Primary and Secondary Prevention of CVD Donald M





























- Slides: 89

Primary and Secondary Prevention of CVD Donald M. Lloyd-Jones MD Sc. M FACC FAHA

Spectrum of Prevention for CVD • Secondary Prevention - MI/ACS - Chronic CHD, PAD, DM(? ), CKD(? ) - Post-stroke/TIA • Primary Prevention - High, intermediate, low global risk - Individual risk factors • Primordial Prevention - Prevention of RF development

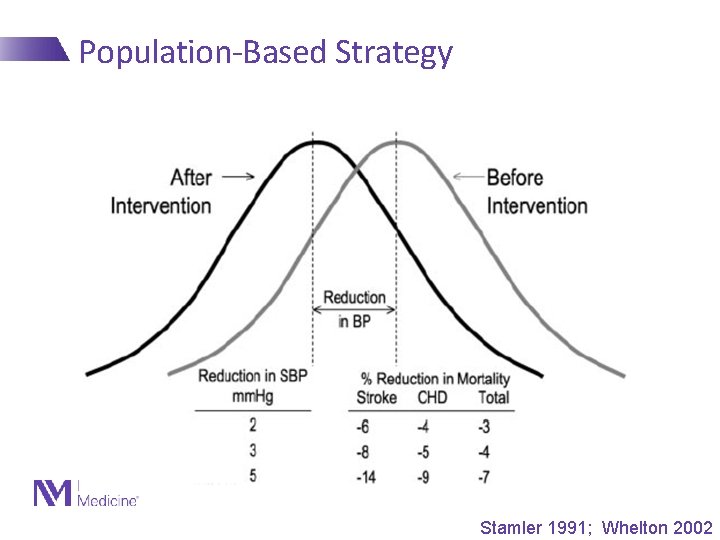
Guiding Concepts in Prevention • Thomas Strasser, 1978 - “Primordial prevention” - Prevention of the development of risk factors, not just disease • Once risk factors develop, risk for disease is high and difficult to reduce substantially • Geoffrey Rose, 1981 - “High-risk” vs. “population” strategies for prevention of disease - Current guidelines focus mostly on “high-risk” strategies, where relative risk is high - Shifting the population distribution of a risk factor can have dramatic impact on disease

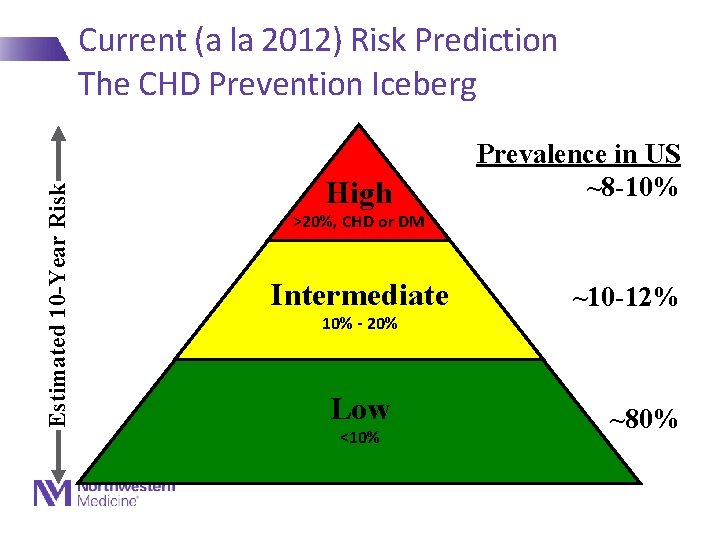
Estimated 10 -Year Risk Current (a la 2012) Risk Prediction The CHD Prevention Iceberg High Prevalence in US ~8 -10% >20%, CHD or DM Intermediate 10% - 20% Low <10% ~10 -12% ~80%

Population-Based Strategy Stamler 1991; Whelton 2002

Clinical Prevention Trials in CV Disease • The first question: “Why the heck are we talking about clinical trials in a course that is supposed to focus on epidemiology? ” • Answer: “How do we know all of these risk factors actually cause CVD? ”

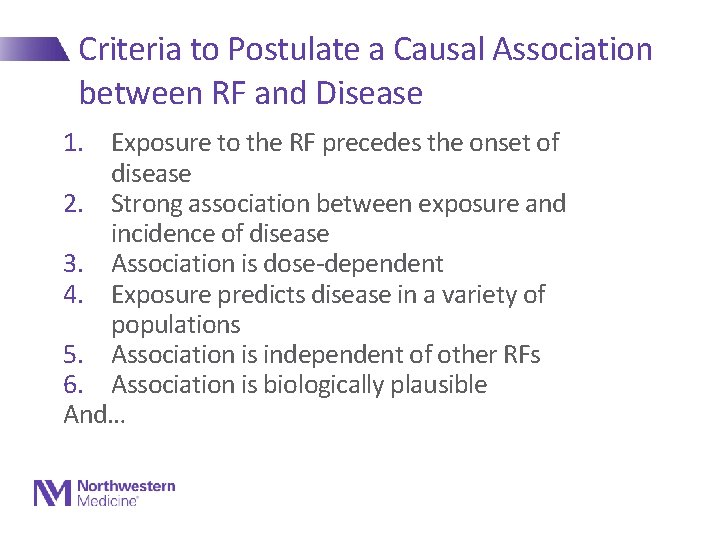
Criteria to Postulate a Causal Association between RF and Disease 1. Exposure to the RF precedes the onset of disease 2. Strong association between exposure and incidence of disease 3. Association is dose-dependent 4. Exposure predicts disease in a variety of populations 5. Association is independent of other RFs 6. Association is biologically plausible And…

Criteria to Postulate a Causal Association between RF and Disease 7. Intervention to modify or abolish the RF results (in large samples) in a lower incidence of disease Here is where clinical trials come in… They are just extensions of epidemiology, but we have more control over the environment/exposure

What is a clinical trial? Critical features of design • Hypothesis to be tested: e. g. , lowering cholesterol will lead to a reduction in CHD events • Equipoise exists – is it ethical to do a trial?

What is a clinical trial? Critical features of design • Appropriate selection of patients • Easily definable phenotype on which to intervene • Relative homogeneity of study sample • People who have some risk of disease – With whom should you start to test your hypothesis? • Secondary>primary high risk>primary low risk

What is a clinical trial? Critical features of design • Define intervention: YOU CONTROL THE EXPOSURE! - What is it? - How do you decide who gets it? - How long do you deliver it?

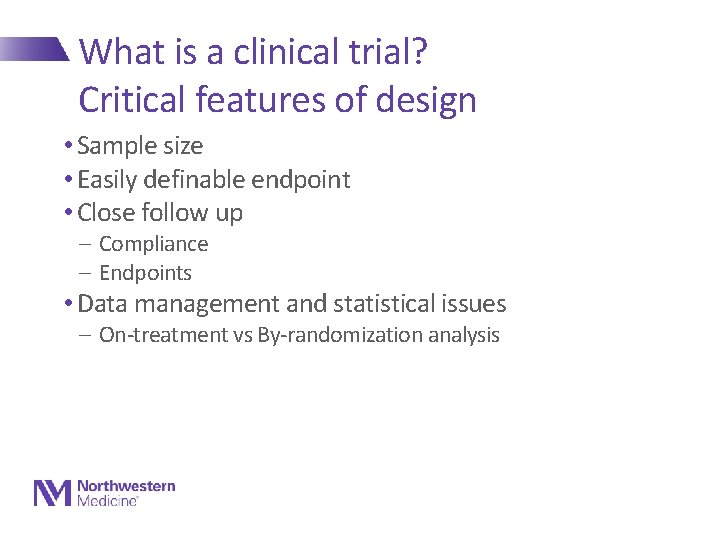
What is a clinical trial? Critical features of design • Sample size • Easily definable endpoint • Close follow up - Compliance - Endpoints • Data management and statistical issues - On-treatment vs By-randomization analysis

What is a clinical trial? Critical features of design • Which leads us to the Prospective, Randomized, Double-blind, Placebocontrolled Clinical Trial • Considered the highest form of clinical evidence

Secondary Prevention

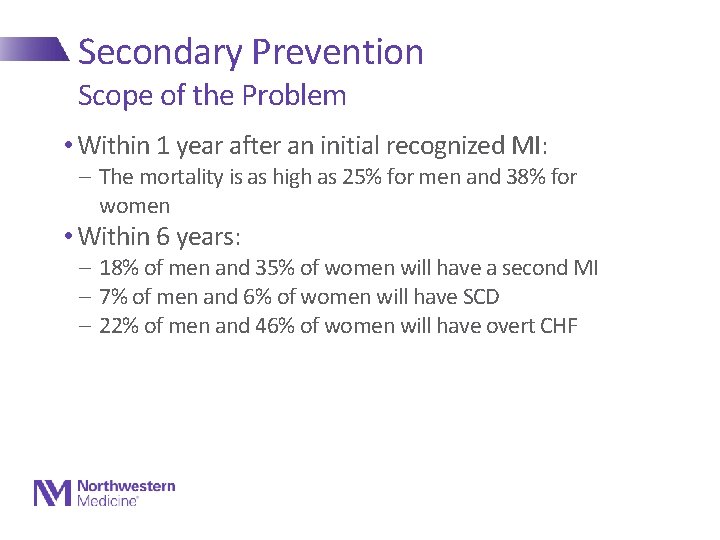
Secondary Prevention Scope of the Problem • Within 1 year after an initial recognized MI: - The mortality is as high as 25% for men and 38% for women • Within 6 years: - 18% of men and 35% of women will have a second MI - 7% of men and 6% of women will have SCD - 22% of men and 46% of women will have overt CHF

Secondary Prevention Scope of the Problem • Outcomes similar after unrecognized MI and after unstable angina • The elderly are at highest risk for recurrent events and death • Patients with clinical CHD have a large burden of subclinical disease - Not necessarily evident/impressive at angiography - Prone to plaque rupture - Goal of secondary prevention is to stabilize/regress residual disease

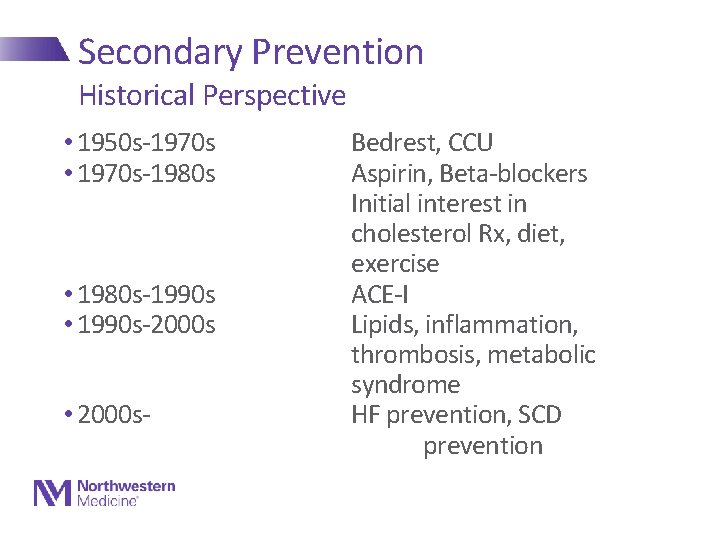
Secondary Prevention Historical Perspective • 1950 s-1970 s • 1970 s-1980 s • 1980 s-1990 s • 1990 s-2000 s • 2000 s- Bedrest, CCU Aspirin, Beta-blockers Initial interest in cholesterol Rx, diet, exercise ACE-I Lipids, inflammation, thrombosis, metabolic syndrome HF prevention, SCD prevention

AHA/ACC Secondary Prevention Guidelines 2011 JACC/Circ Nov, 2011


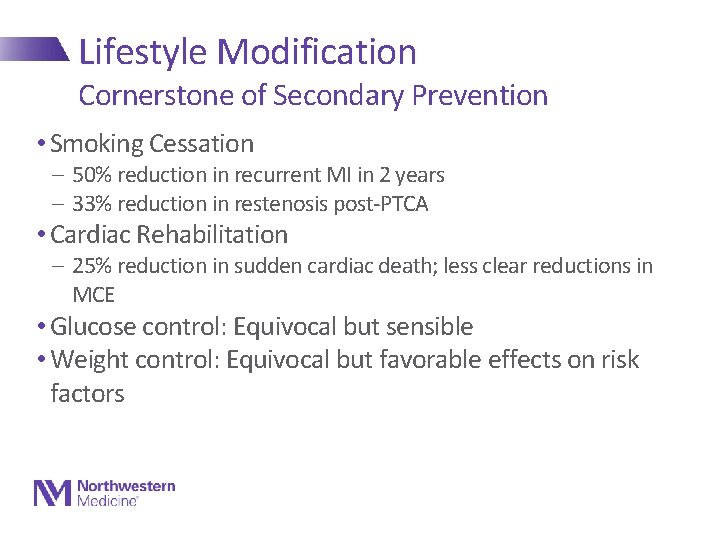
Lifestyle Modification Cornerstone of Secondary Prevention • Smoking Cessation - 50% reduction in recurrent MI in 2 years - 33% reduction in restenosis post-PTCA • Cardiac Rehabilitation - 25% reduction in sudden cardiac death; less clear reductions in MCE • Glucose control: Equivocal but sensible • Weight control: Equivocal but favorable effects on risk factors

Secondary Prevention “Standard” Therapies for Overt CHD • Aspirin: 50% reduction in death/NFMI • Other anti-platelet agent (for most) • Beta-blockers: 25% reduction in CHD death, especially SCD • ACE-inhibitors: 15 -20% reductions in mortality/HF depending on setting • Statin

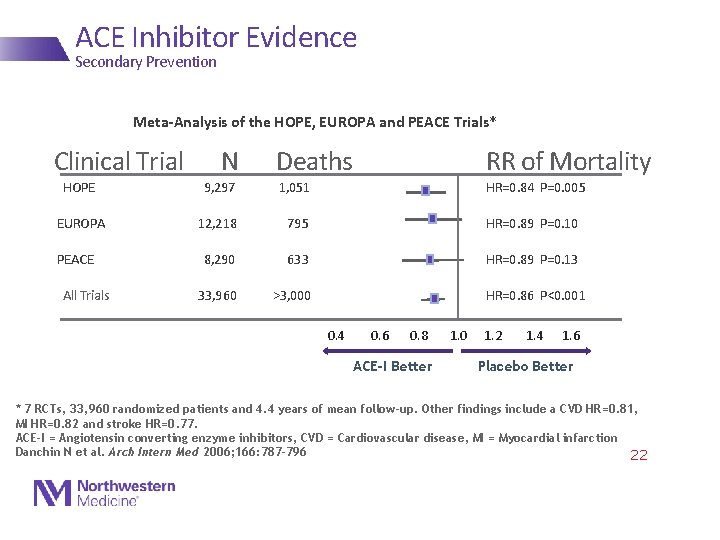
ACE Inhibitor Evidence Secondary Prevention Meta-Analysis of the HOPE, EUROPA and PEACE Trials* Clinical Trial N Deaths RR of Mortality HOPE 9, 297 EUROPA 12, 218 795 HR=0. 89 P=0. 10 8, 290 633 HR=0. 89 P=0. 13 33, 960 >3, 000 PEACE All Trials 1, 051 HR=0. 84 P=0. 005 HR=0. 86 P<0. 001 0. 4 0. 6 0. 8 ACE-I Better 1. 0 1. 2 1. 4 1. 6 Placebo Better * 7 RCTs, 33, 960 randomized patients and 4. 4 years of mean follow-up. Other findings include a CVD HR=0. 81, MI HR=0. 82 and stroke HR=0. 77. ACE-I = Angiotensin converting enzyme inhibitors, CVD = Cardiovascular disease, MI = Myocardial infarction Danchin N et al. Arch Intern Med 2006; 166: 787 -796 22

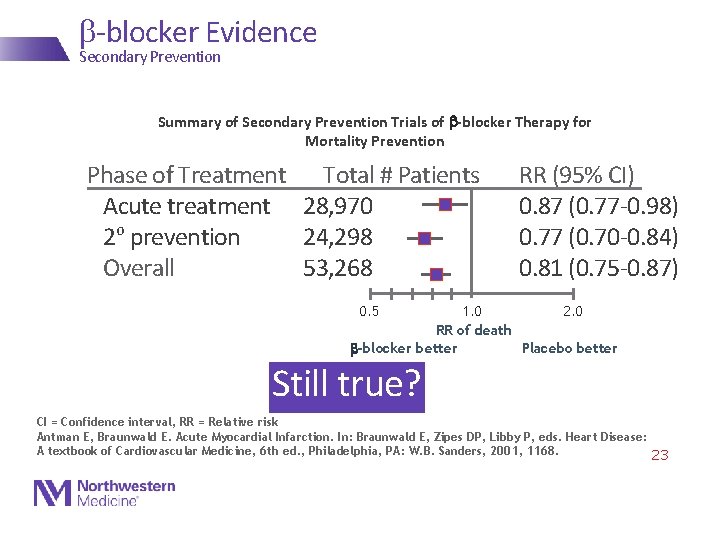
b-blocker Evidence Secondary Prevention Summary of Secondary Prevention Trials of b-blocker Therapy for Mortality Prevention Phase of Treatment Total # Patients Acute treatment 28, 970 2 o prevention 24, 298 Overall 53, 268 RR (95% CI) 0. 87 (0. 77 -0. 98) 0. 77 (0. 70 -0. 84) 0. 81 (0. 75 -0. 87) 0. 5 1. 0 2. 0 RR of death b-blocker better Placebo better Still true? CI = Confidence interval, RR = Relative risk Antman E, Braunwald E. Acute Myocardial Infarction. In: Braunwald E, Zipes DP, Libby P, eds. Heart Disease: A textbook of Cardiovascular Medicine, 6 th ed. , Philadelphia, PA: W. B. Sanders, 2001, 1168. 23

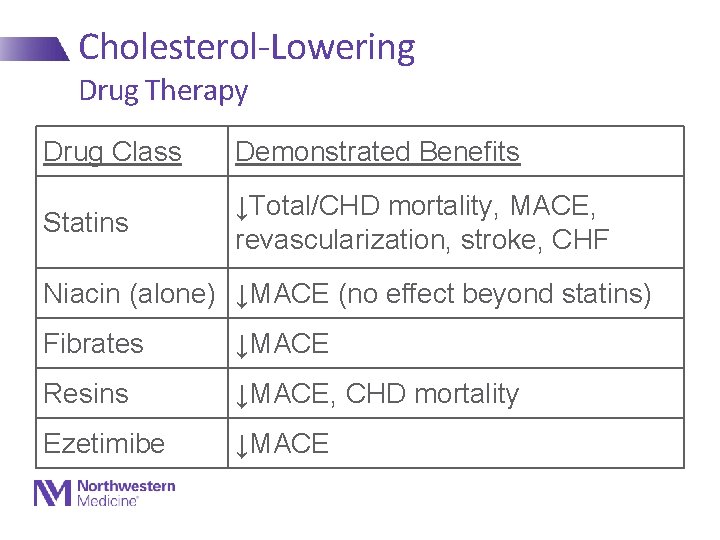
Cholesterol-Lowering Drug Therapy Drug Class Demonstrated Benefits Statins ↓Total/CHD mortality, MACE, revascularization, stroke, CHF Niacin (alone) ↓MACE (no effect beyond statins) Fibrates ↓MACE Resins ↓MACE, CHD mortality Ezetimibe ↓MACE

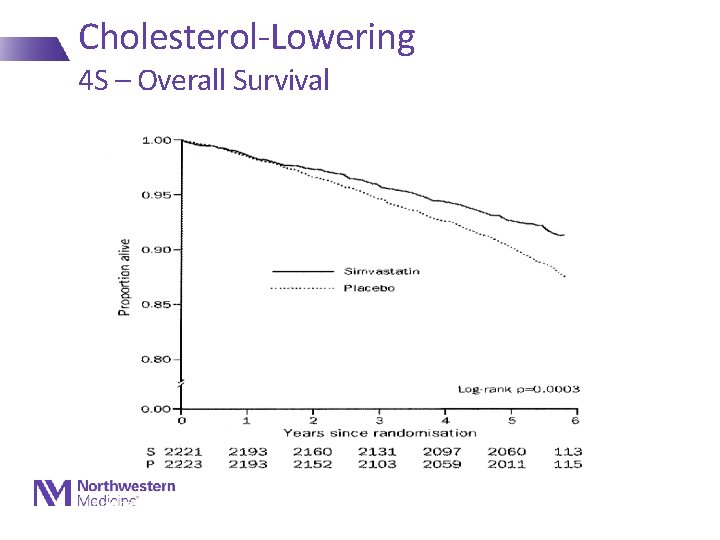
Cholesterol-Lowering 4 S – Overall Survival Lancet 1994; 344: 1383

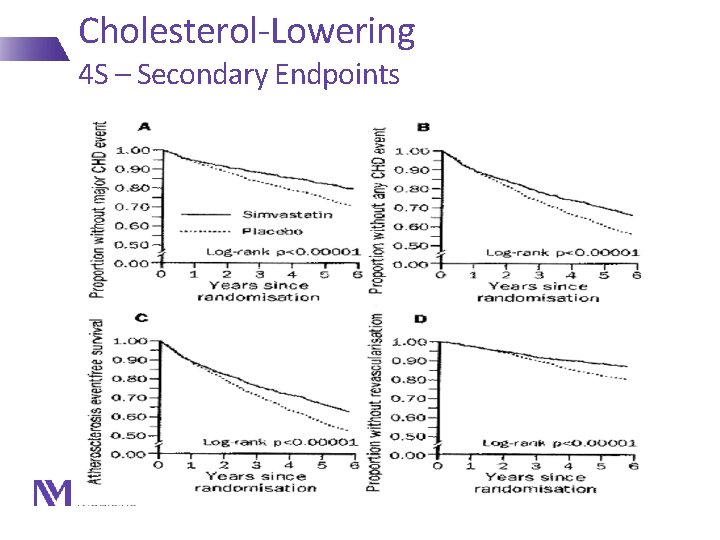
Cholesterol-Lowering 4 S – Secondary Endpoints Lancet 1994; 344: 1383

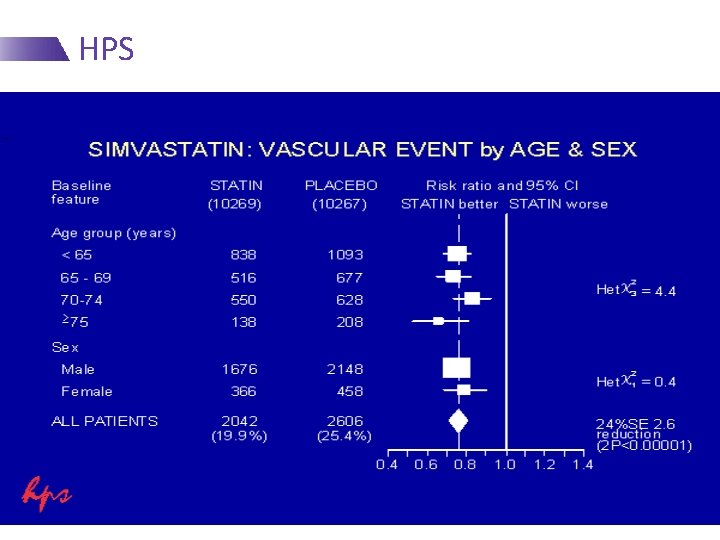
HPS

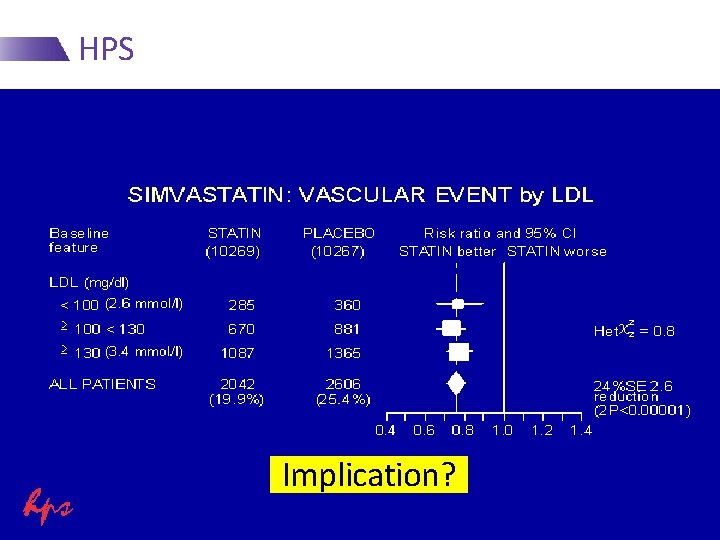
HPS Implication?

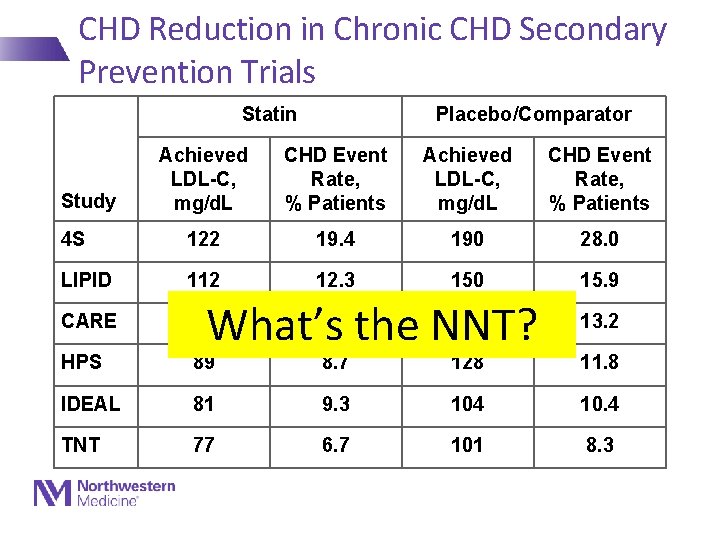
CHD Reduction in Chronic CHD Secondary Prevention Trials Statin Placebo/Comparator Achieved LDL-C, mg/d. L CHD Event Rate, % Patients 4 S 122 19. 4 190 28. 0 LIPID 112 12. 3 150 15. 9 CARE 98 HPS 89 8. 7 128 11. 8 IDEAL 81 9. 3 104 10. 4 TNT 77 6. 7 101 8. 3 Study 10. 2 135 What’s the NNT? 13. 2

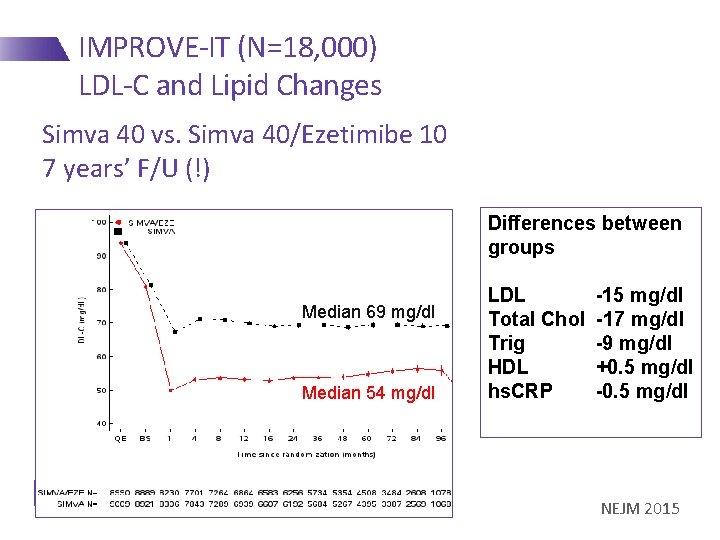
IMPROVE-IT (N=18, 000) LDL-C and Lipid Changes Simva 40 vs. Simva 40/Ezetimibe 10 7 years’ F/U (!) Differences between groups Median 69 mg/dl Median 54 mg/dl LDL Total Chol Trig HDL hs. CRP -15 mg/dl -17 mg/dl -9 mg/dl +0. 5 mg/dl -0. 5 mg/dl NEJM 2015

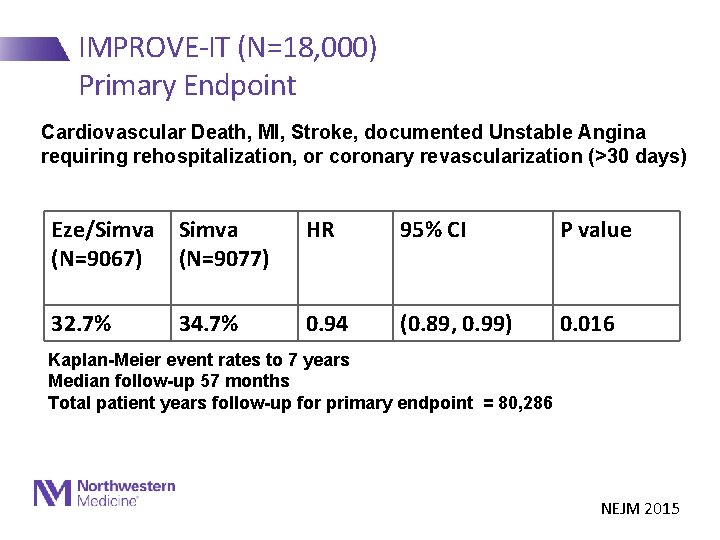
IMPROVE-IT (N=18, 000) Primary Endpoint Cardiovascular Death, MI, Stroke, documented Unstable Angina requiring rehospitalization, or coronary revascularization (>30 days) Eze/Simva (N=9067) (N=9077) HR 95% CI P value 32. 7% 0. 94 (0. 89, 0. 99) 0. 016 34. 7% Kaplan-Meier event rates to 7 years Median follow-up 57 months Total patient years follow-up for primary endpoint = 80, 286 NEJM 2015

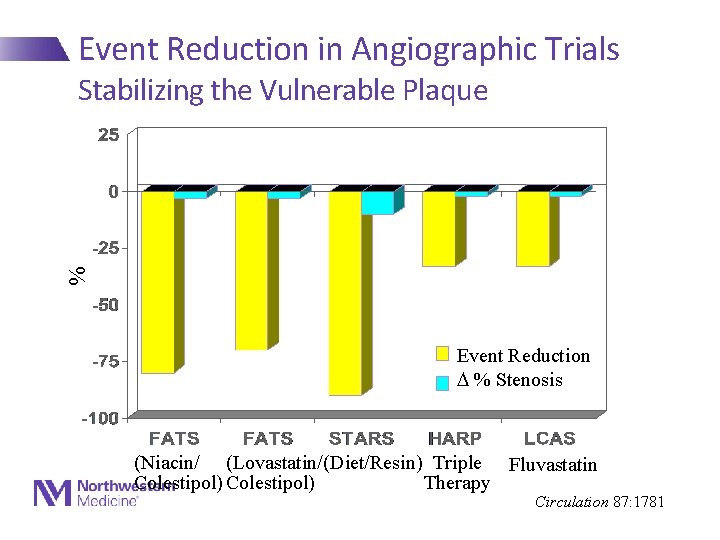
Event Reduction in Angiographic Trials % Stabilizing the Vulnerable Plaque Event Reduction % Stenosis (Niacin/ (Lovastatin/ (Diet/Resin) Triple Fluvastatin Colestipol) Therapy Circulation 87: 1781

Secondary Prevention LDL-Lowering vs PCI • Atorvastatin Versus Revascularization Treatment (AVERT) - Pts with stable CAD and LDL>115 - Atorvastatin 80 mg/day vs Angioplasty + Usual Care - Primary endpoint: Total ischemic events in 18 months

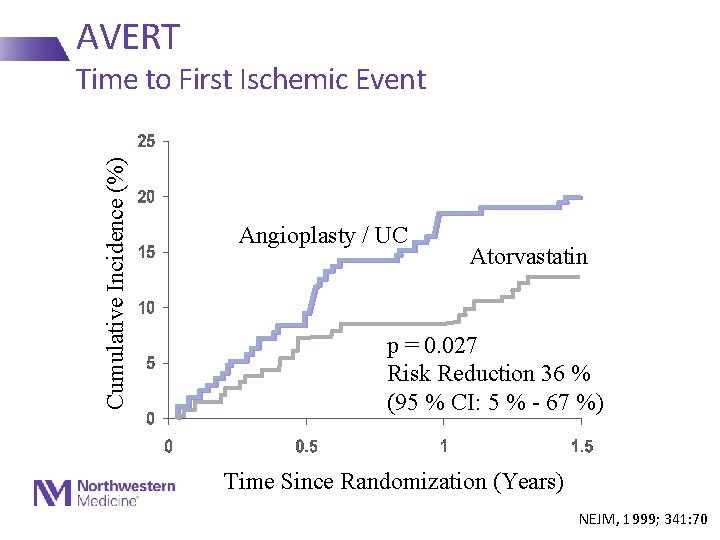
AVERT Cumulative Incidence (%) Time to First Ischemic Event Angioplasty / UC Atorvastatin p = 0. 027 Risk Reduction 36 % (95 % CI: 5 % - 67 %) Time Since Randomization (Years) NEJM, 1999; 341: 70

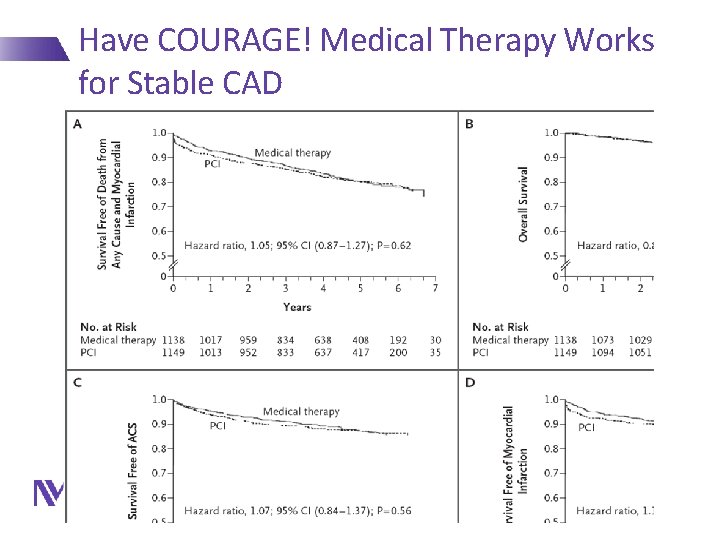
Have COURAGE! Medical Therapy Works for Stable CAD

Should We Raise HDL-C with Pharmacotherapy? • No!

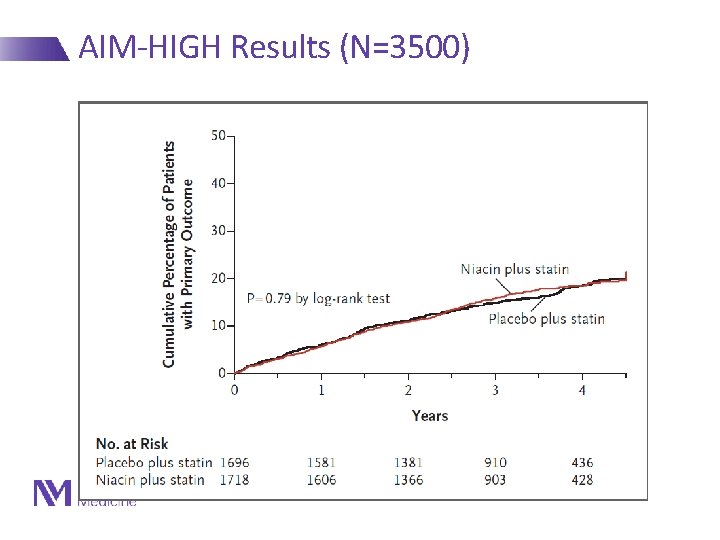
AIM-HIGH Results (N=3500) NEJM 2011

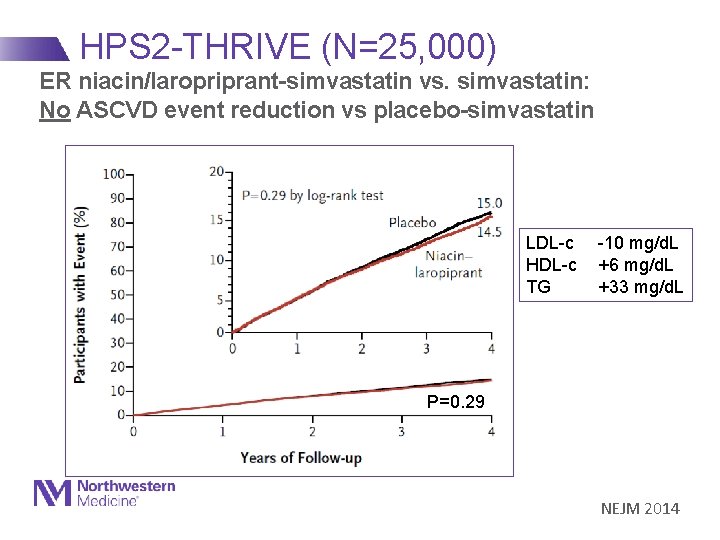
HPS 2 -THRIVE (N=25, 000) ER niacin/laropriprant-simvastatin vs. simvastatin: No ASCVD event reduction vs placebo-simvastatin LDL-c -10 mg/d. L HDL-c +6 mg/d. L TG +33 mg/d. L P=0. 29 NEJM 2014

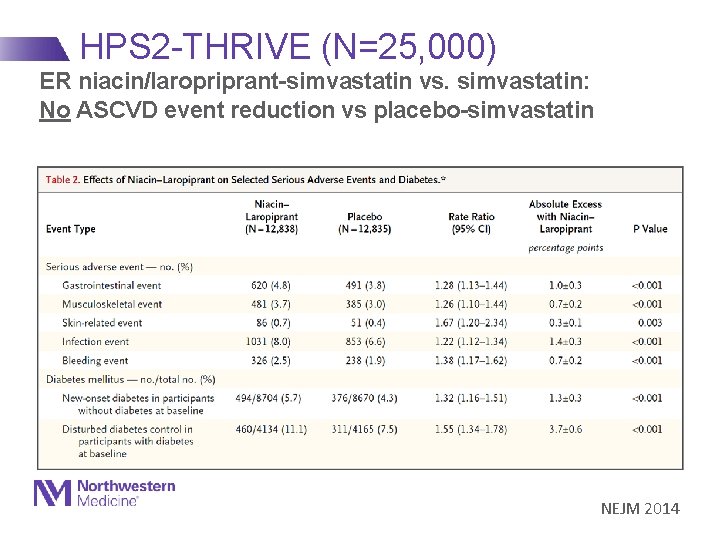
HPS 2 -THRIVE (N=25, 000) ER niacin/laropriprant-simvastatin vs. simvastatin: No ASCVD event reduction vs placebo-simvastatin NEJM 2014

Novel Targets for Intervention? • Homocysteine • Hormone replacement therapy • Antibiotics • Vitamin supplements X X

Primary Prevention

Primary Prevention Potential Targets for Intervention • Diet • Weight • Physical activity • Antiplatelet agents • Dyslipidemia • Blood pressure • Diabetes • Metabolic syndrome


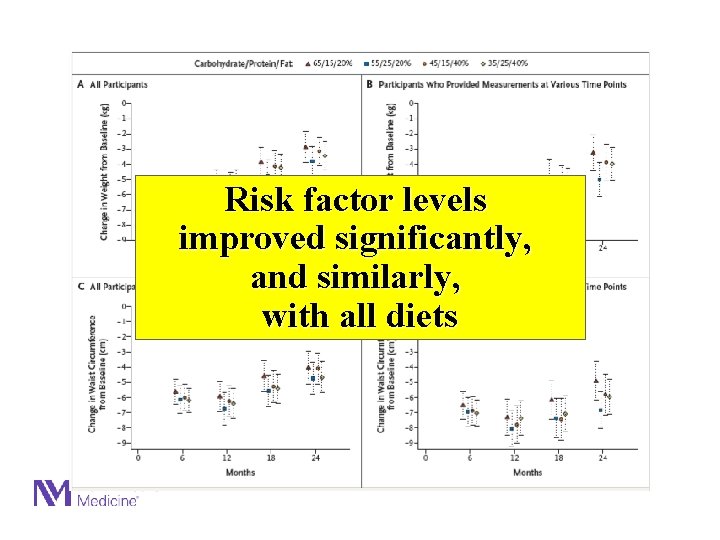
Risk factor levels improved significantly, and similarly, with all diets

Take Home • De-emphasize dietary content (micronutrients) somewhat for weight loss • Focus on calories and find what works (substitution!) • Referral to dietician • Increase physical activity - Concrete recommendations, pedometer - Aim for vigorous, if possible • Set achievable goals - 1 lb/week glidepath - Soft landing • Motivational interviewing • Electronic media/m. Health technology

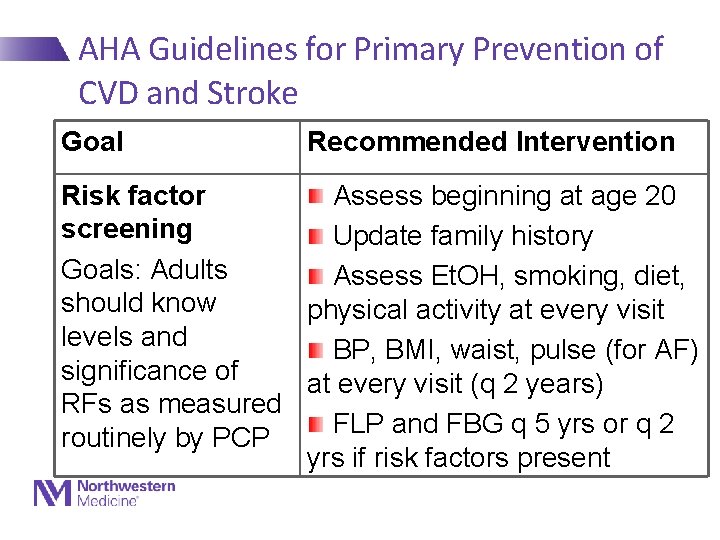
AHA Guidelines for Primary Prevention of CVD and Stroke Goal Recommended Intervention Risk factor Assess beginning at age 20 screening Update family history Goals: Adults Assess Et. OH, smoking, diet, should know physical activity at every visit levels and BP, BMI, waist, pulse (for AF) significance of at every visit (q 2 years) RFs as measured FLP and FBG q 5 yrs or q 2 routinely by PCP yrs if risk factors present

Primary Prevention - Lipids

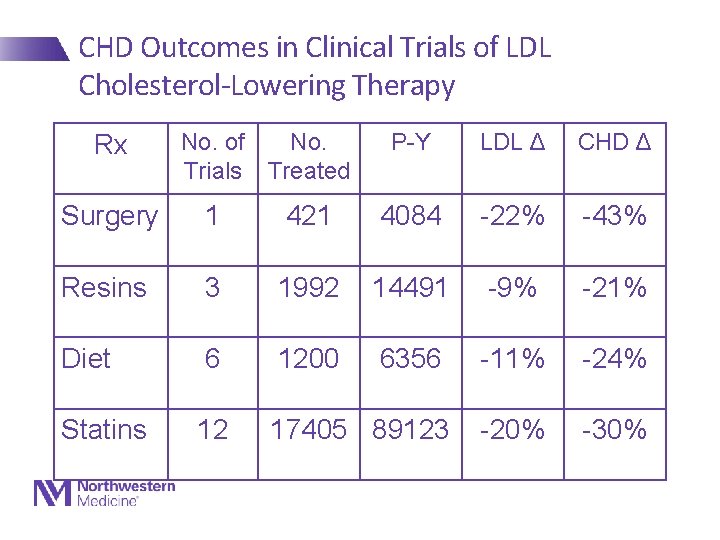
CHD Outcomes in Clinical Trials of LDL Cholesterol-Lowering Therapy Rx No. of No. Trials Treated P-Y LDL Δ CHD Δ Surgery 1 421 4084 -22% -43% Resins 3 1992 14491 -9% -21% Diet 6 1200 6356 -11% -24% Statins 12 17405 89123 -20% -30%

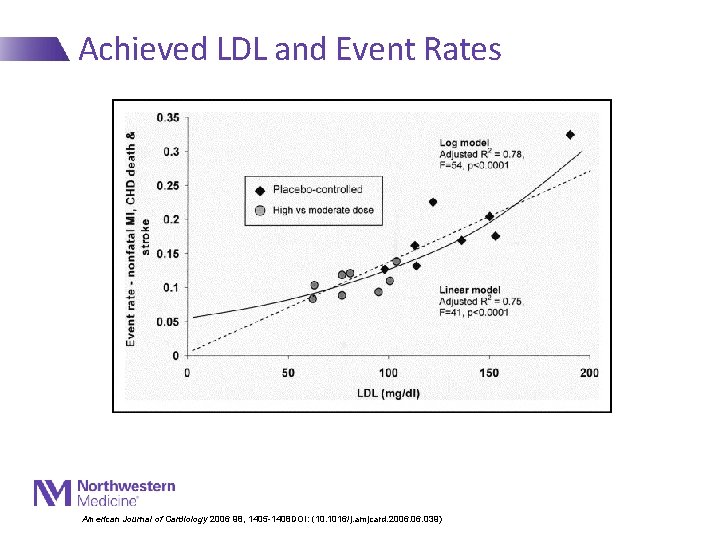
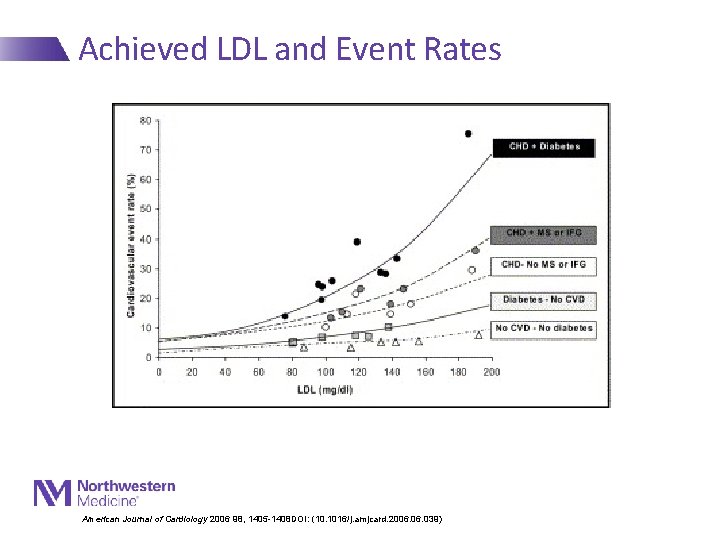
Achieved LDL and Event Rates American Journal of Cardiology 2006 98, 1405 -1408 DOI: (10. 1016/j. amjcard. 2006. 039)

Achieved LDL and Event Rates American Journal of Cardiology 2006 98, 1405 -1408 DOI: (10. 1016/j. amjcard. 2006. 039)

Prevention Paradigm - Lipids • “The intensity of prevention efforts should match the absolute risk of the patient”

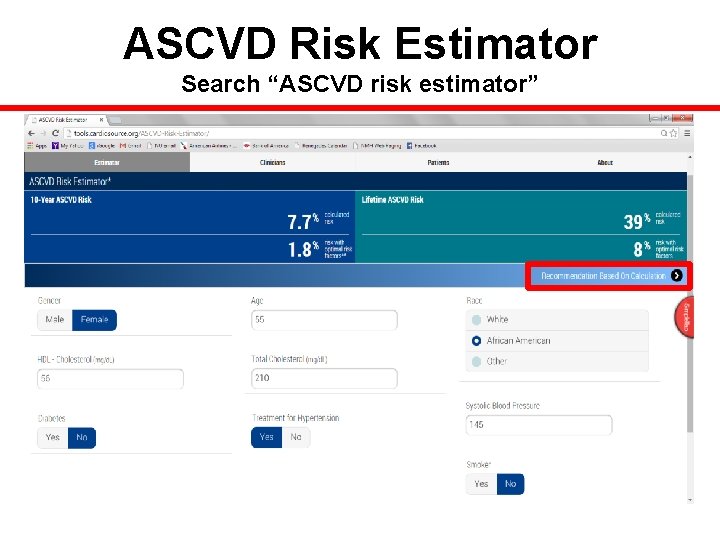
ASCVD Risk Estimator Search “ASCVD risk estimator”

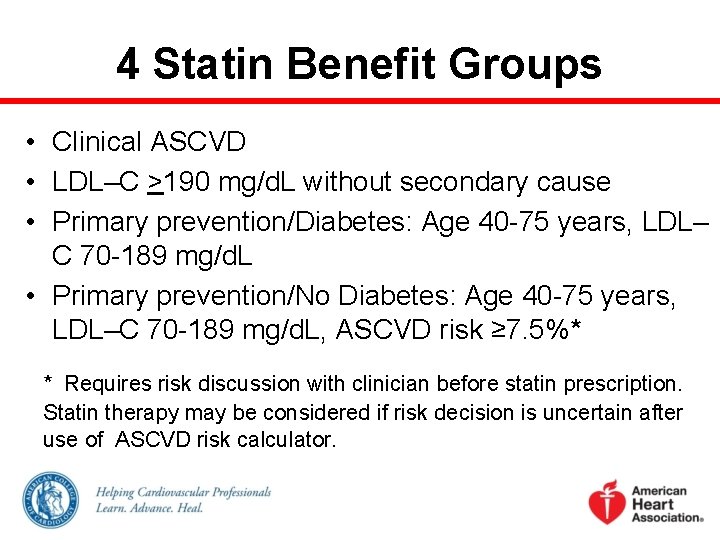
4 Statin Benefit Groups • Clinical ASCVD • LDL–C >190 mg/d. L without secondary cause • Primary prevention/Diabetes: Age 40 -75 years, LDL– C 70 -189 mg/d. L • Primary prevention/No Diabetes: Age 40 -75 years, LDL–C 70 -189 mg/d. L, ASCVD risk ≥ 7. 5%* * Requires risk discussion with clinician before statin prescription. Statin therapy may be considered if risk decision is uncertain after use of ASCVD risk calculator.

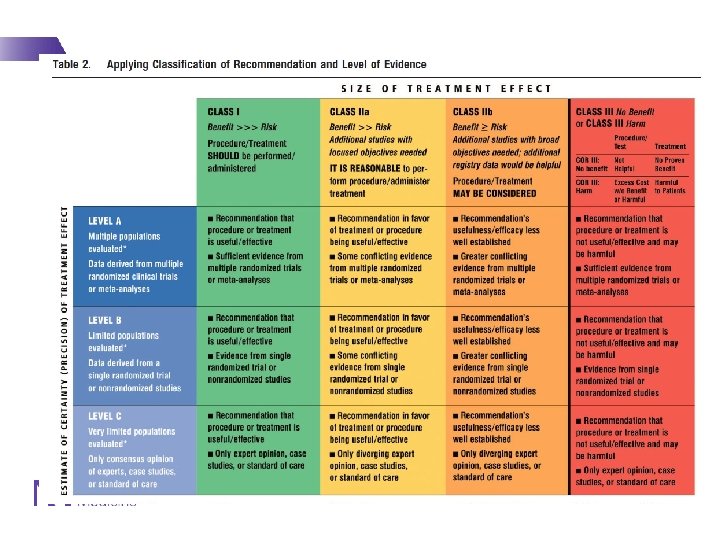
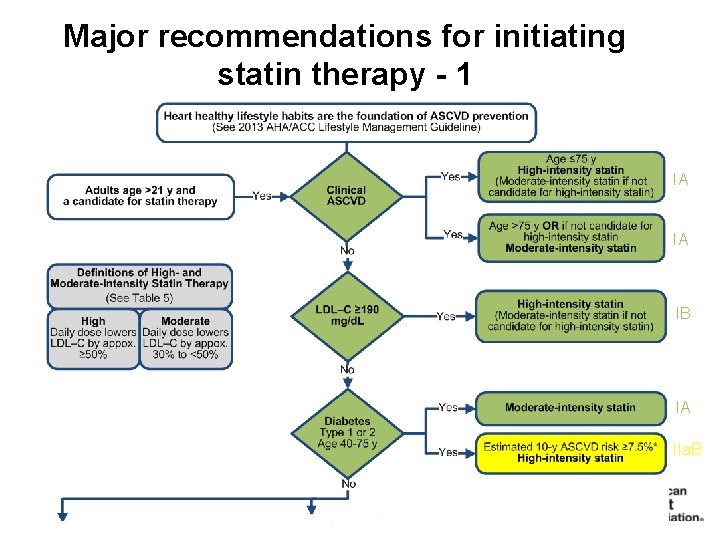
Major recommendations for initiating statin therapy - 1 IA IA IB IA IIa. B 1

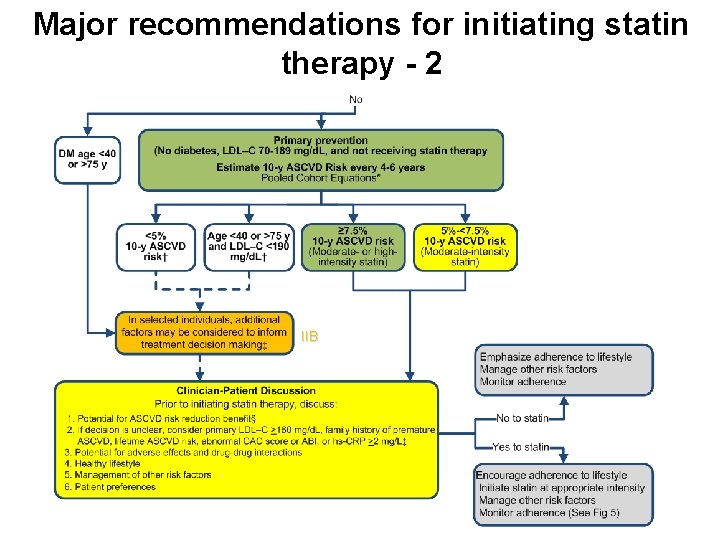
Major recommendations for initiating statin therapy - 2 IIB


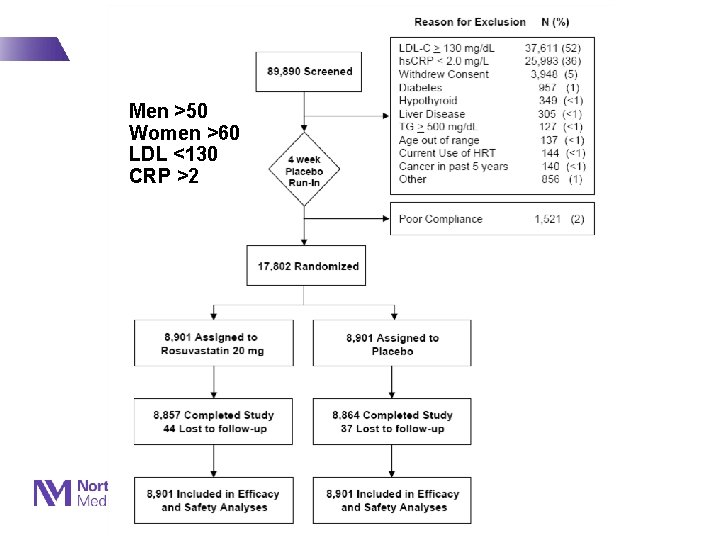
Men >50 Women >60 LDL <130 CRP >2

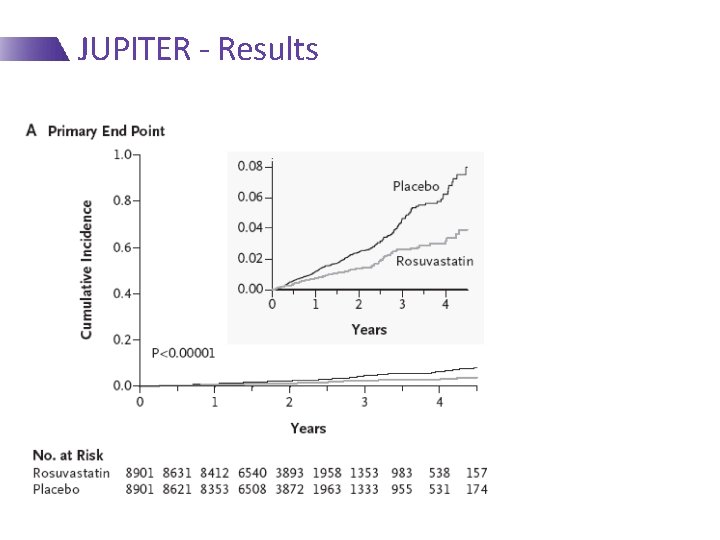
JUPITER - Results At 2 Yrs: 44% RRR 1. 36/100 p-y vs. 0. 77/100 p-y NNT 100 -150

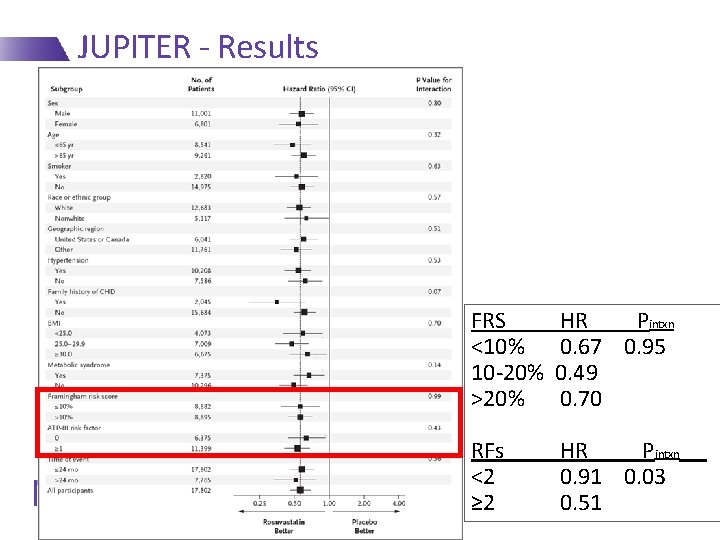
JUPITER - Results FRS <10% 10 -20% >20% HR Pintxn 0. 67 0. 95 0. 49 0. 70 RFs <2 ≥ 2 HR Pintxn 0. 91 0. 03 0. 51

Primary Prevention - BP

Prevention Paradigm - BP • “All high BP is bad; treat all high BP”

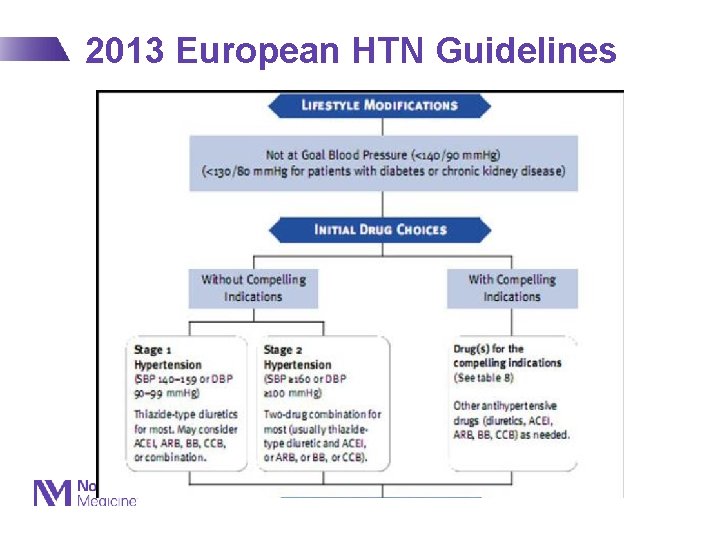
2013 European HTN Guidelines Eur J Hypertens 2013; 31(7): 1281 -1357

2013 European HTN Guidelines Eur J Hypertens 2013; 31(7): 1281 -1357

Progression of BP-Lowering Trials • Started with very high DBP 1960 s • Then moderately high DBP • By 1980 s, epidemiological data suggested SBP was more important • Isolated systolic hypertension trials – 1991 -1999 64 2/20/2021

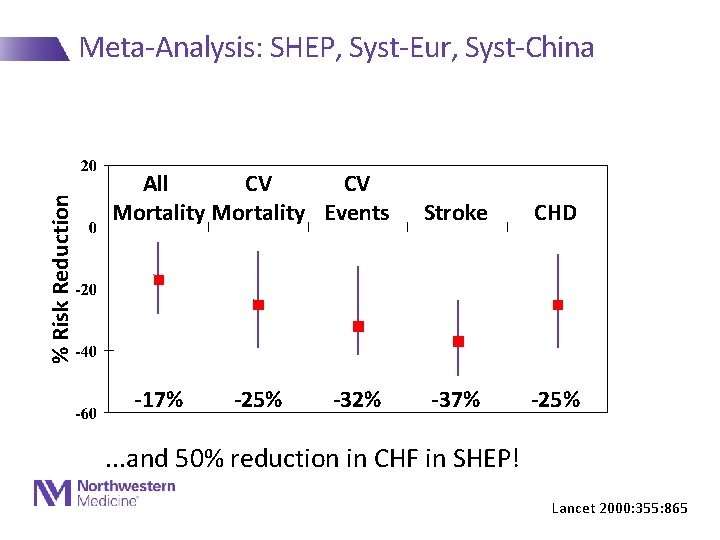
% Risk Reduction Meta-Analysis: SHEP, Syst-Eur, Syst-China All CV CV Mortality Events -17% -25% -32% Stroke CHD -37% -25% . . . and 50% reduction in CHF in SHEP! Lancet 2000: 355: 865

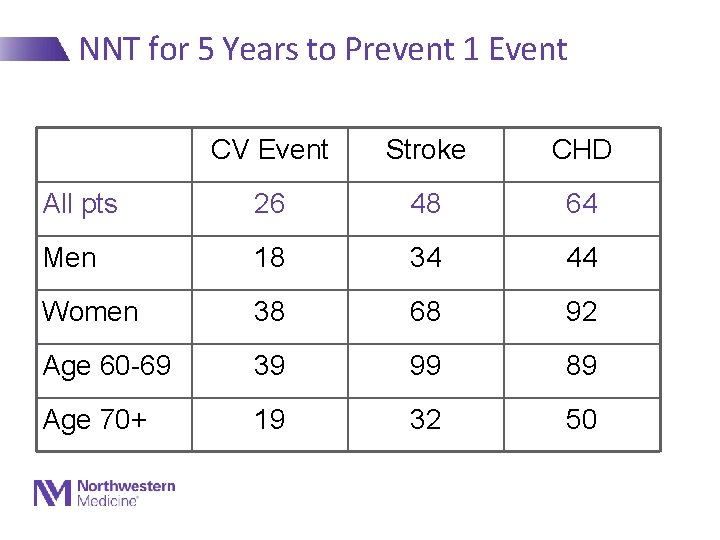
NNT for 5 Years to Prevent 1 Event CV Event Stroke CHD All pts 26 48 64 Men 18 34 44 Women 38 68 92 Age 60 -69 39 99 89 Age 70+ 19 32 50 Staessen, Lancet 2000: 355: 865

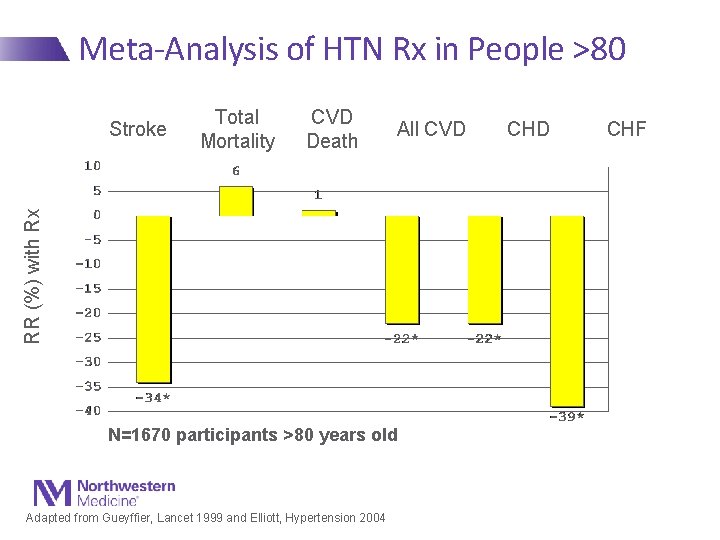
Meta-Analysis of HTN Rx in People >80 Total Mortality CVD Death All CVD RR (%) with Rx Stroke N=1670 participants >80 years old Adapted from Gueyffier, Lancet 1999 and Elliott, Hypertension 2004 CHD CHF

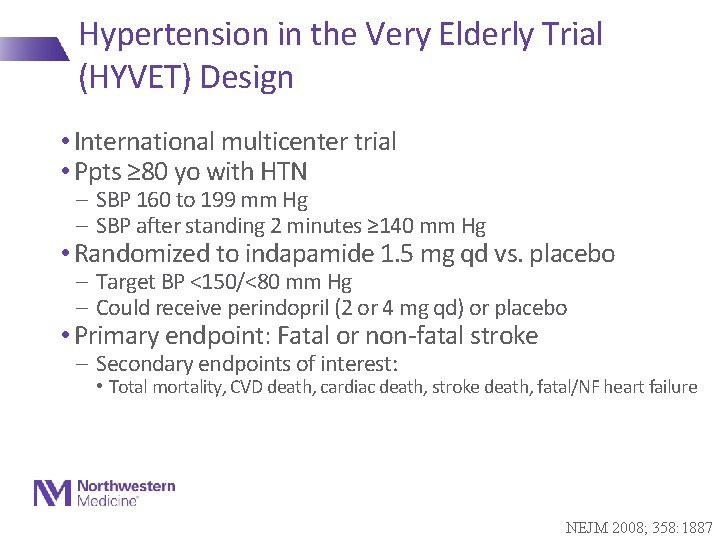
Hypertension in the Very Elderly Trial (HYVET) Design • International multicenter trial • Ppts ≥ 80 yo with HTN - SBP 160 to 199 mm Hg - SBP after standing 2 minutes ≥ 140 mm Hg • Randomized to indapamide 1. 5 mg qd vs. placebo - Target BP <150/<80 mm Hg - Could receive perindopril (2 or 4 mg qd) or placebo • Primary endpoint: Fatal or non-fatal stroke - Secondary endpoints of interest: • Total mortality, CVD death, cardiac death, stroke death, fatal/NF heart failure NEJM 2008; 358: 1887

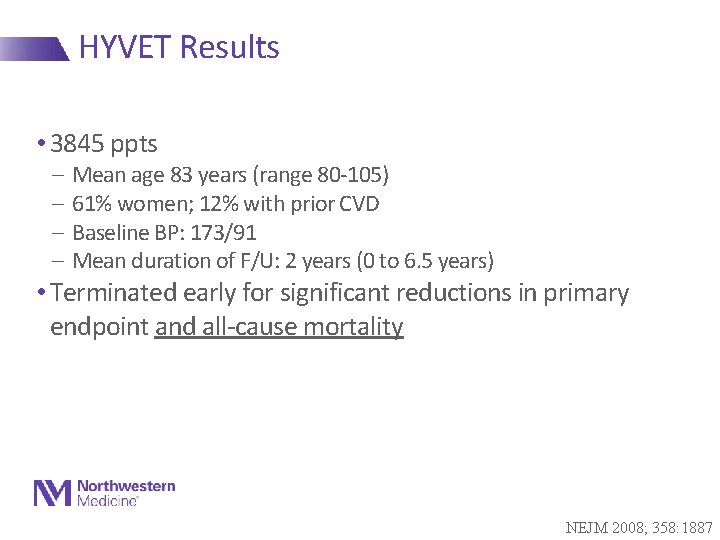
HYVET Results • 3845 ppts - Mean age 83 years (range 80 -105) - 61% women; 12% with prior CVD - Baseline BP: 173/91 - Mean duration of F/U: 2 years (0 to 6. 5 years) • Terminated early for significant reductions in primary endpoint and all-cause mortality NEJM 2008; 358: 1887

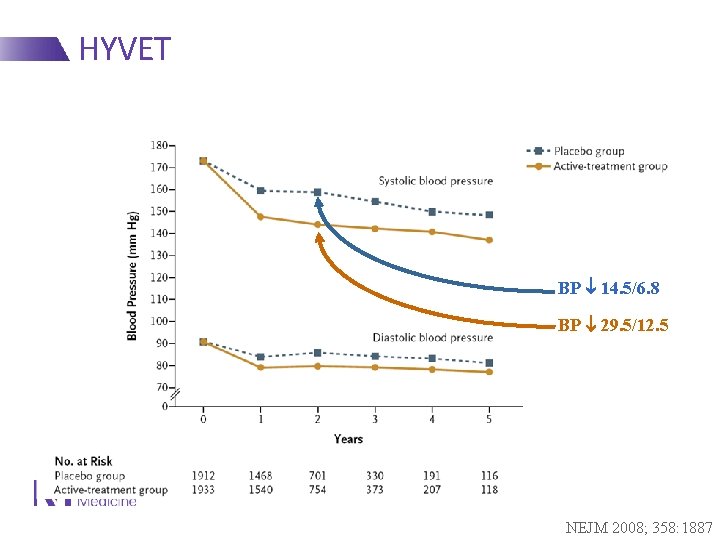
HYVET BP 14. 5/6. 8 BP 29. 5/12. 5 NEJM 2008; 358: 1887

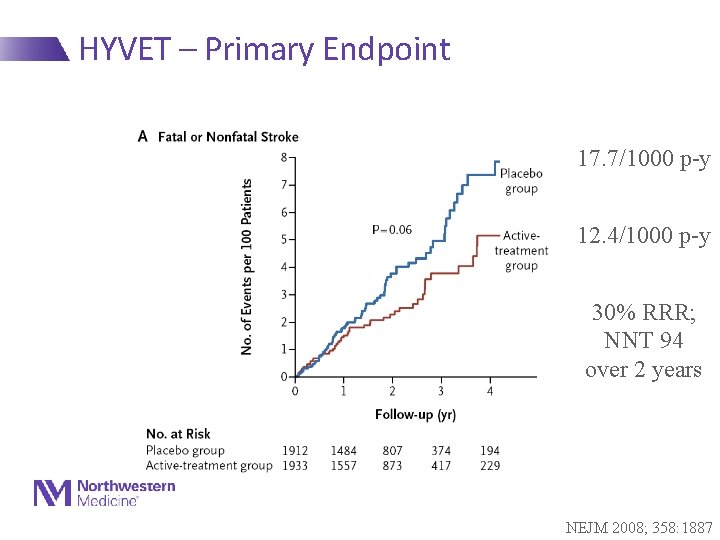
HYVET – Primary Endpoint 17. 7/1000 p-y 12. 4/1000 p-y 30% RRR; NNT 94 over 2 years NEJM 2008; 358: 1887

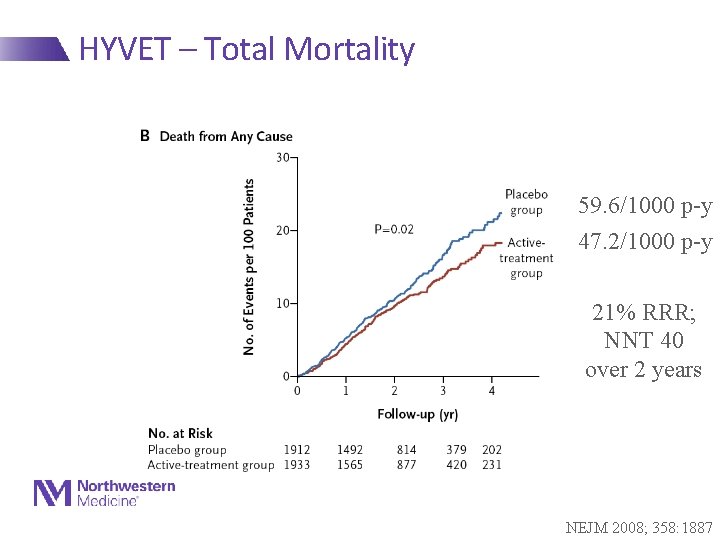
HYVET – Total Mortality 59. 6/1000 p-y 47. 2/1000 p-y 21% RRR; NNT 40 over 2 years NEJM 2008; 358: 1887

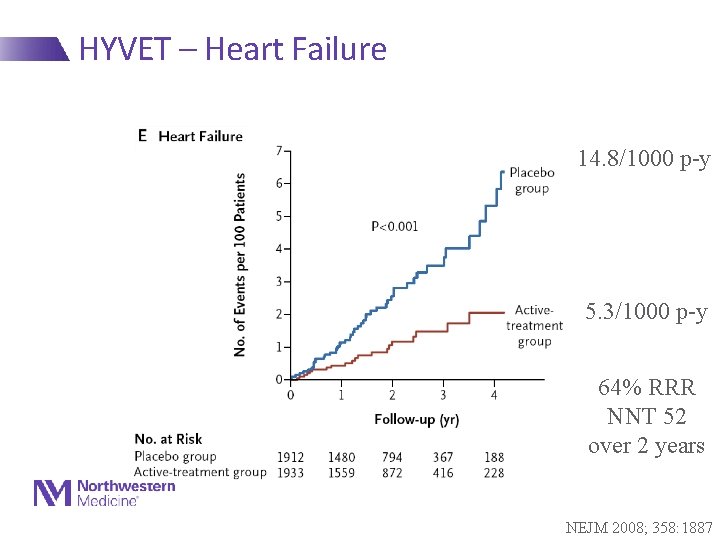
HYVET – Heart Failure 14. 8/1000 p-y 5. 3/1000 p-y 64% RRR NNT 52 over 2 years NEJM 2008; 358: 1887

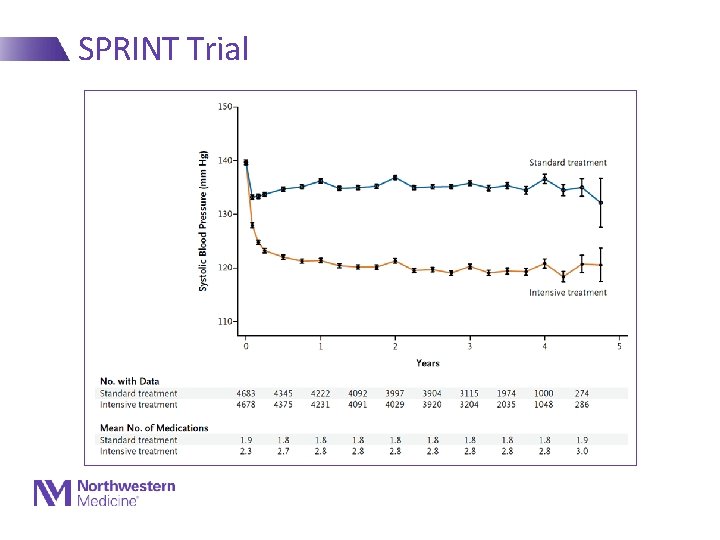
SPRINT Trial 74 2/20/2021

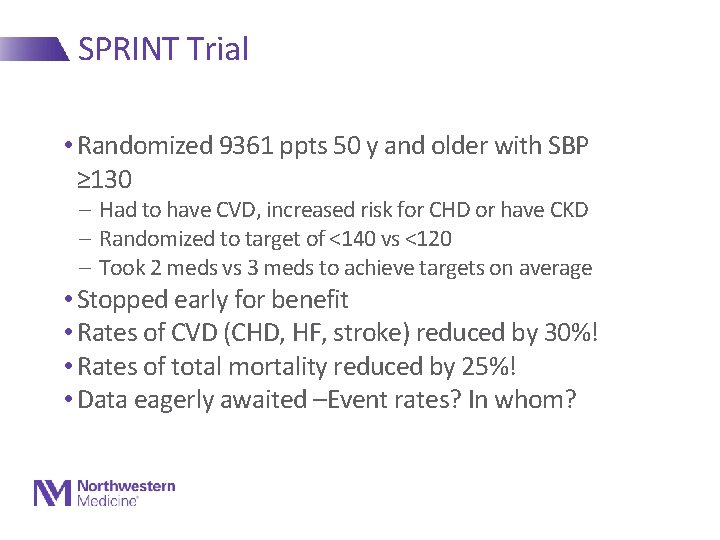
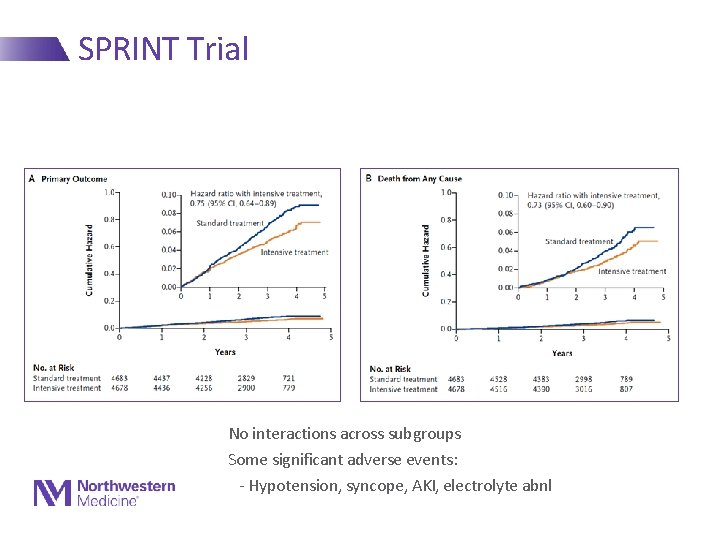
SPRINT Trial • Randomized 9361 ppts 50 y and older with SBP ≥ 130 - Had to have CVD, increased risk for CHD or have CKD - Randomized to target of <140 vs <120 - Took 2 meds vs 3 meds to achieve targets on average • Stopped early for benefit • Rates of CVD (CHD, HF, stroke) reduced by 30%! • Rates of total mortality reduced by 25%! • Data eagerly awaited –Event rates? In whom?

SPRINT Trial

SPRINT Trial No interactions across subgroups Some significant adverse events: - Hypotension, syncope, AKI, electrolyte abnl

Ancillary Treatment to Reduce Risk in HTN • Comprehensive Lifestyle Modification • ASA - HOT Trial: 36% reduction in MI with ASA 75 mg / day • Statins

Ancillary Treatment to Reduce Risk in HTN • Comprehensive Lifestyle Modification • ASA - HOT Trial: 36% reduction in MI with ASA 75 mg / day • Statins

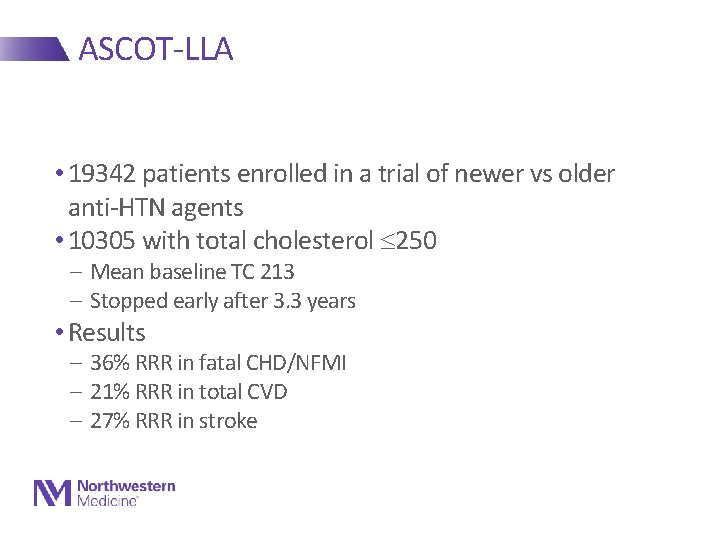
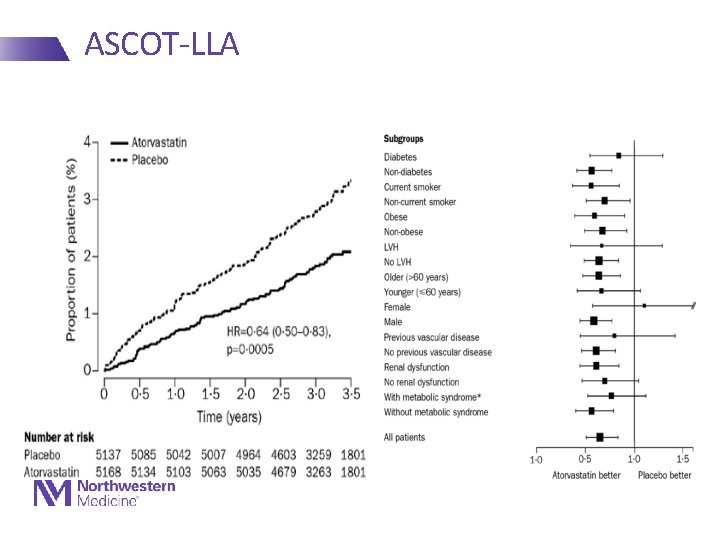
ASCOT-LLA • 19342 patients enrolled in a trial of newer vs older anti-HTN agents • 10305 with total cholesterol 250 - Mean baseline TC 213 - Stopped early after 3. 3 years • Results - 36% RRR in fatal CHD/NFMI - 21% RRR in total CVD - 27% RRR in stroke

ASCOT-LLA

Primary Prevention - Diabetes

CVD Prevention in Diabetes • Elevated blood sugar increases risk for CVD (all types) • RFs cluster, especially in DM, increasing risk • Diabetics are at high risk, and have worse outcomes when they have an event • Treatment of blood glucose does not effectively reduce macrovascular ASCVD events; just microvascular complications • Focus is on treating other RFs aggressivelt (BP, lipids, weight)

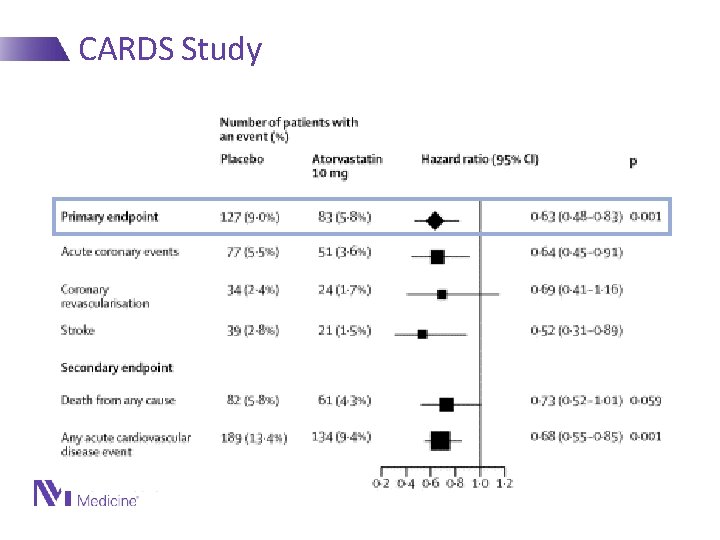
CARDS Study Lancet 2004

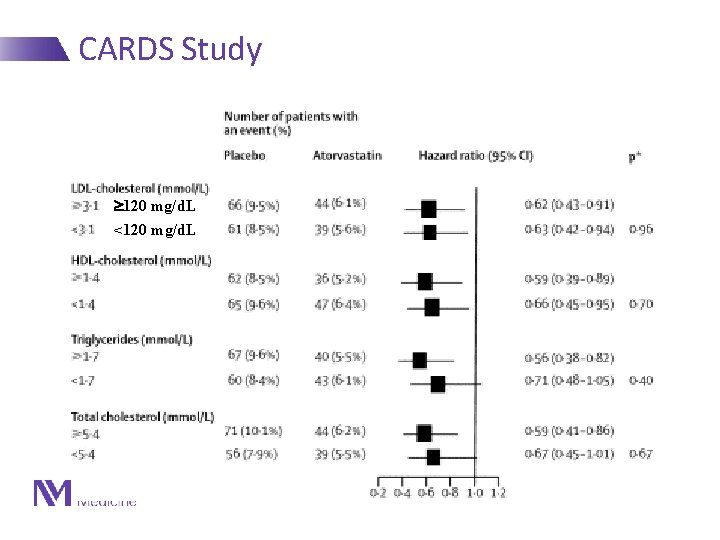
CARDS Study 120 mg/d. L <120 mg/d. L Lancet 2004

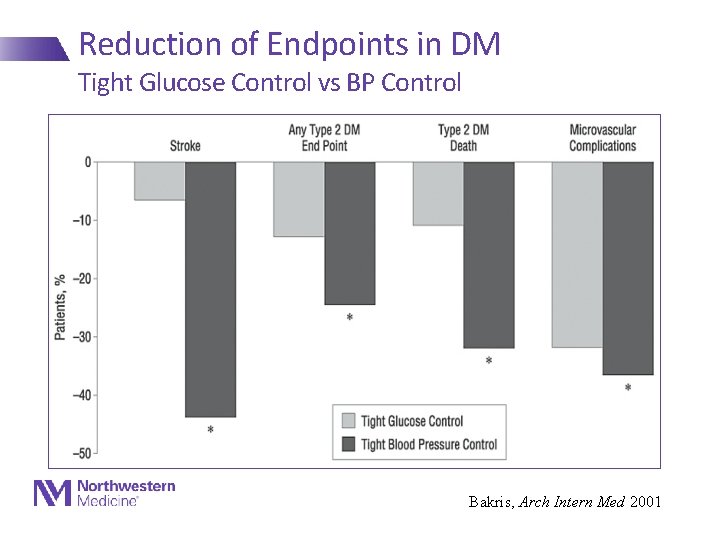
Reduction of Endpoints in DM Tight Glucose Control vs BP Control Bakris, Arch Intern Med 2001

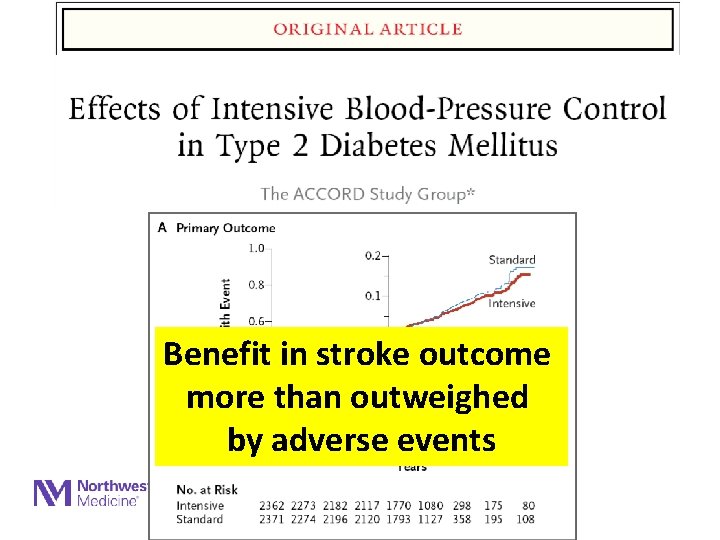
Benefit in stroke outcome more than outweighed by adverse events

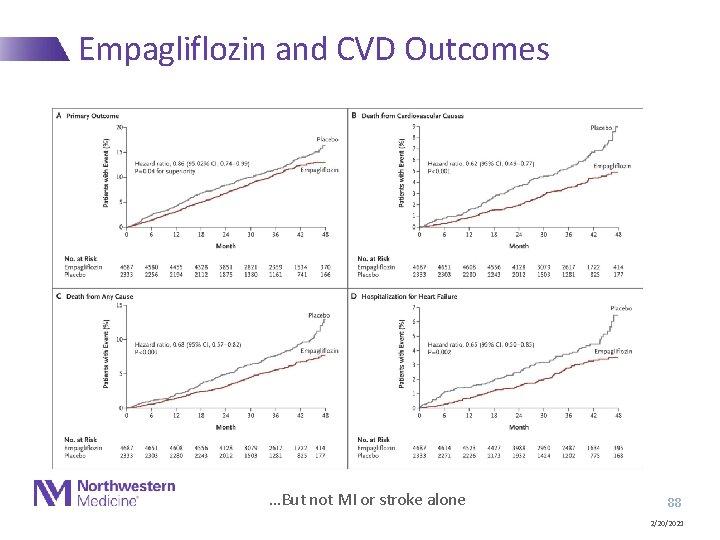
Empagliflozin and CVD Outcomes …But not MI or stroke alone 88 2/20/2021

Questions? 89 2/20/2021
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Pvd cvd coating
Pvd cvd coating Pvd vs cvd
Pvd vs cvd Nufab northwestern
Nufab northwestern Pvd, cvd
Pvd, cvd Commvault firewall ports
Commvault firewall ports Contoh specific protection
Contoh specific protection Recurvte
Recurvte Secondary prevention
Secondary prevention Secondary prevention
Secondary prevention Secondary prevention
Secondary prevention Secondary prevention
Secondary prevention Primary prevention examples
Primary prevention examples Primary prevention examples
Primary prevention examples Primary prevention of poliomyelitis
Primary prevention of poliomyelitis Primary and secondary effects of a tectonic hazard
Primary and secondary effects of a tectonic hazard Impact of business decisions on stakeholders
Impact of business decisions on stakeholders Planting more trees is called
Planting more trees is called Primary volcanic hazards
Primary volcanic hazards Use case primary and secondary actors
Use case primary and secondary actors Use case primary and secondary actors
Use case primary and secondary actors Static efficiency
Static efficiency Thyroid storm tsh levels
Thyroid storm tsh levels Primary and secondary retroperitoneal organs
Primary and secondary retroperitoneal organs Tolerance model of succession
Tolerance model of succession Oxidation of secondary alcohol to carboxylic acid
Oxidation of secondary alcohol to carboxylic acid Examples of primary and secondary stakeholders
Examples of primary and secondary stakeholders Agents of socialization
Agents of socialization Merton’s typology
Merton’s typology Bronchiole
Bronchiole Differentiate primary and secondary tillage
Differentiate primary and secondary tillage Primary and secondary tertiary sources
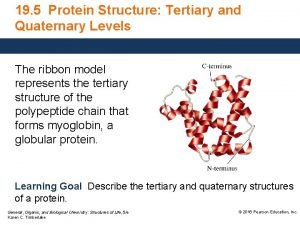
Primary and secondary tertiary sources Primary secondary and tertiary protein structure
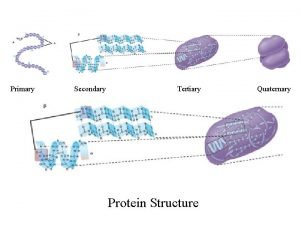
Primary secondary and tertiary protein structure Is autobiography a primary source
Is autobiography a primary source Secondary sources of light
Secondary sources of light Positive reinforcement vs negative reinforcement
Positive reinforcement vs negative reinforcement Antiporters
Antiporters Primary needs and secondary needs
Primary needs and secondary needs Primary and secondary lesions of skin
Primary and secondary lesions of skin Primary growth and secondary growth in plants
Primary growth and secondary growth in plants Active transport
Active transport Levels of care primary secondary tertiary
Levels of care primary secondary tertiary Fixed interval schedule
Fixed interval schedule What are secondary keywords
What are secondary keywords Primary energy and secondary energy
Primary energy and secondary energy Food web
Food web Primary target market and secondary target market
Primary target market and secondary target market Primary and secondary flare in inlay
Primary and secondary flare in inlay What is commercial energy source
What is commercial energy source Primary pollutants
Primary pollutants Primary lysosome vs secondary lysosome
Primary lysosome vs secondary lysosome Satisfaction
Satisfaction Primary needs and secondary needs
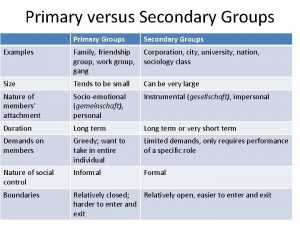
Primary needs and secondary needs Secondary group
Secondary group What is secondary food processing
What is secondary food processing Primary food processing examples
Primary food processing examples Auxilliary view
Auxilliary view Difference between primary battery and secondary battery
Difference between primary battery and secondary battery Hyperthyroidism primary and secondary
Hyperthyroidism primary and secondary Primary and secondary emotions
Primary and secondary emotions Primary and secondary coil
Primary and secondary coil Electrical supply system in building
Electrical supply system in building Primary secondary and tertiary sector
Primary secondary and tertiary sector Secondary economic activities definition
Secondary economic activities definition Primary succession facts
Primary succession facts Primary and secondary deviance
Primary and secondary deviance Tertiary sector examples
Tertiary sector examples What is social process theory
What is social process theory Primary and secondary deviance
Primary and secondary deviance Primary deviance definition
Primary deviance definition What are primary and secondary reinforcers
What are primary and secondary reinforcers Primary growth and secondary growth in plants
Primary growth and secondary growth in plants Vascular ray
Vascular ray Sewage treatment primary secondary and tertiary
Sewage treatment primary secondary and tertiary Benjamin cummings
Benjamin cummings Hyperthyroidism primary and secondary
Hyperthyroidism primary and secondary Circumferential tie in inlay
Circumferential tie in inlay Specific and pervasive boundaries for behavior
Specific and pervasive boundaries for behavior Primary and secondary succession weblab
Primary and secondary succession weblab Difference between primary battery and secondary battery
Difference between primary battery and secondary battery Primary and secondary cells
Primary and secondary cells Primary and secondary fracture lines in glass
Primary and secondary fracture lines in glass Primary pollutants and secondary pollutants
Primary pollutants and secondary pollutants What is primary and secondary air pollution
What is primary and secondary air pollution Primary ecological succession
Primary ecological succession Epiplon definicion
Epiplon definicion Primary secondary and tertiary protein structure
Primary secondary and tertiary protein structure Secondary deviance examples
Secondary deviance examples Secondary storage vs primary storage
Secondary storage vs primary storage Inverse transducer converts
Inverse transducer converts