PODSTAWY CHEMII SUPRAMOLEKULARNEJ Z ELEMENTAMI NANO NIEKONWENCJONALNIE PODSTAWOWE





































![ROTAKSANY Figure 2. Cartoon representation of a [2]rotaxane. ROTAKSANY Figure 2. Cartoon representation of a [2]rotaxane.](https://slidetodoc.com/presentation_image_h2/ad3f1762e4f064ff2454fa70aee51a3a/image-71.jpg)

![KATENANDY Figure 1. Cartoon representation of a [2]catenane. KATENANDY Figure 1. Cartoon representation of a [2]catenane.](https://slidetodoc.com/presentation_image_h2/ad3f1762e4f064ff2454fa70aee51a3a/image-73.jpg)




- Slides: 81

PODSTAWY CHEMII SUPRAMOLEKULARNEJ Z ELEMENTAMI NANO – NIEKONWENCJONALNIE PODSTAWOWE POJĘCIA Marek Pietraszkiewicz, Instytut Chemii Fizycznej PAN, 01 -224 Warszawa, Kasprzaka 44/52, tel: 3433416 E-mail: pietrasz@ichf. edu. pl









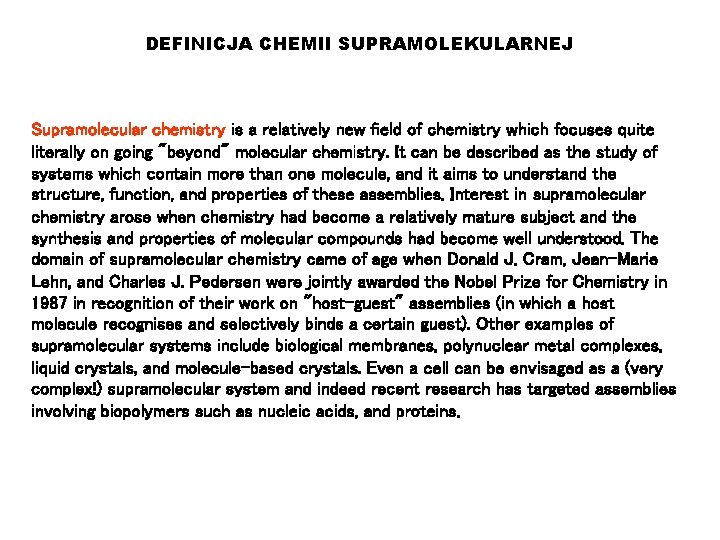
DEFINICJA CHEMII SUPRAMOLEKULARNEJ Supramolecular chemistry is a relatively new field of chemistry which focuses quite literally on going "beyond" molecular chemistry. It can be described as the study of systems which contain more than one molecule, and it aims to understand the structure, function, and properties of these assemblies. Interest in supramolecular chemistry arose when chemistry had become a relatively mature subject and the synthesis and properties of molecular compounds had become well understood. The domain of supramolecular chemistry came of age when Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen were jointly awarded the Nobel Prize for Chemistry in 1987 in recognition of their work on "host-guest" assemblies (in which a host molecule recognises and selectively binds a certain guest). Other examples of supramolecular systems include biological membranes, polynuclear metal complexes, liquid crystals, and molecule-based crystals. Even a cell can be envisaged as a (very complex!) supramolecular system and indeed recent research has targeted assemblies involving biopolymers such as nucleic acids, and proteins.

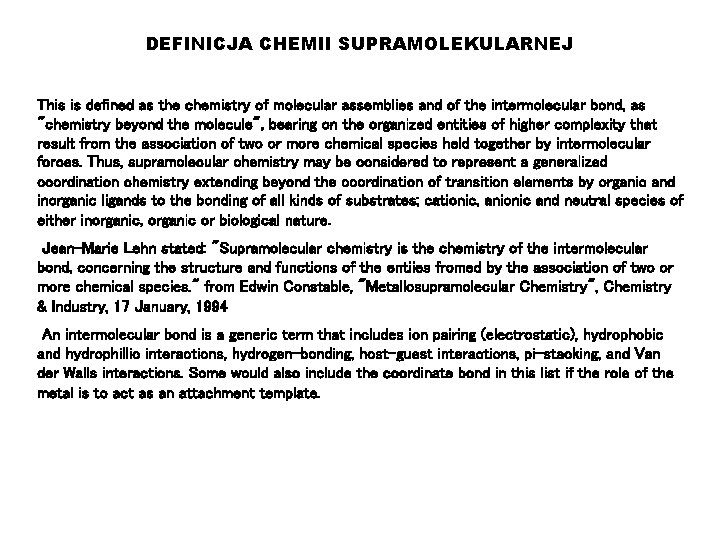
DEFINICJA CHEMII SUPRAMOLEKULARNEJ This is defined as the chemistry of molecular assemblies and of the intermolecular bond, as "chemistry beyond the molecule", bearing on the organized entities of higher complexity that result from the association of two or more chemical species held together by intermolecular forces. Thus, supramolecular chemistry may be considered to represent a generalized coordination chemistry extending beyond the coordination of transition elements by organic and inorganic ligands to the bonding of all kinds of substrates; cationic, anionic and neutral species of either inorganic, organic or biological nature. Jean-Marie Lehn stated: "Supramolecular chemistry is the chemistry of the intermolecular bond, concerning the structure and functions of the entiies fromed by the association of two or more chemical species. " from Edwin Constable, "Metallosupramolecular Chemistry", Chemistry & Industry, 17 January, 1994 An intermolecular bond is a generic term that includes ion pairing (electrostatic), hydrophobic and hydrophillic interactions, hydrogen-bonding, host-guest interactions, pi-stacking, and Van der Walls interactions. Some would also include the coordinate bond in this list if the role of the metal is to act as an attachment template.

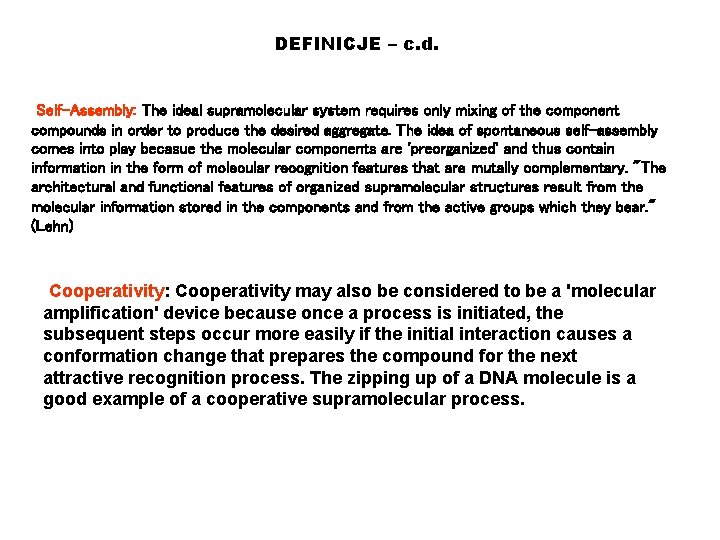
DEFINICJE – c. d. Self-Assembly: The ideal supramolecular system requires only mixing of the component compounds in order to produce the desired aggregate. The idea of spontaneous self-assembly comes into play becasue the molecular components are 'preorganized' and thus contain information in the form of molecular recognition features that are mutally complementary. "The architectural and functional features of organized supramolecular structures result from the molecular information stored in the components and from the active groups which they bear. " (Lehn) Cooperativity: Cooperativity may also be considered to be a 'molecular amplification' device because once a process is initiated, the subsequent steps occur more easily if the initial interaction causes a conformation change that prepares the compound for the next attractive recognition process. The zipping up of a DNA molecule is a good example of a cooperative supramolecular process.

DEFINICJE – c. d. Lattice Energy: The lattice energy, U, of an ionic solid is generally defined asthe energy change associated with the process of going from solid to gas: MX(s) --> M+(g) + X-(g) The lattice energy receives contributions from electrostatic, repulsive, dispersive, and the zero-point energy. (Seddon) Synthon: "Supermolecular synthons are structural units within supermolecules which can be formed and/or assembled by known or conceivable synthetic operations involving intermolecular interactions. " (Desiraju) The recognition and use of these spacial arrangements of intermolecular interactions follows the same lines as in conventional organic synthesis.

ELEKTROUJEMNOŚĆ The affinity for electrons. The atoms of the various elements differ in their affinity for electrons. This image distorts the conventional periodic table of the elements so that the greater the electronegativity of an atom, the higher its position in the table.

ELEKTROUJEMNOŚĆ

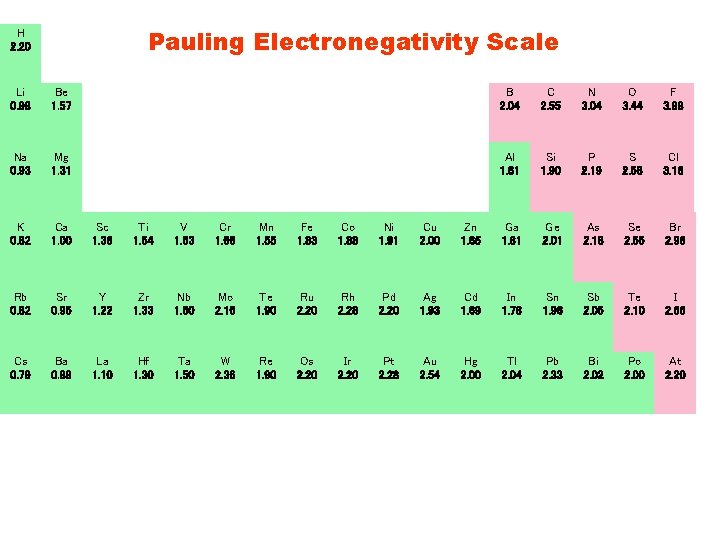
Pauling Electronegativity Scale H 2. 20 Li 0. 98 Be 1. 57 B 2. 04 C 2. 55 N 3. 04 O 3. 44 F 3. 98 Na 0. 93 Mg 1. 31 Al 1. 61 Si 1. 90 P 2. 19 S 2. 58 Cl 3. 16 K 0. 82 Ca 1. 00 Sc 1. 36 Ti 1. 54 V 1. 63 Cr 1. 66 Mn 1. 55 Fe 1. 83 Co 1. 88 Ni 1. 91 Cu 2. 00 Zn 1. 65 Ga 1. 81 Ge 2. 01 As 2. 18 Se 2. 55 Br 2. 96 Rb 0. 82 Sr 0. 95 Y 1. 22 Zr 1. 33 Nb 1. 60 Mo 2. 16 Te 1. 90 Ru 2. 20 Rh 2. 28 Pd 2. 20 Ag 1. 93 Cd 1. 69 In 1. 78 Sn 1. 96 Sb 2. 05 Te 2. 10 I 2. 66 Cs 0. 79 Ba 0. 89 La 1. 10 Hf 1. 30 Ta 1. 50 W 2. 36 Re 1. 90 Os 2. 20 Ir 2. 20 Pt 2. 28 Au 2. 54 Hg 2. 00 Tl 2. 04 Pb 2. 33 Bi 2. 02 Po 2. 00 At 2. 20

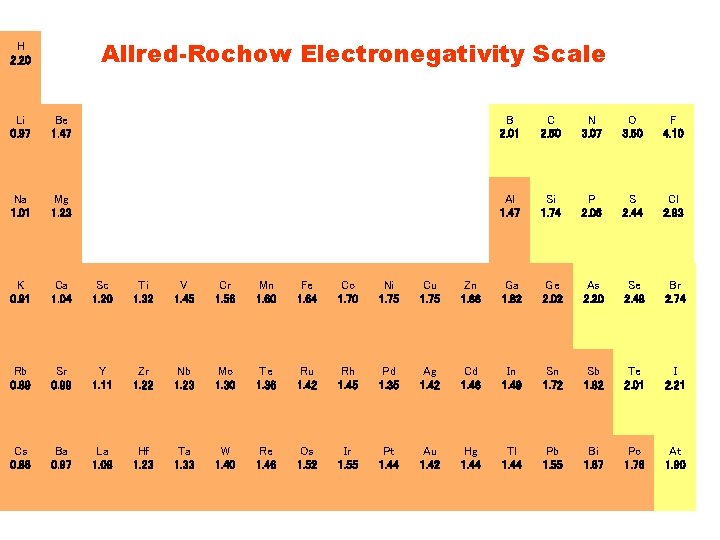
Allred-Rochow Electronegativity Scale H 2. 20 Li 0. 97 Be 1. 47 B 2. 01 C 2. 50 N 3. 07 O 3. 50 F 4. 10 Na 1. 01 Mg 1. 23 Al 1. 47 Si 1. 74 P 2. 06 S 2. 44 Cl 2. 83 K 0. 91 Ca 1. 04 Sc 1. 20 Ti 1. 32 V 1. 45 Cr 1. 56 Mn 1. 60 Fe 1. 64 Co 1. 70 Ni 1. 75 Cu 1. 75 Zn 1. 66 Ga 1. 82 Ge 2. 02 As 2. 20 Se 2. 48 Br 2. 74 Rb 0. 89 Sr 0. 99 Y 1. 11 Zr 1. 22 Nb 1. 23 Mo 1. 30 Te 1. 36 Ru 1. 42 Rh 1. 45 Pd 1. 35 Ag 1. 42 Cd 1. 46 In 1. 49 Sn 1. 72 Sb 1. 82 Te 2. 01 I 2. 21 Cs 0. 86 Ba 0. 97 La 1. 08 Hf 1. 23 Ta 1. 33 W 1. 40 Re 1. 46 Os 1. 52 Ir 1. 55 Pt 1. 44 Au 1. 42 Hg 1. 44 Tl 1. 44 Pb 1. 55 Bi 1. 67 Po 1. 76 At 1. 90

Although fluorine (F) is the most electronegative element, it is the electronegativity of runner-up oxygen (O) that is exploited by life. • The relative electronegativity of two interacting atoms also plays a major part in determining what kind of chemical bond forms between them.

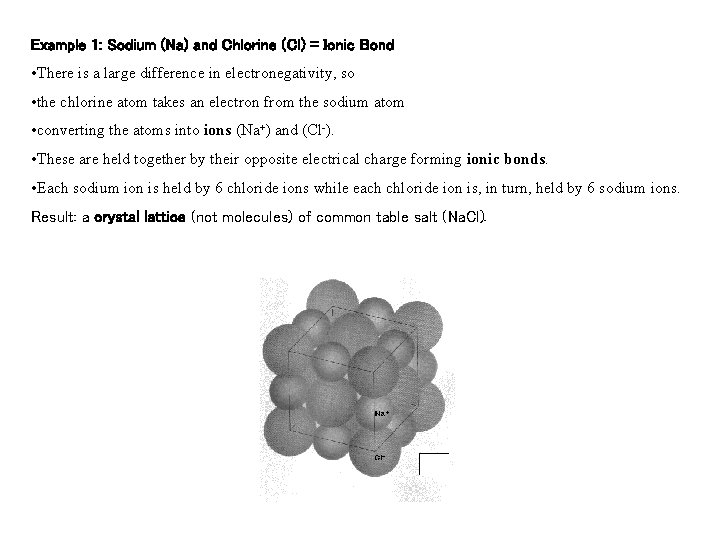
Example 1: Sodium (Na) and Chlorine (Cl) = Ionic Bond • There is a large difference in electronegativity, so • the chlorine atom takes an electron from the sodium atom • converting the atoms into ions (Na+) and (Cl-). • These are held together by their opposite electrical charge forming ionic bonds. • Each sodium ion is held by 6 chloride ions while each chloride ion is, in turn, held by 6 sodium ions. Result: a crystal lattice (not molecules) of common table salt (Na. Cl).

Example 2: Carbon (C) and Oxygen (O) = Covalent Bond • there is only a small difference in electronegativity, so • the two atoms share the electrons • Result: a covalent bond (depicted as C: H or C-H) • atoms held together by the mutual affinity for their shared electrons • an array of atoms held together by covalent bonds forms a true molecule. Example 3: Hydrogen (H) and Oxygen (O) = Polar Covalent Bond • moderate difference in electronegativity, so • oxygen atom pulls the electron of the hydrogen atom closer to itself • Result: a polar covalent bond • Oxygen does this with 2 hydrogen atoms to form a molecule of water

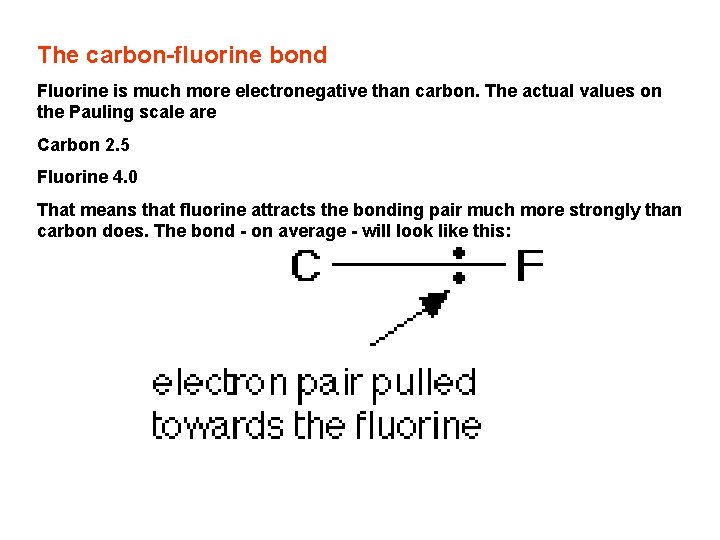
The carbon-fluorine bond Fluorine is much more electronegative than carbon. The actual values on the Pauling scale are Carbon 2. 5 Fluorine 4. 0 That means that fluorine attracts the bonding pair much more strongly than carbon does. The bond - on average - will look like this:

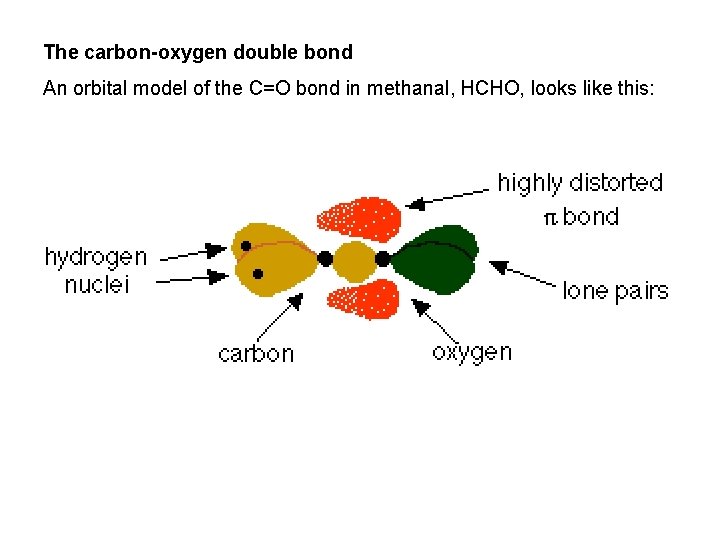
The carbon-oxygen double bond An orbital model of the C=O bond in methanal, HCHO, looks like this:

POLARYZOWALNOŚĆ Ability of an ion to distort the electron cloud of another. Large amount of polarization - electrons shared between elements - covalent bonding. A small positive ion favours covalency very high charge/volume ratio highly polarizing attracts electron density of negative ion e. g. , Li. H more nearly covalent than Na. H

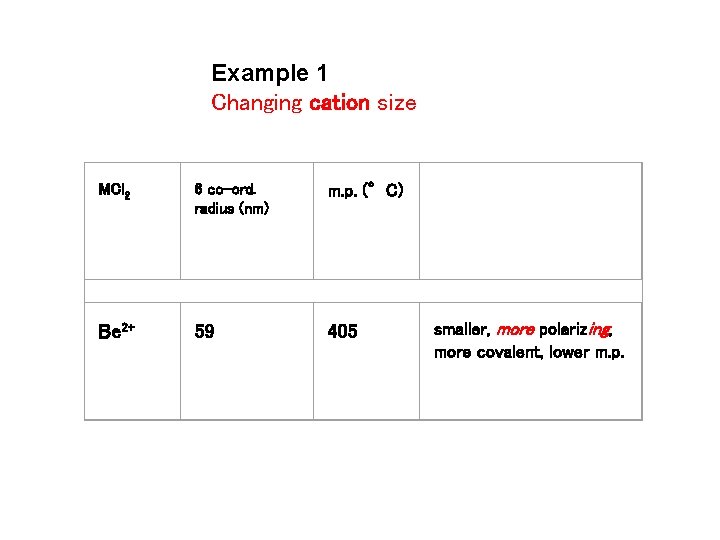
Example 1 Changing cation size MCl 2 6 co-ord. radius (nm) m. p. (°C) Be 2+ 59 405 smaller, more polarizing, more covalent, lower m. p.

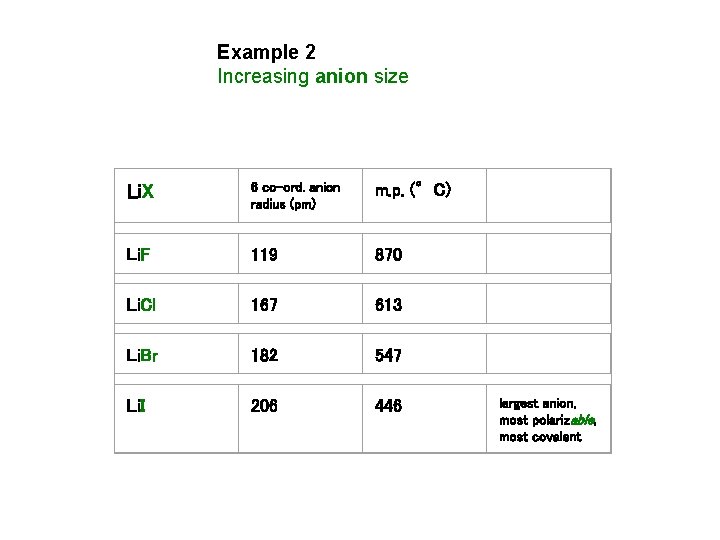
Example 2 Increasing anion size Li. X 6 co-ord. anion radius (pm) m. p. (°C) Li. F 119 870 Li. Cl 167 613 Li. Br 182 547 Li. I 206 446 largest anion, most polarizable, most covalent

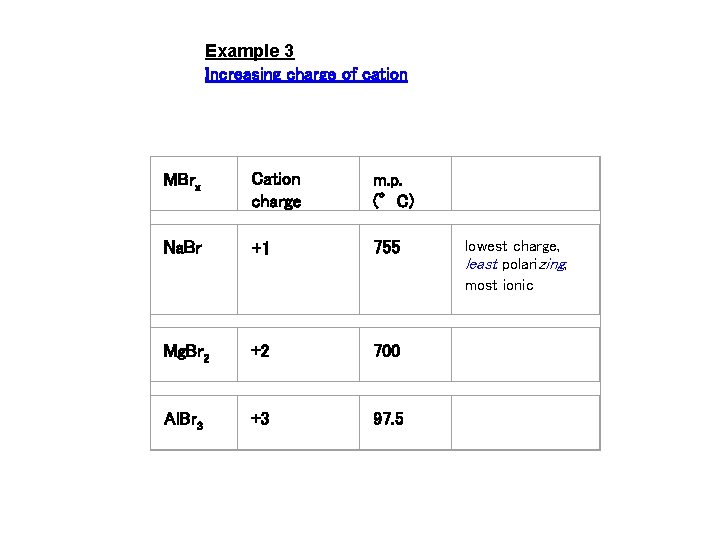
Example 3 Increasing charge of cation MBrx Cation charge m. p. (°C) Na. Br +1 755 Mg. Br 2 +2 700 Al. Br 3 +3 97. 5 lowest charge, least polarizing, most ionic


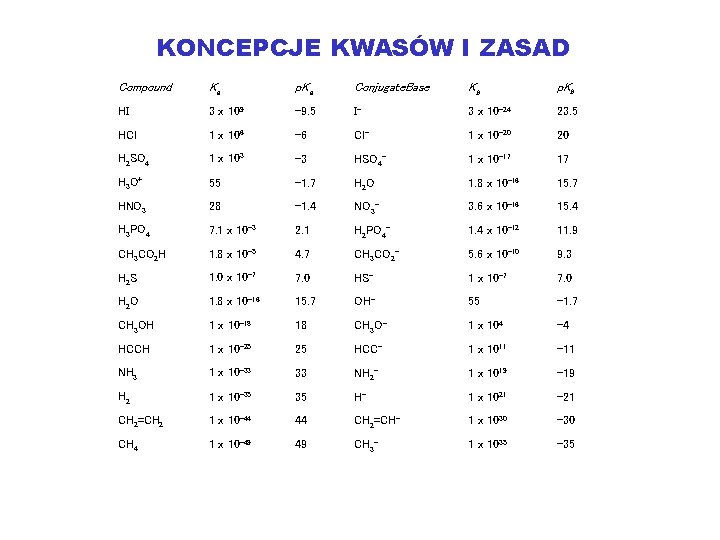
KONCEPCJE KWASÓW I ZASAD Compound Ka p. Ka Conjugate. Base Kb p. Kb HI 3 x 109 -9. 5 I- 3 x 10 -24 23. 5 HCl 1 x 106 -6 Cl- 1 x 10 -20 20 H 2 SO 4 1 x 103 -3 HSO 4 - 1 x 10 -17 17 H 3 O+ 55 -1. 7 H 2 O 1. 8 x 10 -16 15. 7 HNO 3 28 -1. 4 NO 3 - 3. 6 x 10 -16 15. 4 H 3 PO 4 7. 1 x 10 -3 2. 1 H 2 PO 4 - 1. 4 x 10 -12 11. 9 CH 3 CO 2 H 1. 8 x 10 -5 4. 7 CH 3 CO 2 - 5. 6 x 10 -10 9. 3 H 2 S 1. 0 x 10 -7 7. 0 HS- 1 x 10 -7 7. 0 H 2 O 1. 8 x 10 -16 15. 7 OH- 55 -1. 7 CH 3 OH 1 x 10 -18 18 CH 3 O- 1 x 104 -4 HCCH 1 x 10 -25 25 HCC- 1 x 1011 -11 NH 3 1 x 10 -33 33 NH 2 - 1 x 1019 -19 H 2 1 x 10 -35 35 H- 1 x 1021 -21 CH 2=CH 2 1 x 10 -44 44 CH 2=CH- 1 x 1030 -30 CH 4 1 x 10 -49 49 CH 3 - 1 x 1035 -35

Koncepcja Lewisa kwasów i zasad In the Lewis theory of acid-base reactions, bases donate pairs of electrons and acids accept pairs of electrons. A Lewis acid is therefore any substance, such as the H+ ion, that can accept a pair of nonbonding electrons. In other words, a Lewis acid is an electron-pair acceptor. A Lewis base is any substance, such as the OH- ion, that can donate a pair of nonbonding electrons. A Lewis base is therefore an electron-pair donor.

WIĄZANIA CHEMICZNE Covalent Bonding Ionic substances: • usually brittle • high melting point • organized into an ordered lattice of atoms, which can be cleaved along a smooth line the electrostatic forces organize the ions of ionic substances into a rigid, organized three-dimensional arrangement The vast majority of chemical substances are not ionic in nature • gases and liquids, in addition to solids • low melting temperatures Bond energy is always a positive value - it takes energy to break a covalent bond (conversely energy is released during bond formation)

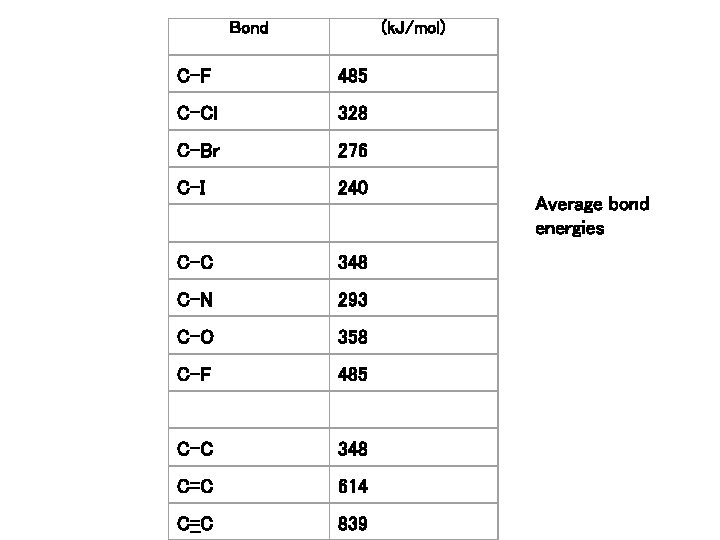
Bond (k. J/mol) C-F 485 C-Cl 328 C-Br 276 C-I 240 C-C 348 C-N 293 C-O 358 C-F 485 C-C 348 C=C 614 C=C 839 Average bond energies

RODZAJE ODDZIAŁYWAŃ MIĘDZYCZĄSTECZKOWYCH • WIĄZANIE WODOROWE • WIĄZANIE KOORDYNACYJNE • WIĄZANIE JONOWE • ODDZIAŁYWANIA VAN DER WAALSA • ODDZIAŁYWANIA PI-STAKINGOWE • SIŁY POLIDYSPERSYJNE

RODZAJE ODDZIAŁYWAŃ MIĘDZYCZĄSTECZKOWYCH dipole-dipole interaction. dipole-dipole force. Electrostatic attraction between oppositely charged poles of two or more dipoles electric dipole moment. (µ) dipole moment. A measure of the degree of polarity of a polar molecule*. Dipole moment is a vector with magnitude equal to charge separation times the distance between the centers of positive and negative charges. Chemists point the vector from the positive to the negative pole; physicists point it the opposite way. Dipole moments are often expressed in units called Debyes hydrogen bonding. An especially strong dipole-dipole* force between molecules X-H. . . Y, where X and Y are small electronegative atoms (usually F, N, or O) and. . . denotes the hydrogen bond. Hydrogen bonds are responsible for the unique properties of water and they loosely pin biological polymers like proteins and DNA into their characteristic shapes.

RODZAJE ODDZIAŁYWAŃ MIĘDZYCZĄSTECZKOWYCH intermolecular force. An attraction or repulsion between molecules. Intermolecular forces are much weaker than chemical bonds. Hydrogen bonds, dipole-dipole interactions, and London forces are examples of intermolecular forces. London force. dispersion force. An intermolecular attractive force that arises from a cooperative oscillation of electron clouds on a collection of molecules at close range. van der Waals force. A force acting between nonbonded atoms or molecules. Includes dipole-dipole, dipole-induced dipole, and London forces.

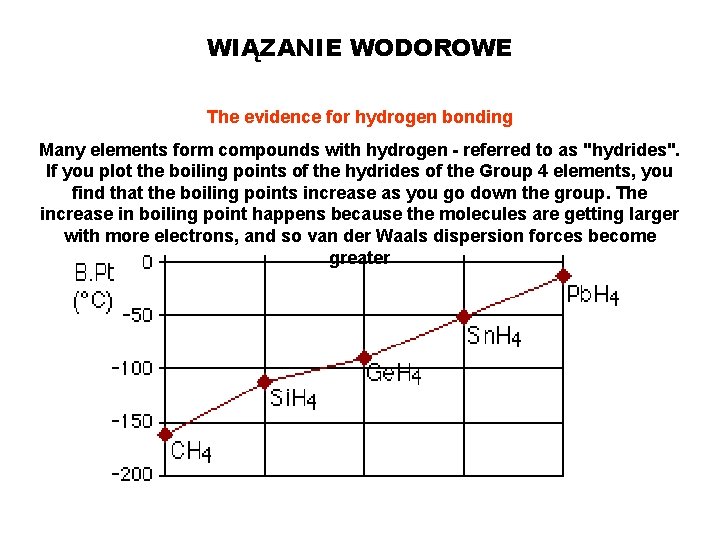
WIĄZANIE WODOROWE The evidence for hydrogen bonding Many elements form compounds with hydrogen - referred to as "hydrides". If you plot the boiling points of the hydrides of the Group 4 elements, you find that the boiling points increase as you go down the group. The increase in boiling point happens because the molecules are getting larger with more electrons, and so van der Waals dispersion forces become greater

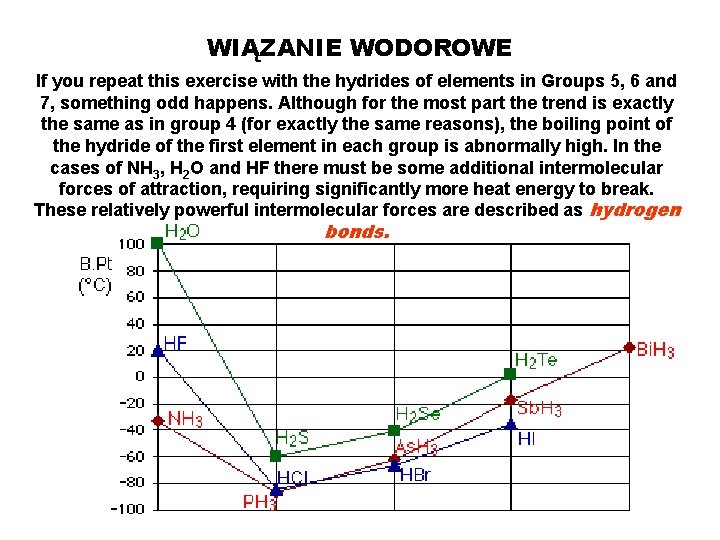
WIĄZANIE WODOROWE If you repeat this exercise with the hydrides of elements in Groups 5, 6 and 7, something odd happens. Although for the most part the trend is exactly the same as in group 4 (for exactly the same reasons), the boiling point of the hydride of the first element in each group is abnormally high. In the cases of NH 3, H 2 O and HF there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. These relatively powerful intermolecular forces are described as hydrogen bonds.

WIĄZANIE WODOROWE GRUPY ATOMÓW ZAANGAŻOWANE W WIĄZANIA WODOROWE: N-H, O-H, F-H, C-H CH-kwasy: CH 3 NO 2, CH 2(CO 2 Et)2

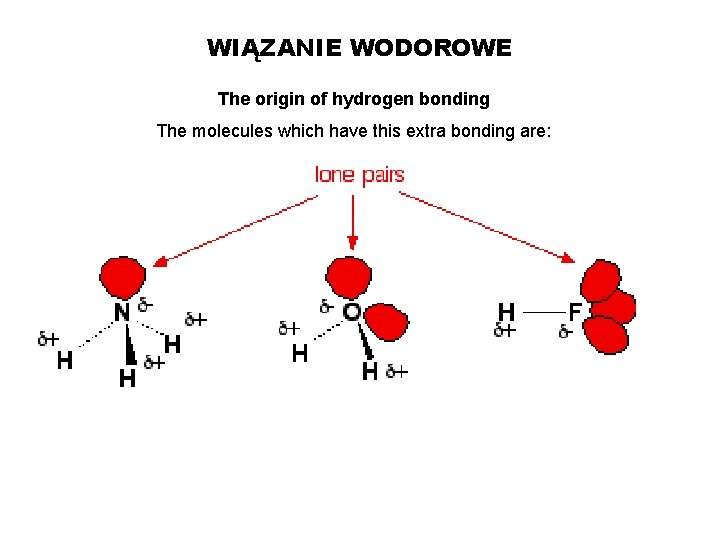
WIĄZANIE WODOROWE The origin of hydrogen bonding The molecules which have this extra bonding are:

WIĄZANIE HYDROFOBOWE The Hydrophobic bond ΔG= ΔH−TΔS Equilibrium when ΔG = 0. G is Gibbs’free energy, the enthalpy is H = E + PV, Tis absolute temperature and S is the entropy. The process goes spontaneously from left to right when ΔG< 0. Find the position of thermodynamic equilibrium for a well -known example of insolubility: CH 4 in benzene→CH 4 in H 2 O The experimental data show (all units in calories per mol): ΔG = ΔH −T ΔS + 2600 = − 2800 − 298(− 18) +2600 = − 2800 + 5400 Conclusion: Insolubility of paraffin in water due to entropy loss, not to enthalpy change!

ODDZIAŁYWANIA VAN DER WAALSA Dipole-Dipole forces are one of van der Waals' three forces. Dipole forces occur in polar molecules, that is, molecules that have an unequal sharing of electrons. For example, HCl comprised of the atom Hydrogen and Chlorine is polar. The Chlorine atom has an extra electron, which came from the hydrogen atom. Because of this, the chlorine part of the molecule is negatively charged, and the hydrogen side of the molecule is positively charged. ie. H - Cl + - So in a solution where there are thousands of these molecules around that are slightly charged on each side, the molecules naturally orient themselves the accommodate the charge. The positive part of one molecule will move until it is next to the negative part of a neighboring molecule. These forces between molecules tend to make them 'stick' together.

ODDZIAŁYWANIA VAN DER WAALSA Dispersion forces are another of van der Waals' three forces. They exist between nonpolar molecules. For example, chlorine gas is made up of two chlorine atoms. In this bond, the electrons are equally shared and are not dominant on one side of the molecule as is the case in HCl. The atom looks like this Cl - Cl

ODDZIAŁYWANIA VAN DER WAALSA van der Waals forces: dispersion forces Dispersion forces (one of the two types of van der Waals force we are dealing with on this page) are also known as "London forces" (named after Fritz London who first suggested how they might arise). The origin of van der Waals dispersion forces Temporary fluctuating dipoles Attractions are electrical in nature. In a symmetrical molecule like hydrogen, however, there doesn't seem to be any electrical distortion to produce positive or negative parts. But that's only true on average.

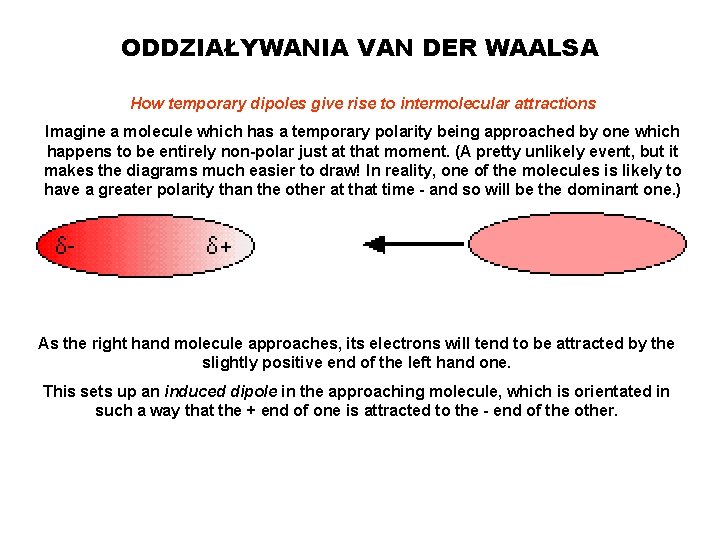
ODDZIAŁYWANIA VAN DER WAALSA How temporary dipoles give rise to intermolecular attractions Imagine a molecule which has a temporary polarity being approached by one which happens to be entirely non-polar just at that moment. (A pretty unlikely event, but it makes the diagrams much easier to draw! In reality, one of the molecules is likely to have a greater polarity than the other at that time - and so will be the dominant one. ) As the right hand molecule approaches, its electrons will tend to be attracted by the slightly positive end of the left hand one. This sets up an induced dipole in the approaching molecule, which is orientated in such a way that the + end of one is attracted to the - end of the other.

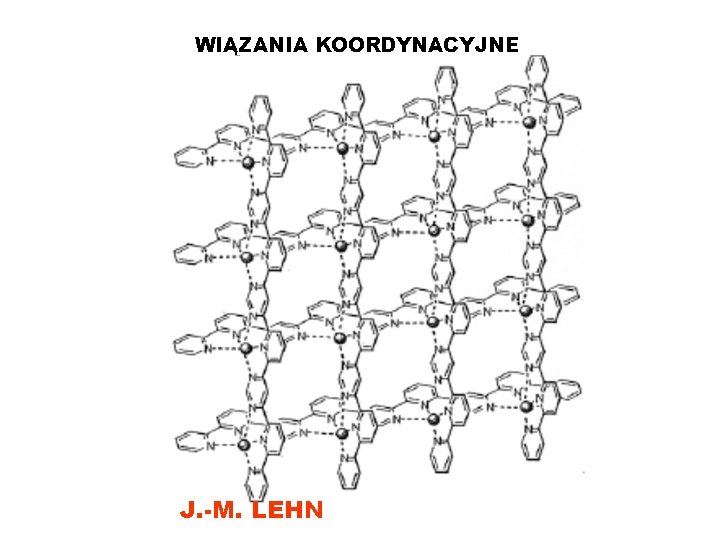
WIĄZANIA KOORDYNACYJNE J. -M. LEHN

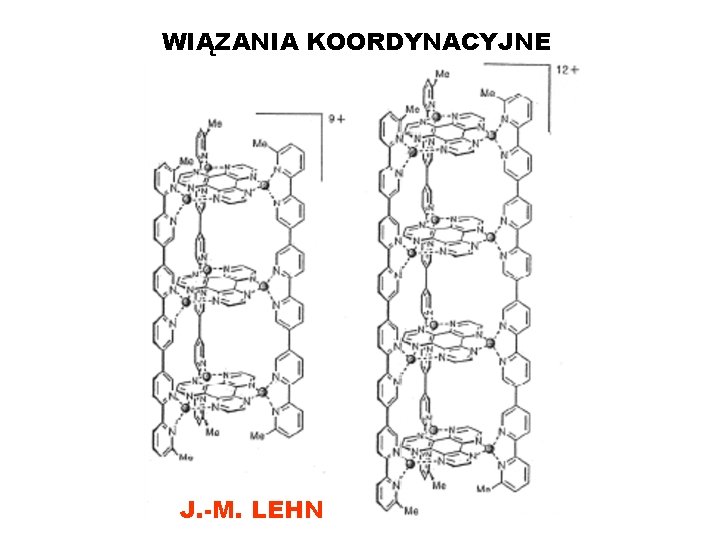
WIĄZANIA KOORDYNACYJNE J. -M. LEHN

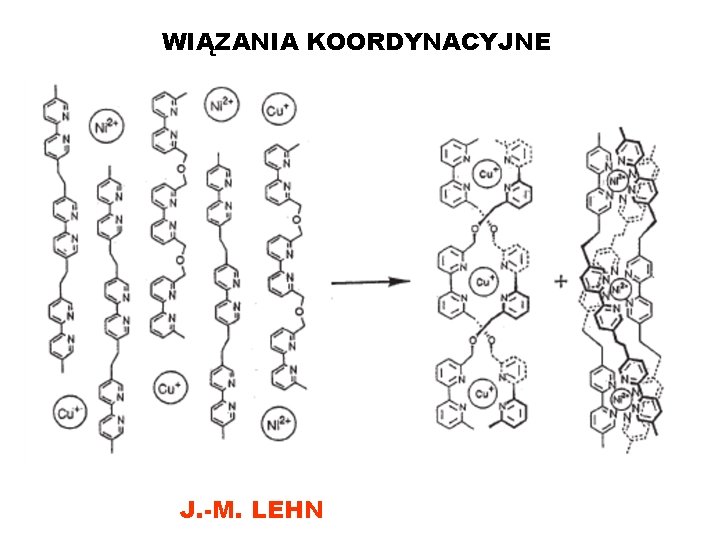
WIĄZANIA KOORDYNACYJNE J. -M. LEHN

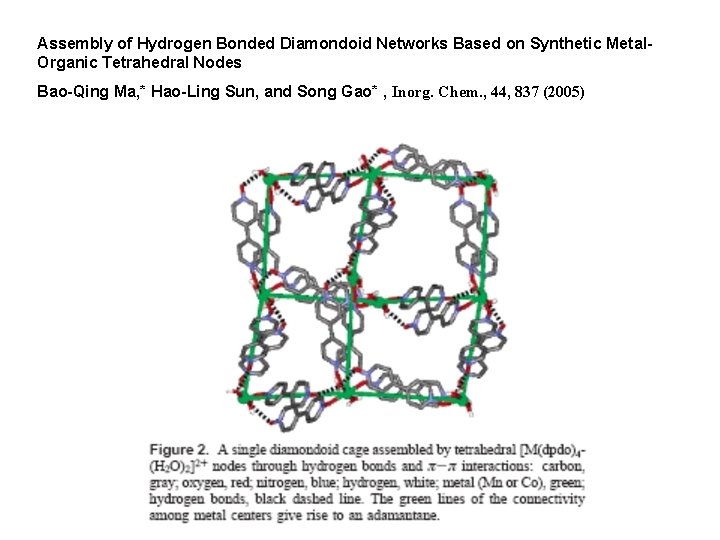
Assembly of Hydrogen Bonded Diamondoid Networks Based on Synthetic Metal. Organic Tetrahedral Nodes Bao-Qing Ma, * Hao-Ling Sun, and Song Gao* , Inorg. Chem. , 44, 837 (2005)

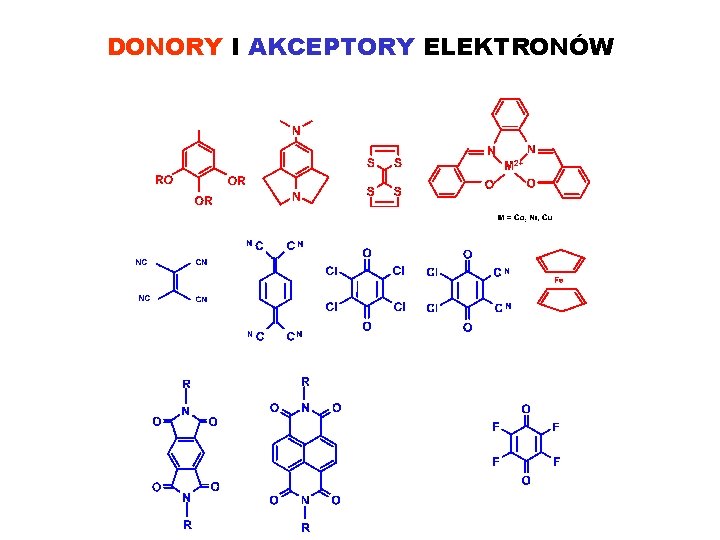
DONORY I AKCEPTORY ELEKTRONÓW

ODDZIAŁYWANIA PI-STAKINGOWE

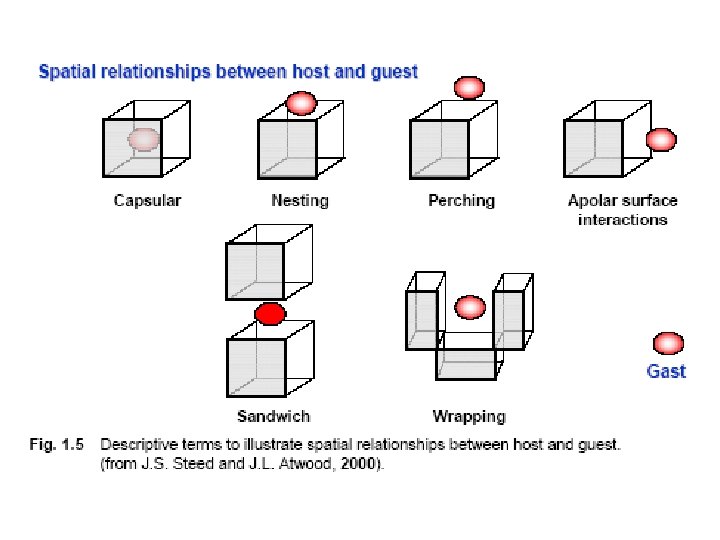
KONCEPCJA: RECEPTOR MOLEKULARNY – SUBSTRAT RECEPTOR: CZĄSTECZKA ZDOLNA DO „WCHŁONIĘCIA” MNIEJSZEJ CZĄSTECZKI DZIEKI ODDZIAŁYWANIOM NIEKOWALENCYJNYM Projektowanie, synteza, architektura molekularna zdolna do wykonywania określonych wcześniej funkcji SUBSTRAT: MAŁA CZĄSTECZKA, KOMPLEMENTARNA ROZMIAREM Z LUKĄ RECEPTORA Substrat: jony nieorganiczne i organiczne, cząsteczki obojętne, kompleksy koordynacyjne, związki metaloorganiczne

KONCEPCJA: RECEPTOR MOLEKULARNY – SUBSTRAT GRUPY FUNKCYJNE: ELEKTRONODONOROWE: OR, OAr, Cl, Br, J, NR, S, P ELEKTROAKCEPTOROWE: F, NO 2, CO 2 H, SO 3 H









PRZEGLĄD RECEPTORÓW MOLEKULARNYCH • RECEPTORY ACYKLICZNE • ETERY KORONOWE • PODANDY • MAKROCYKLICZNE POLIAMINY • KRYPTANDY • CYKLOFANY • SFERANDY • TORANDY • CYKLODEKSTRYNY • . ROTAKSANY • KATENANDY • WĘZŁY MOLEKULARNE • KALIKSARENY • HELIKATY

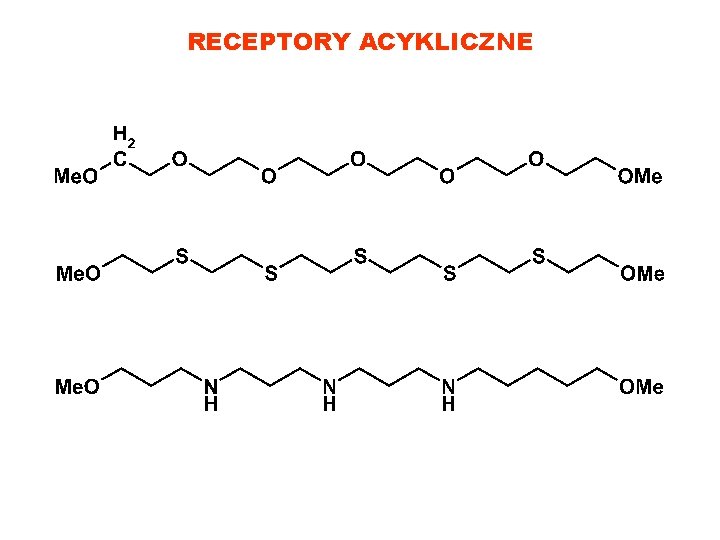
RECEPTORY ACYKLICZNE

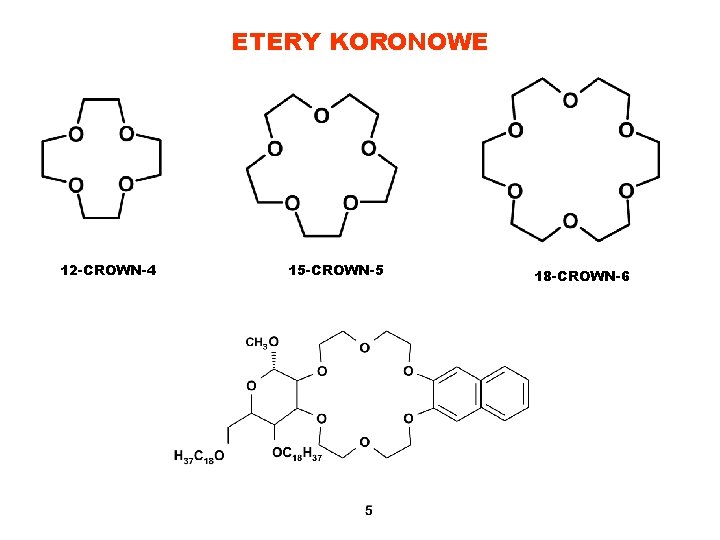
ETERY KORONOWE 12 -CROWN-4 15 -CROWN-5 18 -CROWN-6

PODANDY

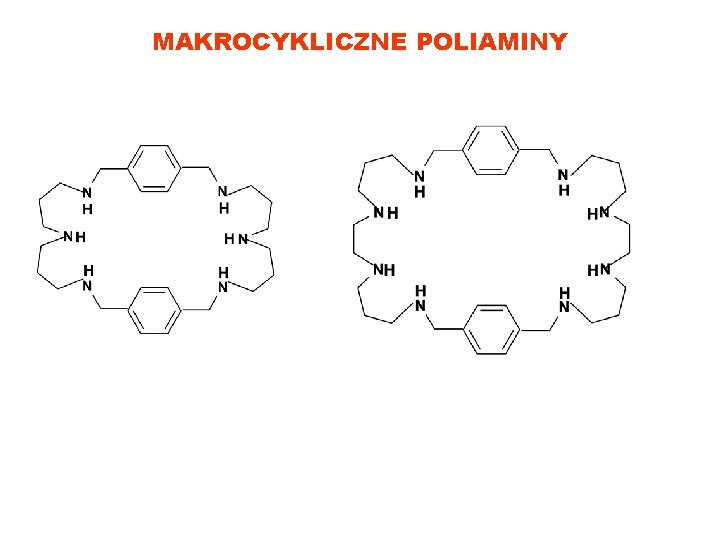
MAKROCYKLICZNE POLIAMINY

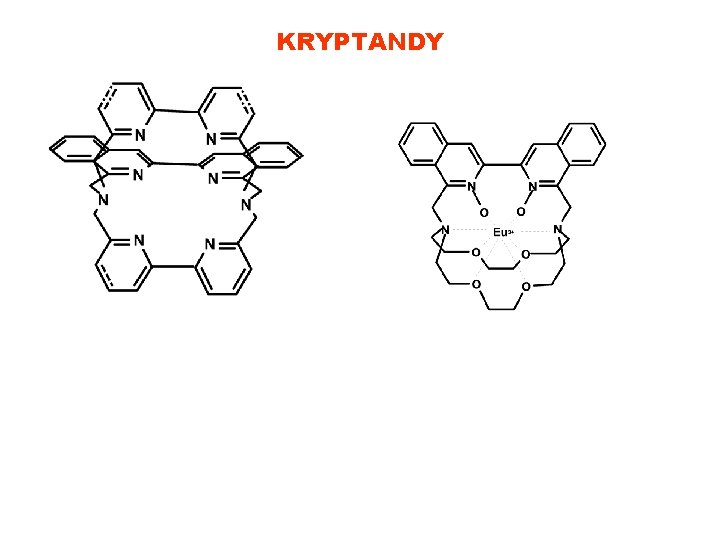
KRYPTANDY

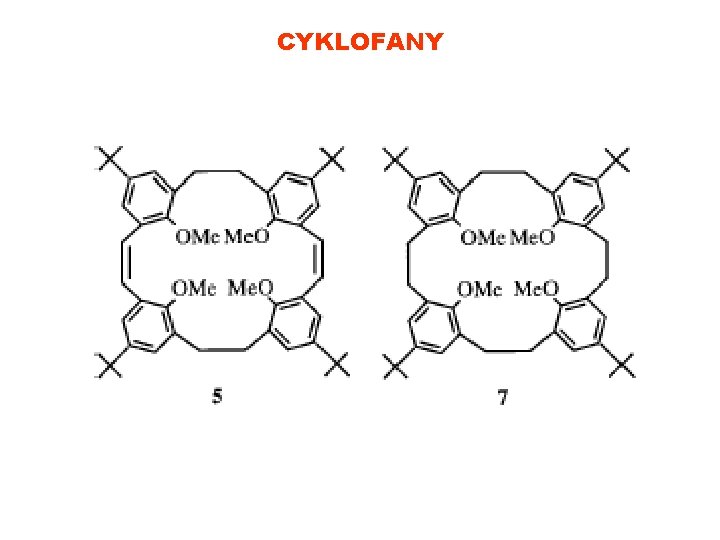
CYKLOFANY

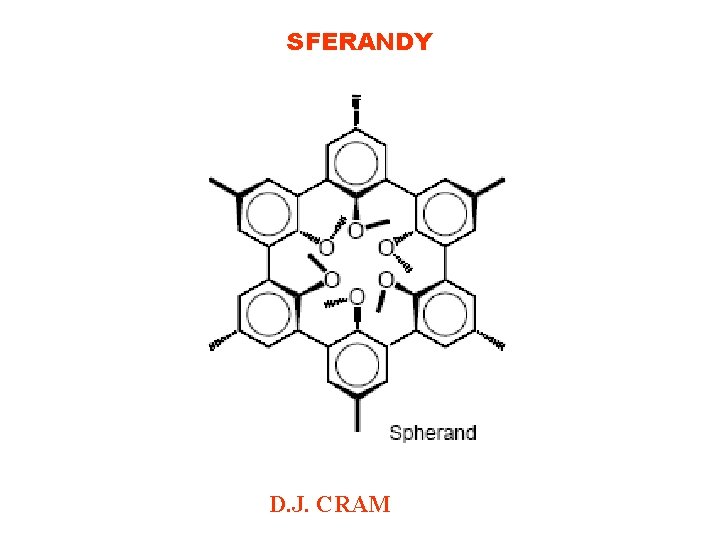
SFERANDY D. J. CRAM

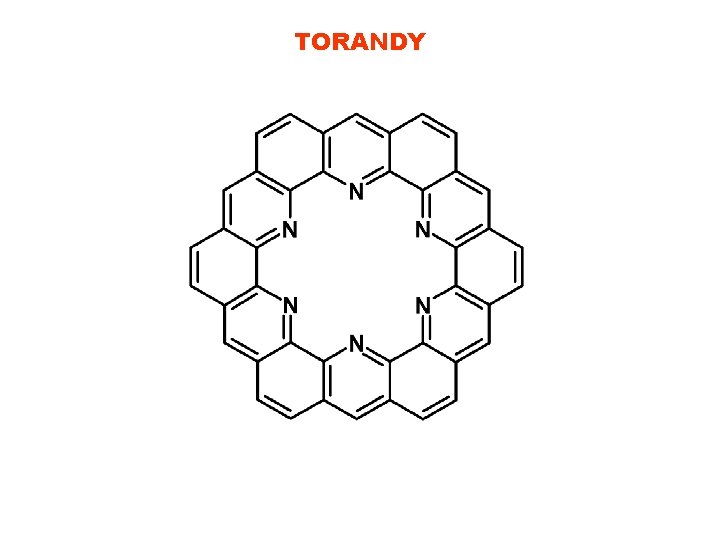
TORANDY

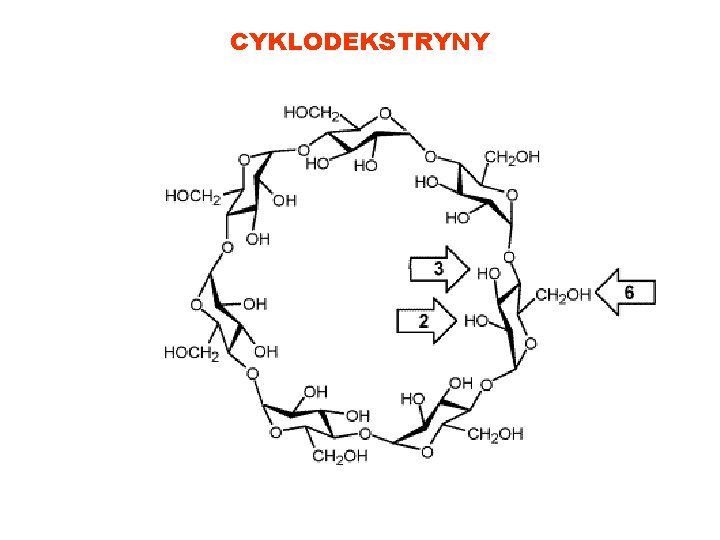
CYKLODEKSTRYNY What are cyclodextrins?

![ROTAKSANY Figure 2 Cartoon representation of a 2rotaxane ROTAKSANY Figure 2. Cartoon representation of a [2]rotaxane.](https://slidetodoc.com/presentation_image_h2/ad3f1762e4f064ff2454fa70aee51a3a/image-71.jpg)
ROTAKSANY Figure 2. Cartoon representation of a [2]rotaxane.

![KATENANDY Figure 1 Cartoon representation of a 2catenane KATENANDY Figure 1. Cartoon representation of a [2]catenane.](https://slidetodoc.com/presentation_image_h2/ad3f1762e4f064ff2454fa70aee51a3a/image-73.jpg)
KATENANDY Figure 1. Cartoon representation of a [2]catenane.


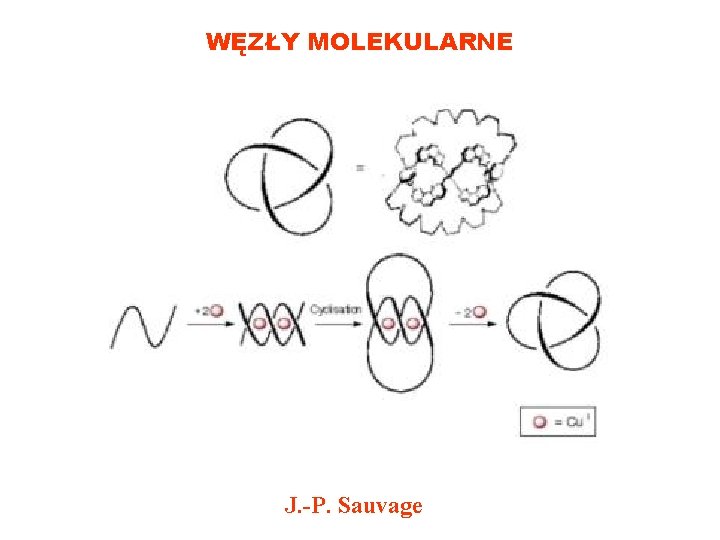
WĘZŁY MOLEKULARNE J. -P. Sauvage

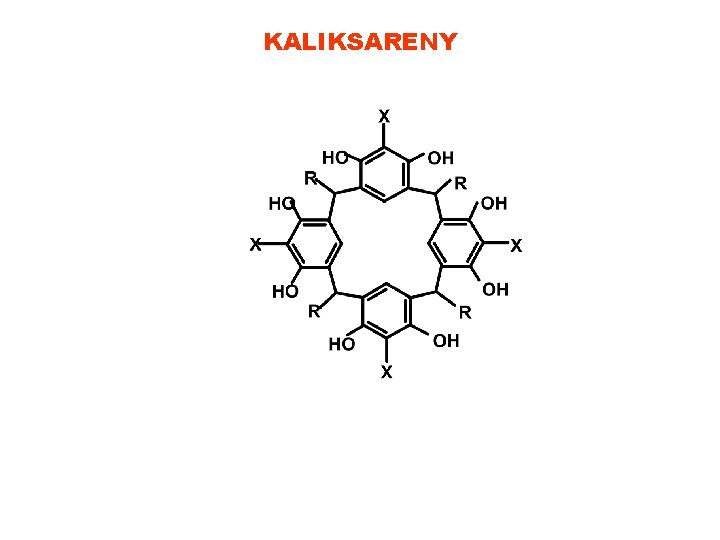
KALIKSARENY

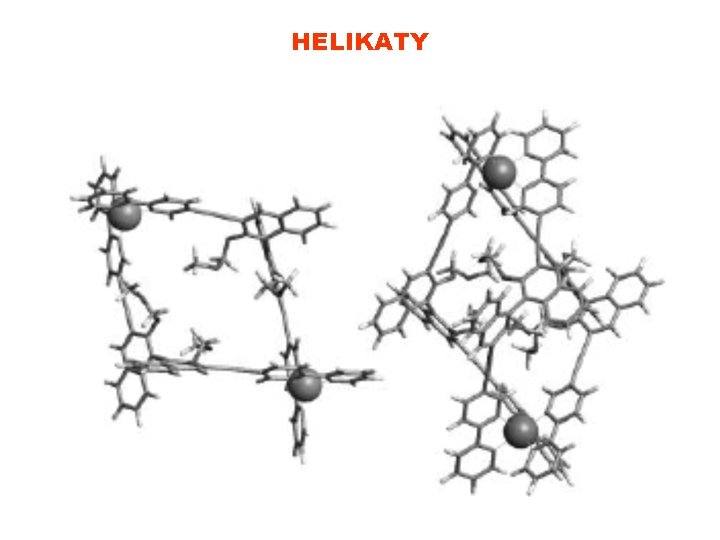
HELIKATY

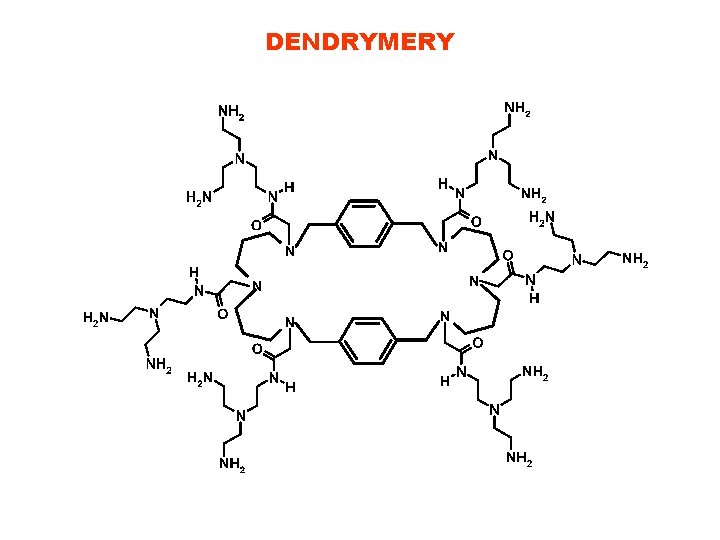
DENDRYMERY

FUNKCJE RECEPTORÓW MOLEKULARNYCH • TRANSPORT • KATALIZA • ROZPOZNANIE • STABILIZACJA RZADKICH STANÓW REDOX • TRANSFORMACJA SUBSTRATU • REAKCJA FOTOCHEMICZNA • KONWERSJA ENERGII ŚWIETLNEJ • ALLOSTERIA

INTERNETOWE PORTALE SPECJALISTYCZNE – POKAZ MOŻLIWOŚCI http: //nanotechweb. org http: //www. scirus. com http: //google. scholar. com http: //nanotechnologie. pagina. nl/

ĆWICZENIA
 Radiochemia
Radiochemia Wkład polaków w rozwój chemii
Wkład polaków w rozwój chemii Kolory w chemii nieorganicznej
Kolory w chemii nieorganicznej Budowa zestawu komputerowego
Budowa zestawu komputerowego Zastosowanie sztućców klasycznych
Zastosowanie sztućców klasycznych Język romski podstawowe zwroty
Język romski podstawowe zwroty Podstawowe elementy zestawu komputerowego
Podstawowe elementy zestawu komputerowego Podstawowe figury geometryczne
Podstawowe figury geometryczne Respektuj zasady grupy
Respektuj zasady grupy Podstawowe gałęzie transportu
Podstawowe gałęzie transportu Figura wklęsła
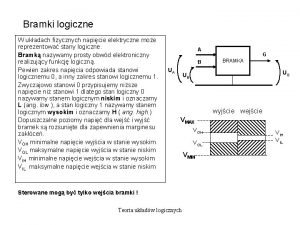
Figura wklęsła Stany logiczne
Stany logiczne Relacyjne bazy danych access
Relacyjne bazy danych access Interpersonalny model komunikacji morgana
Interpersonalny model komunikacji morgana Alu informatika
Alu informatika Podstawowe instrukcje
Podstawowe instrukcje Color 051909
Color 051909 Manewry na drodze prezentacja
Manewry na drodze prezentacja ściąganie plików
ściąganie plików Najstarsze przekłady biblii
Najstarsze przekłady biblii Dopisz wyrazy podstawowe i pochodne według wzoru
Dopisz wyrazy podstawowe i pochodne według wzoru Substancje wzorcowe w alkacymetrii
Substancje wzorcowe w alkacymetrii Transformacja encji
Transformacja encji Nakrycie proste
Nakrycie proste Fazy klasycznego cyklu koniunkturalnego
Fazy klasycznego cyklu koniunkturalnego Jednostki fizyczne
Jednostki fizyczne Modele barw stosowane w grafice komputerowej
Modele barw stosowane w grafice komputerowej Platforma wint odpowiedzi ratownictwo techniczne
Platforma wint odpowiedzi ratownictwo techniczne Colormap matlab
Colormap matlab Typy jednostek mieszkalnych
Typy jednostek mieszkalnych Mała jedynka trygonometryczna
Mała jedynka trygonometryczna Kroki fitness choreografia
Kroki fitness choreografia Barbara tomkowiak
Barbara tomkowiak Menisk wklęsły i wypukły
Menisk wklęsły i wypukły Przykladowy pesel
Przykladowy pesel Spalanie bezpłomieniowe, żarzenie
Spalanie bezpłomieniowe, żarzenie Majuskuła czcionka
Majuskuła czcionka Bpmn xor
Bpmn xor Teoretyczne podstawy informatyki
Teoretyczne podstawy informatyki Savoir-vivre, czyli zasady dobrego wychowania
Savoir-vivre, czyli zasady dobrego wychowania Program do tworzenia stron html
Program do tworzenia stron html Studnia potencjału
Studnia potencjału Reforma podstawy programowej
Reforma podstawy programowej Damian urbańczyk wikipedia
Damian urbańczyk wikipedia Html podstawy
Html podstawy Najnowsza podstawa programowa z religii
Najnowsza podstawa programowa z religii Miernik elektrodynamiczny
Miernik elektrodynamiczny Warunki realizacji podstawy programowej
Warunki realizacji podstawy programowej Vhdl automat
Vhdl automat Podstawy sztucznej inteligencji
Podstawy sztucznej inteligencji Zasada prac przygotowanych belka
Zasada prac przygotowanych belka Monitorowanie realizacji podstawy programowej
Monitorowanie realizacji podstawy programowej Podstawy fizykochemii spalania
Podstawy fizykochemii spalania Podstawy fizykochemii spalania
Podstawy fizykochemii spalania Monitorowanie realizacji podstawy programowej
Monitorowanie realizacji podstawy programowej Podstawy akustyki
Podstawy akustyki Teoretyczne podstawy informatyki
Teoretyczne podstawy informatyki Wzór na pole powierzchni graniastosłupa
Wzór na pole powierzchni graniastosłupa Podstawy kryptografii
Podstawy kryptografii Podstawy hydrauliki
Podstawy hydrauliki Visual basic podstawy
Visual basic podstawy Realizacja podstawy programowej w edukacji wczesnoszkolnej
Realizacja podstawy programowej w edukacji wczesnoszkolnej Język sql - podstawy zapytań
Język sql - podstawy zapytań Osnowa dokumentu
Osnowa dokumentu Hektar na m2
Hektar na m2 Nano ne
Nano ne Nano 101
Nano 101 Milli centi deci chart
Milli centi deci chart S4a instalar
S4a instalar Nanogears
Nanogears Measured quantity
Measured quantity Nano programmed control unit
Nano programmed control unit Si-enheten
Si-enheten Nano micro mili
Nano micro mili Mm micro nano pico
Mm micro nano pico Prefiksi fizika
Prefiksi fizika Silver nano health system samsung
Silver nano health system samsung Mili mikro nano piko značky
Mili mikro nano piko značky Arduino adc
Arduino adc Unp nano
Unp nano Nanoestel
Nanoestel Arduino uno timer interrupt
Arduino uno timer interrupt