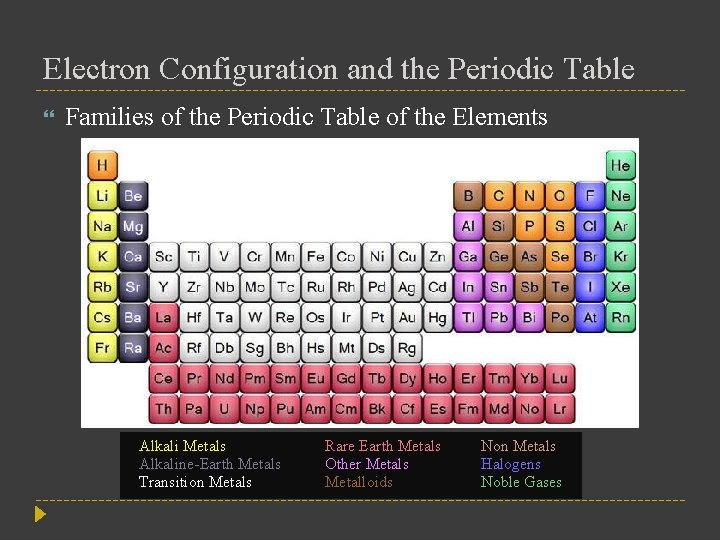
Electron Configuration and the Periodic Table Families of the Periodic Table of the Elements Alkali Metals Alkaline-Earth Metals Transition Metals Rare Earth Metals Other Metals Metalloids Non Metals Halogens Noble Gases

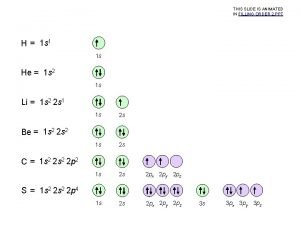
Electron Configuration and the Periodic Table Hydrogen and Helium Hydrogen has an electron configuration of 1 s 1, but despite the ns 1 configuration, it does not share the same properties as the elements of Group 1 Hydrogen is a unique element Like the Group 2 elements, helium has an ns 2 group configuration. Yet it is part of Group 18 Because its highest occupied energy level is filled by two electrons, helium possesses special chemical stability

Electron Configuration and the Periodic Table Alkali Metals The elements of Group 1 of the periodic table are known as the alkali metals lithium, sodium, potassium, rubidium, cesium, and francium In their pure state, all of the alkali metals have a silvery appearance and are soft enough to cut with a knife Highly reactive with oxygen and water

Electron Configuration and the Periodic Table Alkaline-Earth Metals The elements of Group 2 of the periodic table are called the alkaline-earth metals beryllium, magnesium, calcium, strontium, barium, and radium Alkaline-earth metals are less reactive than the alkali metals, but are still too reactive to be found in nature in pure form

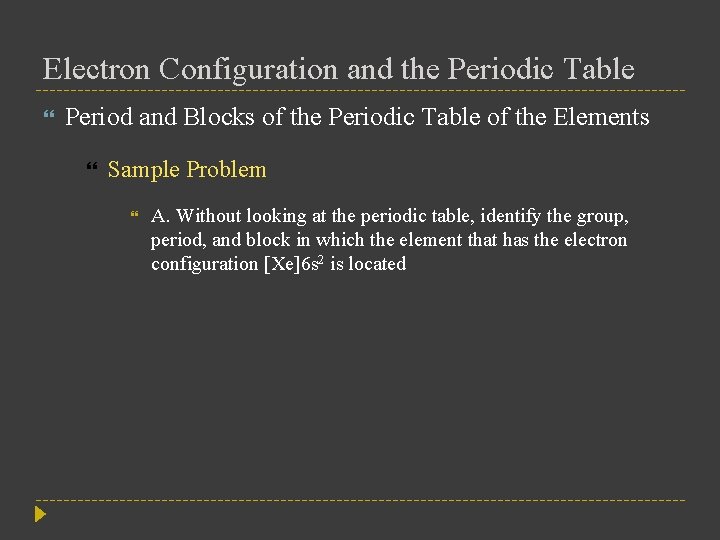
Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem A. Without looking at the periodic table, identify the group, period, and block in which the element that has the electron configuration [Xe]6 s 2 is located

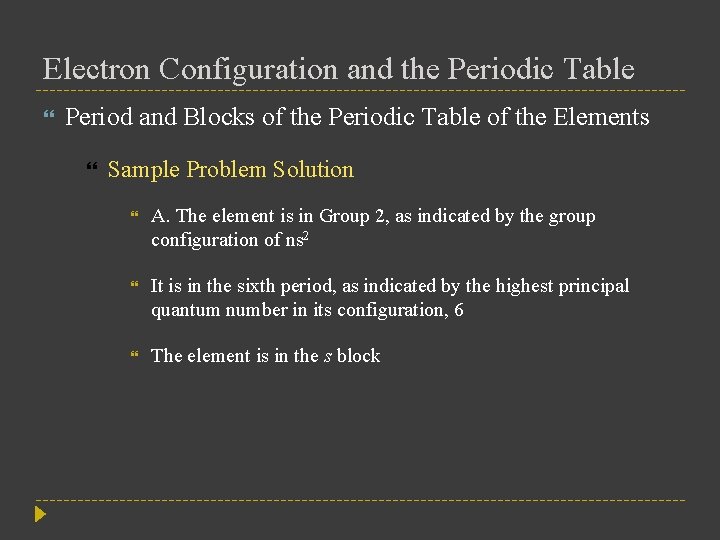
Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Solution A. The element is in Group 2, as indicated by the group configuration of ns 2 It is in the sixth period, as indicated by the highest principal quantum number in its configuration, 6 The element is in the s block

Sample Problem B. Without looking at the periodic table, write the electron configuration for the Group 1 element in the third period. Is this element likely to be more reactive or less reactive than the element described in (A)

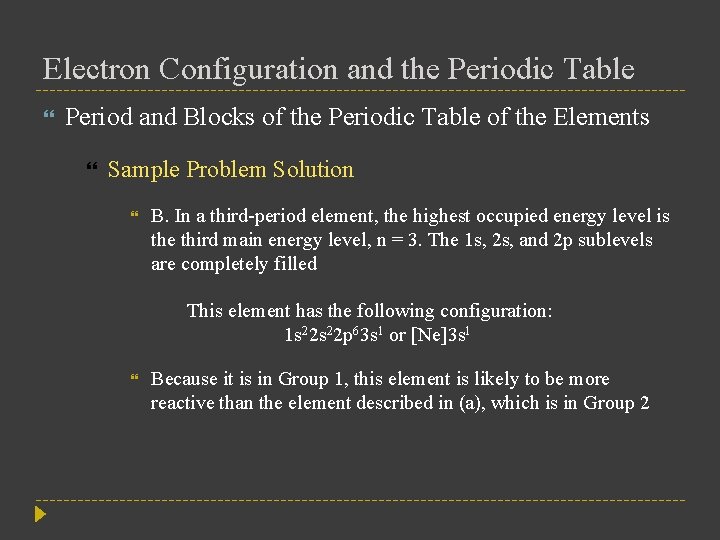
Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Solution B. In a third-period element, the highest occupied energy level is the third main energy level, n = 3. The 1 s, 2 s, and 2 p sublevels are completely filled This element has the following configuration: 1 s 22 p 63 s 1 or [Ne]3 s 1 Because it is in Group 1, this element is likely to be more reactive than the element described in (a), which is in Group 2

Electron Configuration and the Periodic Table Transition Metals The d-block (groups 3 – 12) elements are metals with typical metallic properties and are often referred to as transition elements

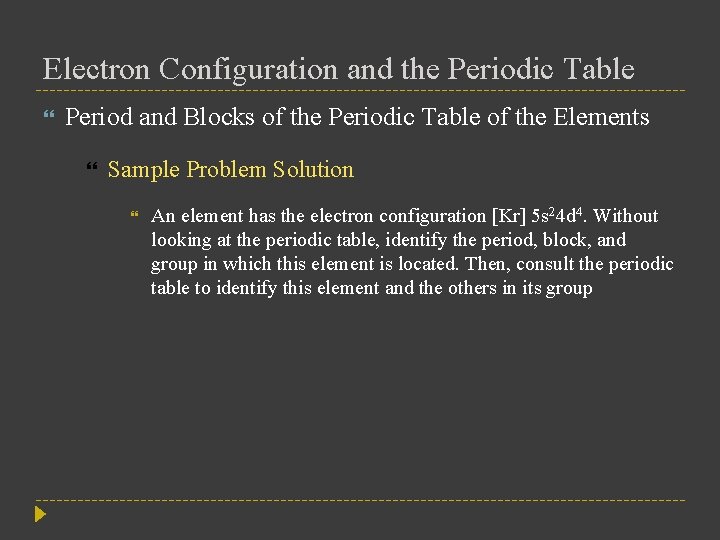
Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Solution An element has the electron configuration [Kr] 5 s 24 d 4. Without looking at the periodic table, identify the period, block, and group in which this element is located. Then, consult the periodic table to identify this element and the others in its group

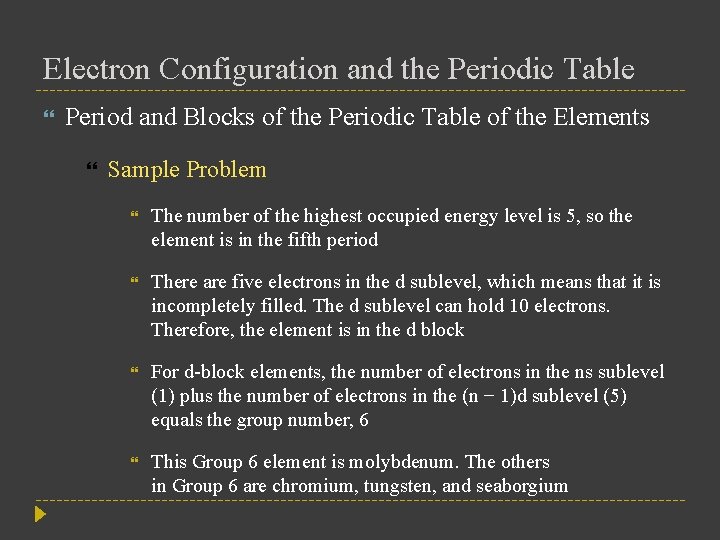
Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem The number of the highest occupied energy level is 5, so the element is in the fifth period There are five electrons in the d sublevel, which means that it is incompletely filled. The d sublevel can hold 10 electrons. Therefore, the element is in the d block For d-block elements, the number of electrons in the ns sublevel (1) plus the number of electrons in the (n − 1)d sublevel (5) equals the group number, 6 This Group 6 element is molybdenum. The others in Group 6 are chromium, tungsten, and seaborgium

Electron Configuration and the Periodic Table Metalloids, Other Metals, Non Metals, Halogens, N Gases The p-block elements consist of all the elements of Groups 13– 18 except helium The p-block elements together with the s-block elements are called the main-group elements The properties of elements of the p block vary greatly

Electron Configuration and the Periodic Table Metalloids Having properties of both metals and non-metals are the metalloids Metalloids have a metal-like appearance and can conduct heat and electricity under certain conditions

Electron Configuration and the Periodic Table Other Metals Other metals elements are elements that are ductile and malleable, but they are not the same as the transition elements aluminum, gallium, indium, tin, thallium, lead, bismuth All of these elements are solid, have a relatively high density, and are opaque

Electron Configuration and the Periodic Table Non Metals Non-metal elements are not able to conduct electricity or heat very well. As opposed to metals, as solids, are nonmetallic elements are very brittle, and cannot be rolled into wires or pounded into sheets carbon, nitrogen, oxygen, phosphorus, sulfur, selenium

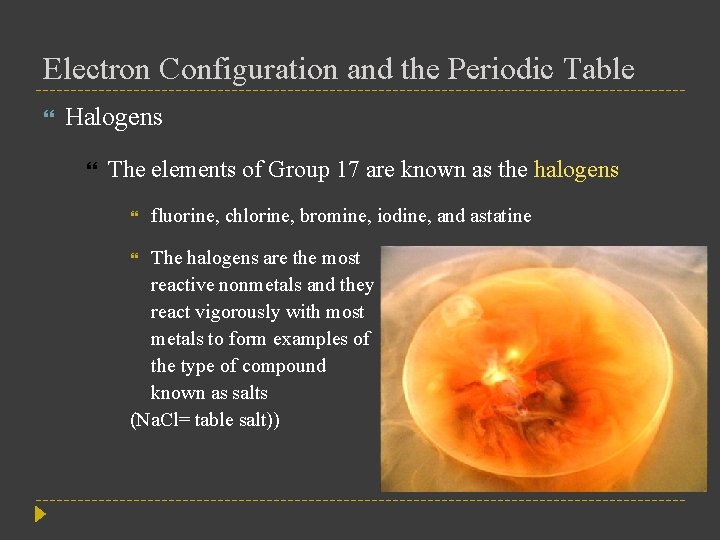
Electron Configuration and the Periodic Table Halogens The elements of Group 17 are known as the halogens fluorine, chlorine, bromine, iodine, and astatine The halogens are the most reactive nonmetals and they react vigorously with most metals to form examples of the type of compound known as salts (Na. Cl= table salt))

Electron Configuration and the Periodic Table Noble Gases Elements of group 18 are known as the noble gases neon, argon, krypton, xenon, and radon The noble gases are the most unreactive elements and all reside as gases in their normal state

Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Without looking at the periodic table, write the outer electron configuration for the Group 14 element in the second period. Then, name the element, and identify it as a metal, nonmetal, or metalloid

Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Solution The group number is higher than 12, so the element is in the p block The total number of electrons in the highest occupied s and p sublevels is therefore equal to the group number minus 10 (14 − 10 = 4) Two electrons are in the s sublevel, so two electrons must also be present in the 2 p sublevel, The outer electron configuration is 2 s 22 p 2 The element is carbon, C, which is a nonmetal

Electron Configuration and the Periodic Table Lanthanides and Actinides In the periodic table, the f-block elements are wedged between Groups 3 and 4 in the sixth and seventh periods The first row of the f block, the lanthanides, are shiny metals similar in reactivity to the Group 2 alkaline metals The second row of the f block, the actinides, are between actinium and rutherfordium. The actinides are all radioactive Their position reflects the fact that they involve the filling of the 4 f sublevel

Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Name the block and group in which each of the following elements is located in the periodic table. Then, use the periodic table to name each element. Identify each element as a metal, nonmetal, or metalloid. Finally, describe whether each element has high reactivity or low reactivity B. [Ne]3 s 23 p 5 C. [Ne]3 s 23 p 6

Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Solution B. The incompletely filled p sublevel shows that this element is in the p block. A total of seven electrons are in the ns and np sublevels, so this element is in Group 17, the halogens The element is chlorine, Cl, and is highly reactive

Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements Sample Problem Solution C. This element has a noble-gas configuration and thus is in Group 18 in the p block The element is argon, Ar, which is an unreactive nonmetal and a noble gas

Electron Configuration and the Periodic Table Period and Blocks of the Periodic Table of the Elements http: //www. ptable. com/
 Electron configuration vs noble gas configuration
Electron configuration vs noble gas configuration Periodic table with electron configuration
Periodic table with electron configuration Electron configuration periodic table
Electron configuration periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 6 the periodic table
6 the periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Quantum numbers and electron configuration
Quantum numbers and electron configuration Iodine valency
Iodine valency N and l electron configuration
N and l electron configuration Orbital description
Orbital description Diamagnetic elements
Diamagnetic elements 42 electron configuration
42 electron configuration 1s 22 s22 p63 s2
1s 22 s22 p63 s2 Stability and electron configuration ch 4
Stability and electron configuration ch 4 Quantum mechanical model and electron configuration
Quantum mechanical model and electron configuration Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law What is absolute configuration
What is absolute configuration Planar chirality
Planar chirality Absolute vs relative configuration
Absolute vs relative configuration Excited state electron configuration
Excited state electron configuration Ohem
Ohem Shape of d orbital class 11
Shape of d orbital class 11 Periodic trends practice problems
Periodic trends practice problems Noble gas configuration for carbon
Noble gas configuration for carbon Li ground state electron configuration
Li ground state electron configuration Unpaired electrons in electron configuration
Unpaired electrons in electron configuration Electron configuration of oxygen
Electron configuration of oxygen Noble gas electron configuration
Noble gas electron configuration Halogen electron configuration
Halogen electron configuration 110
110 Electronic configuration order
Electronic configuration order Bohr rutherford diagram magnesium ion
Bohr rutherford diagram magnesium ion Electronic configuration rule in chemistry
Electronic configuration rule in chemistry Electron configuration long form
Electron configuration long form Electron rules opposite gender only
Electron rules opposite gender only Noble gas notation
Noble gas notation Vbt of cr(nh3)6 3+
Vbt of cr(nh3)6 3+