19 5 Protein Structure Tertiary and Quaternary Levels

- Slides: 18

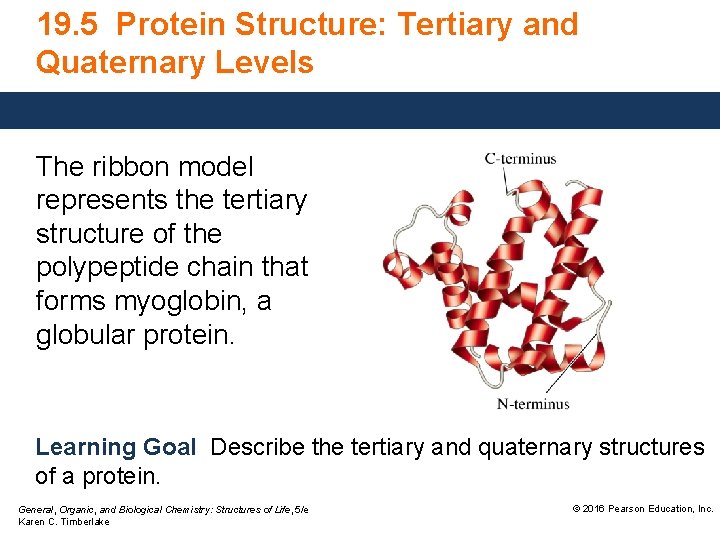
19. 5 Protein Structure: Tertiary and Quaternary Levels The ribbon model represents the tertiary structure of the polypeptide chain that forms myoglobin, a globular protein. Learning Goal Describe the tertiary and quaternary structures of a protein. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

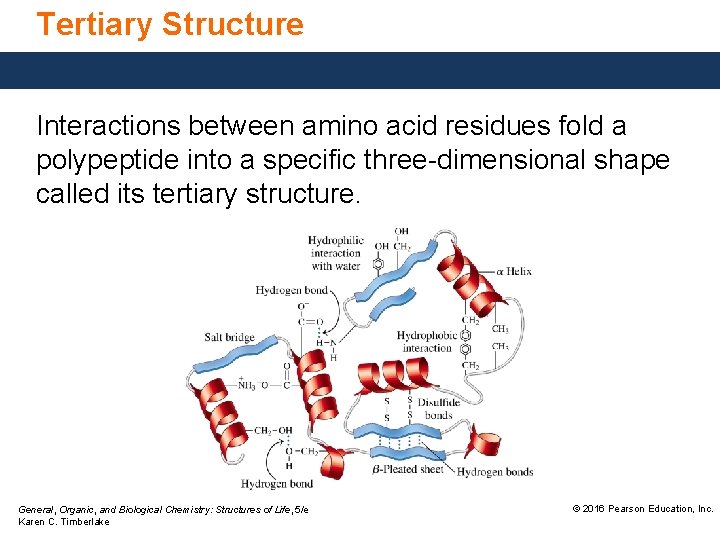
Tertiary Structure The tertiary structure of a protein is an overall threedimensional shape formed by the interactions and repulsions of amino acid residues in different parts of the chain. The fibrous proteins of a-keratin wrap together to form fibrils of hair and wool. Core Chemistry Skill Identifying the Primary, Secondary, Tertiary, and Quaternary Structures of Proteins General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

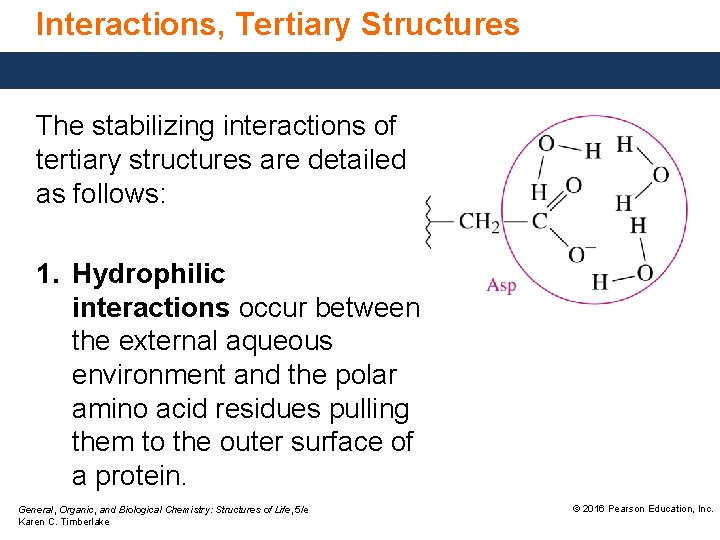
Interactions, Tertiary Structures The stabilizing interactions of tertiary structures are detailed as follows: 1. Hydrophilic interactions occur between the external aqueous environment and the polar amino acid residues pulling them to the outer surface of a protein. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

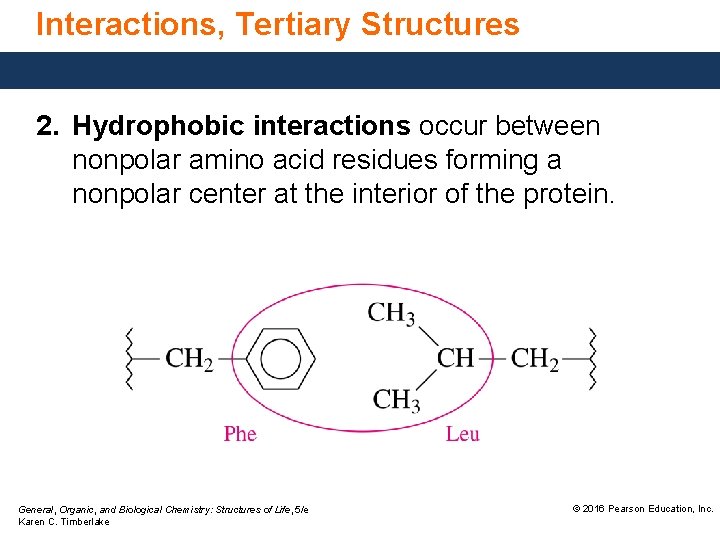
Interactions, Tertiary Structures 2. Hydrophobic interactions occur between nonpolar amino acid residues forming a nonpolar center at the interior of the protein. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

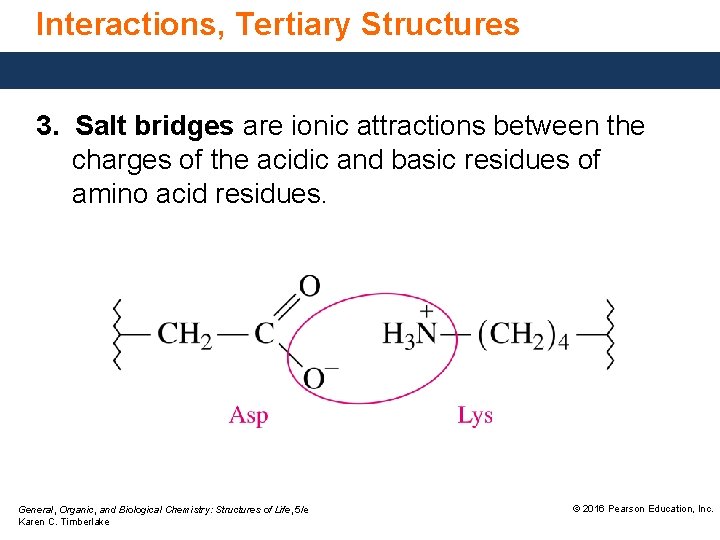
Interactions, Tertiary Structures 3. Salt bridges are ionic attractions between the charges of the acidic and basic residues of amino acid residues. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

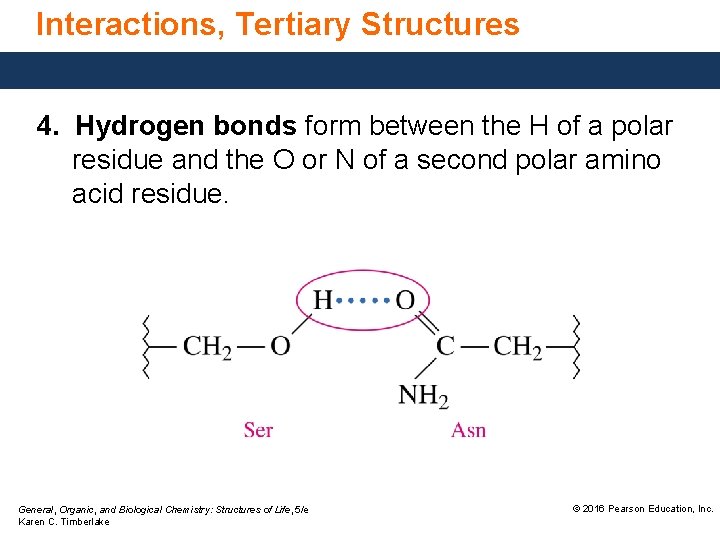
Interactions, Tertiary Structures 4. Hydrogen bonds form between the H of a polar residue and the O or N of a second polar amino acid residue. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

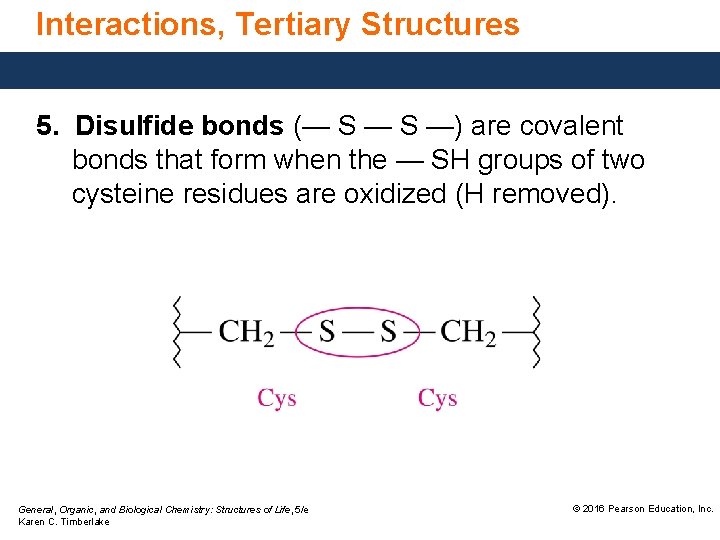
Interactions, Tertiary Structures 5. Disulfide bonds (— S —) are covalent bonds that form when the — SH groups of two cysteine residues are oxidized (H removed). General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Tertiary Structure Interactions between amino acid residues fold a polypeptide into a specific three-dimensional shape called its tertiary structure. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

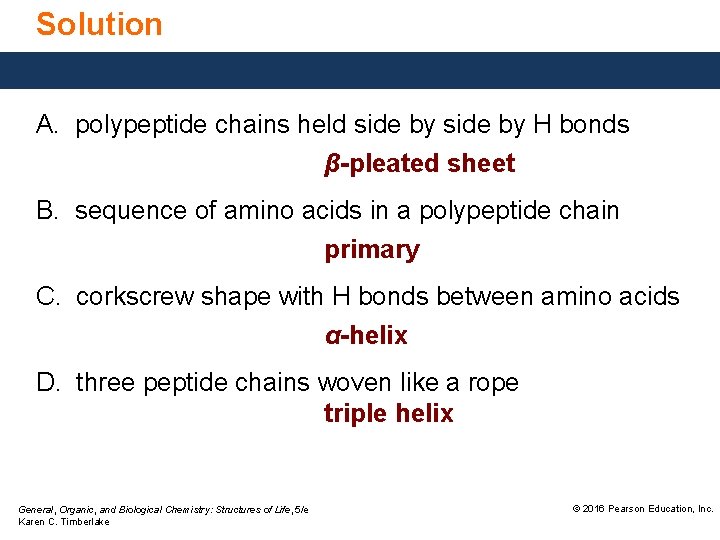
Study Check Indicate the type of protein structure. A. B. C. D. primary α-helix β-pleated sheet triple helix polypeptide chains held side by H bonds sequence of amino acids in a polypeptide chain corkscrew shape with H bonds between amino acids three peptide chains woven like a rope General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution A. polypeptide chains held side by H bonds β-pleated sheet B. sequence of amino acids in a polypeptide chain primary C. corkscrew shape with H bonds between amino acids α-helix D. three peptide chains woven like a rope triple helix General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Globular Proteins, Myoglobin Globular proteins • have compact, spherical shapes formed when sections of the polypeptide chain fold over on top of each other as a result of the interactions between amino acid residues. • carry out the work of the cells, such as synthesis, transport, and metabolism. Myoglobin, a globular protein that stores oxygen in skeletal muscle, contains 153 amino acids in a polypeptide chain, with about three-fourths of the chain in the α-helix secondary structure. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Fibrous Proteins, α- and β-Keratins Fibrous proteins consist of long, thin, fiber-like shapes, involved in the structure of cells and tissues. Two types of fibrous protein include • α-keratins, which make up hair, wool, skin, and nails and contain three α-helices linked by disulfide — S — linkages that coil together the peptide chain like a braid. • β-keratins that are found in the feathers of birds and scales of reptiles and contain large amounts of a β-pleated sheet structure. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

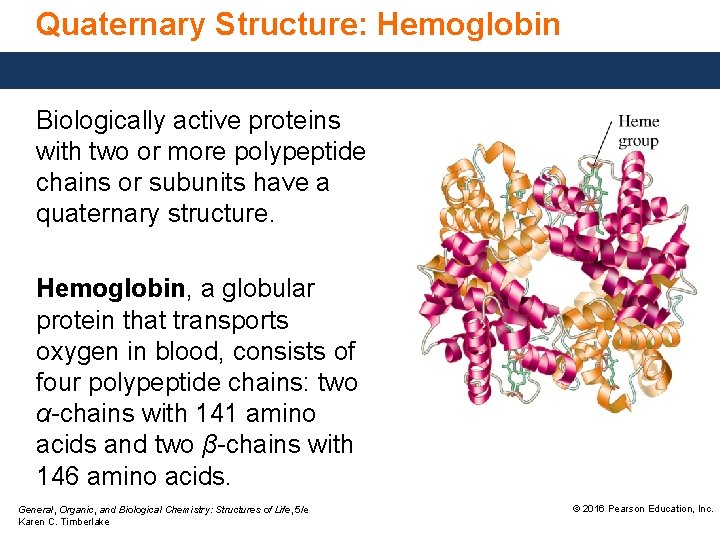
Quaternary Structure: Hemoglobin Biologically active proteins with two or more polypeptide chains or subunits have a quaternary structure. Hemoglobin, a globular protein that transports oxygen in blood, consists of four polypeptide chains: two α-chains with 141 amino acids and two β-chains with 146 amino acids. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Quaternary Structure: Hemoglobin In the quaternary structure of hemoglobin, • the subunits are held together by the same stabilizing interactions in tertiary structures. • each subunit of hemoglobin is a globular protein with an embedded heme group containing an iron center that can bind an oxygen molecule. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Hemoglobin and Myoglobin Hemoglobin and myoglobin • have similar biological functions. • carry oxygen in different parts of the body: hemoglobin carries oxygen in blood, and myoglobin carries oxygen in muscle. • have a different molar mass: myoglobin has a molar mass of 17 000, and hemoglobin has a molar mass of 67 000. • have similar tertiary structures. • carry different amounts of oxygen: myoglobin carries one oxygen molecule, and hemoglobin carries four oxygen molecules. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

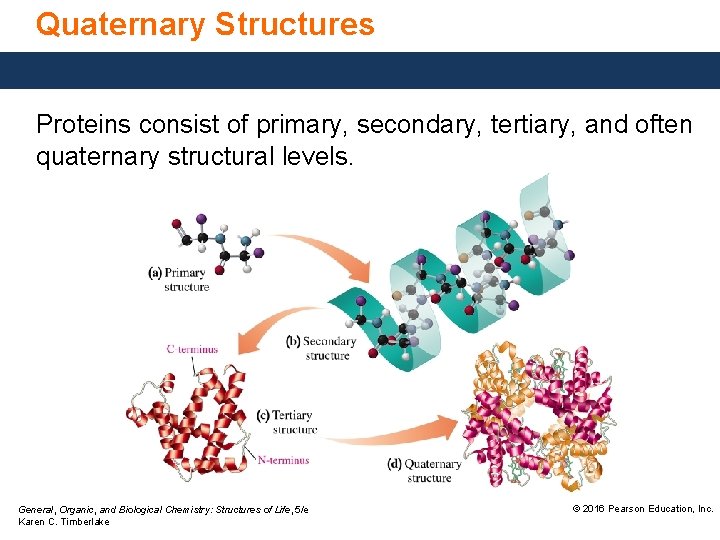
Quaternary Structures Proteins consist of primary, secondary, tertiary, and often quaternary structural levels. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

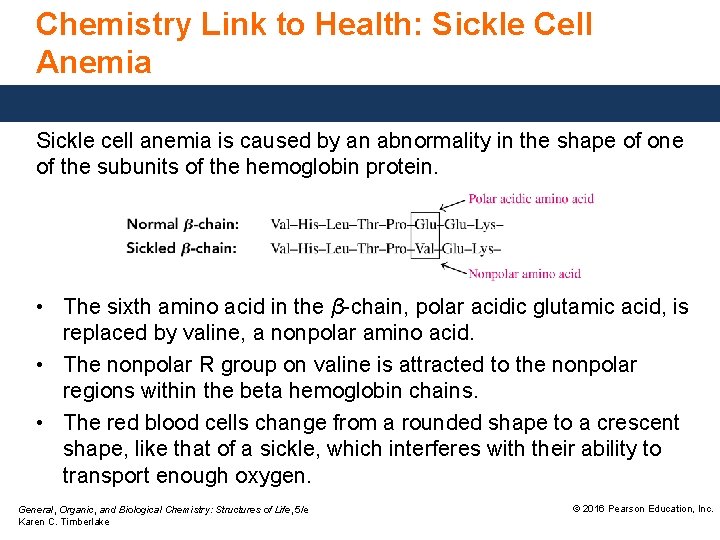
Chemistry Link to Health: Sickle Cell Anemia Sickle cell anemia is caused by an abnormality in the shape of one of the subunits of the hemoglobin protein. • The sixth amino acid in the β-chain, polar acidic glutamic acid, is replaced by valine, a nonpolar amino acid. • The nonpolar R group on valine is attracted to the nonpolar regions within the beta hemoglobin chains. • The red blood cells change from a rounded shape to a crescent shape, like that of a sickle, which interferes with their ability to transport enough oxygen. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

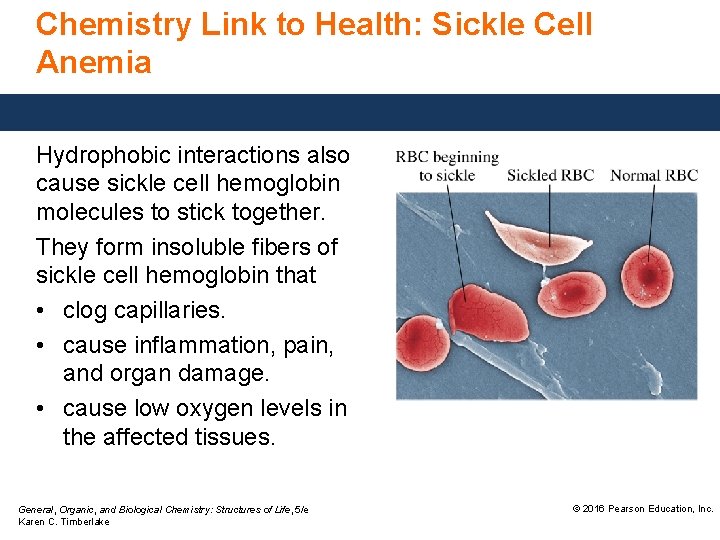
Chemistry Link to Health: Sickle Cell Anemia Hydrophobic interactions also cause sickle cell hemoglobin molecules to stick together. They form insoluble fibers of sickle cell hemoglobin that • clog capillaries. • cause inflammation, pain, and organ damage. • cause low oxygen levels in the affected tissues. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.