Please turn cell phones off 5 5 Chapter







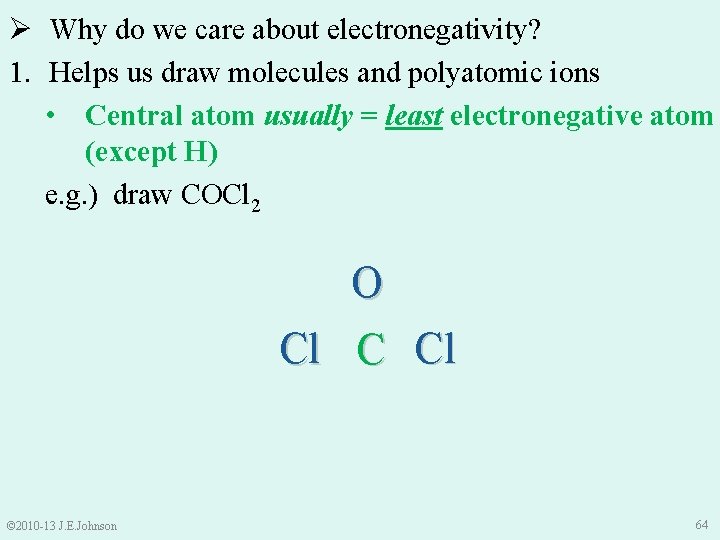










- Slides: 98

Please turn cell phones off 5. 5 Chapter 5 Chemical Bonding and Nomenclature (Worksheets available on Web. CT) © 2010 -13 J. E. Johnson 1

Let’s learn about 3 Types of Chemical Bonding 1) Ionic bonding 2) Covalent bonding 3) Polar covalent bonding Chemical bond: o attractive force between atoms in a compound o involves valence e-s © 2010 -13 J. E. Johnson 2

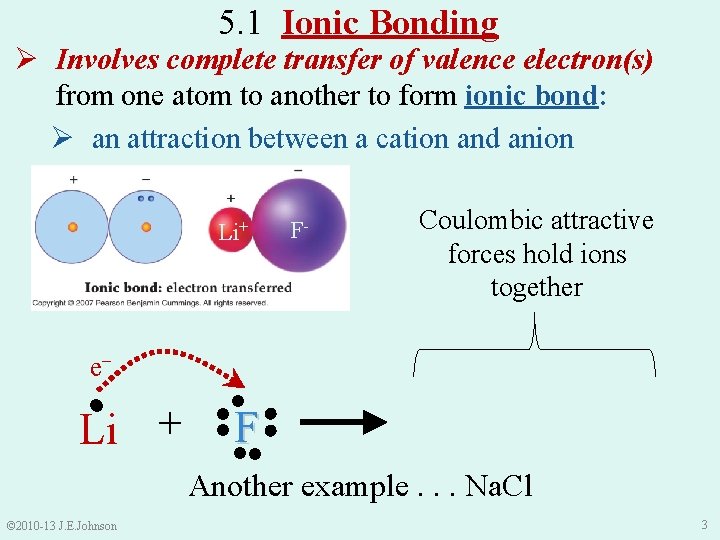
5. 1 Ionic Bonding Ø Involves complete transfer of valence electron(s) from one atom to another to form ionic bond: Ø an attraction between a cation and anion Li+ e− ● Li + ●● ● ● F● ●● F- Coulombic attractive forces hold ions together Li+ + ●●●●● 1●F ●● Another example. . . Na. Cl © 2010 -13 J. E. Johnson 3

5. 1 Ionic Bonding Ionic bond: attraction between a cation and anion Ø When compounds possess ionic bonds, they form ionic compounds: Ø made of cations and anions Ø can be made of metal cations and non-metal anions Ø can be made of polyatomic cations and anions, too Ø We’ll learn about these momentarily © 2010 -13 J. E. Johnson 4

5. 2 Molecules – Covalent Bonding In Chapter 1, we learned: Ø Compound: a pure substance containing 2 or more different types of elements bonded together Molecule: Ø two or more atoms bonded together in a specific arrangement Ø atoms may be same element or different element e. g. ) CH 4, O 3, CH 2 O, N 2, C 6 H 12 O 6 © 2010 -13 J. E. Johnson 5

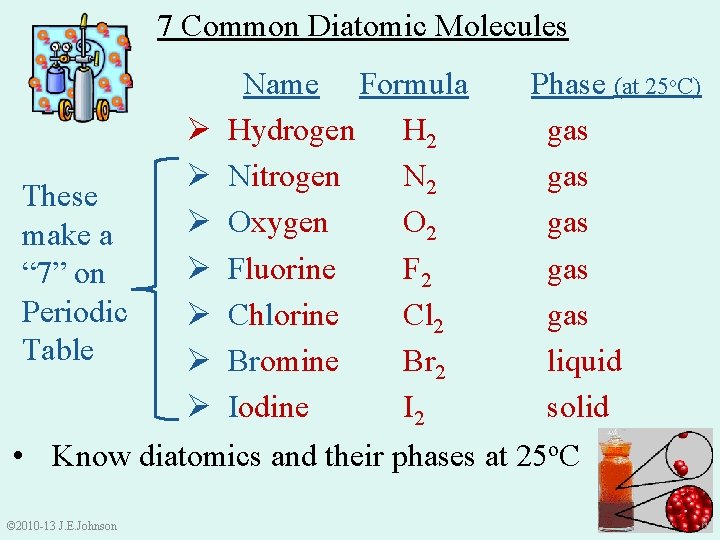
7 Common Diatomic Molecules These make a “ 7” on Periodic Table Ø Ø Ø Ø Name Formula Hydrogen H 2 Nitrogen N 2 Oxygen O 2 Fluorine F 2 Chlorine Cl 2 Bromine Br 2 Iodine I 2 Phase (at 25 o. C) gas gas gas liquid solid • Know diatomics and their phases at 25 o. C © 2010 -13 J. E. Johnson 6 6

Let’s learn about 3 Types of Chemical Bonding 1) Ionic bonding 2) Covalent bonding 3) Polar covalent bonding Chemical bond: o attractive force between atoms in a compound o involves valence e-s © 2010 -13 J. E. Johnson 7

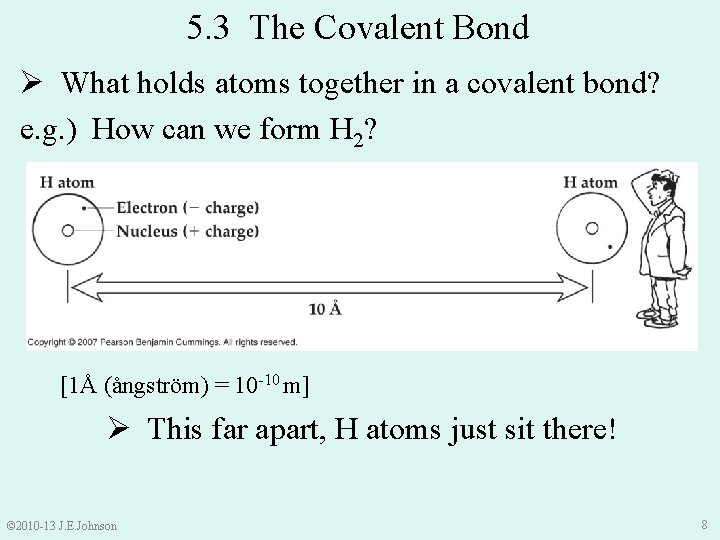
5. 3 The Covalent Bond Ø What holds atoms together in a covalent bond? e. g. ) How can we form H 2? [1Å (ångström) = 10 -10 m] Ø This far apart, H atoms just sit there! © 2010 -13 J. E. Johnson 8

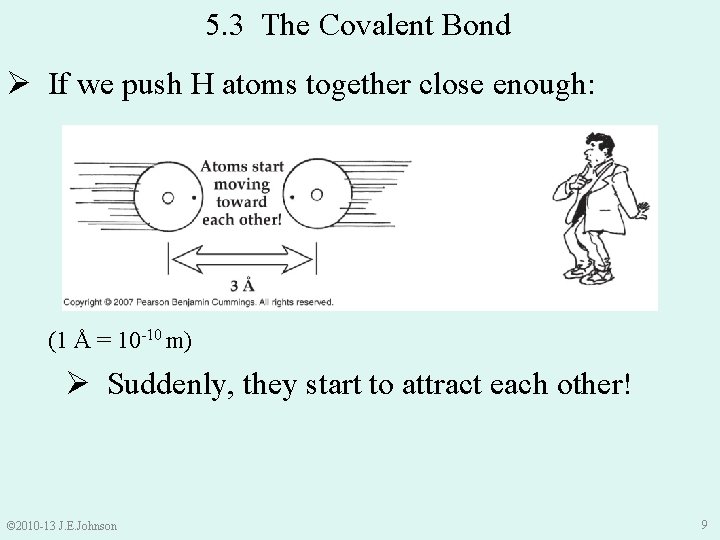
5. 3 The Covalent Bond Ø If we push H atoms together close enough: (1 Å = 10 -10 m) Ø Suddenly, they start to attract each other! © 2010 -13 J. E. Johnson 9

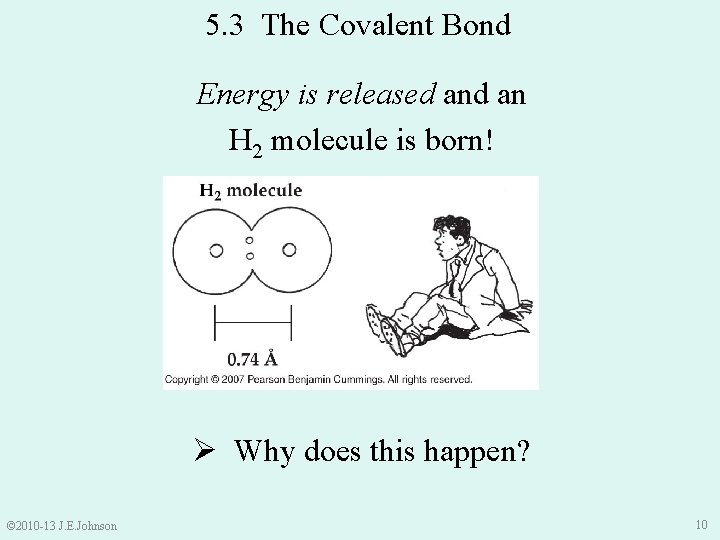
5. 3 The Covalent Bond Energy is released an H 2 molecule is born! Ø Why does this happen? © 2010 -13 J. E. Johnson 10

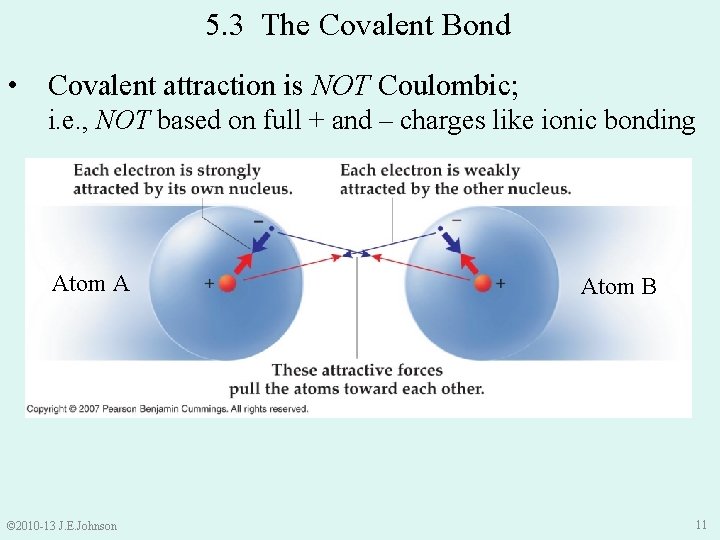
5. 3 The Covalent Bond • Covalent attraction is NOT Coulombic; i. e. , NOT based on full + and – charges like ionic bonding Atom A © 2010 -13 J. E. Johnson Atom B 11

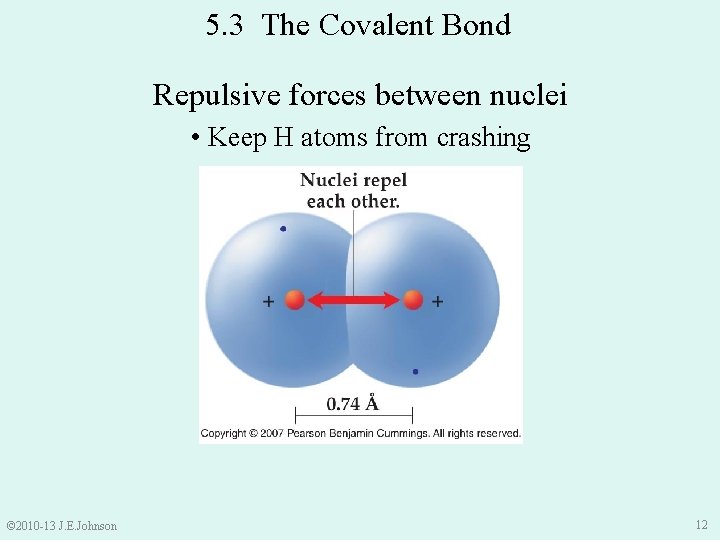
5. 3 The Covalent Bond Repulsive forces between nuclei • Keep H atoms from crashing © 2010 -13 J. E. Johnson 12

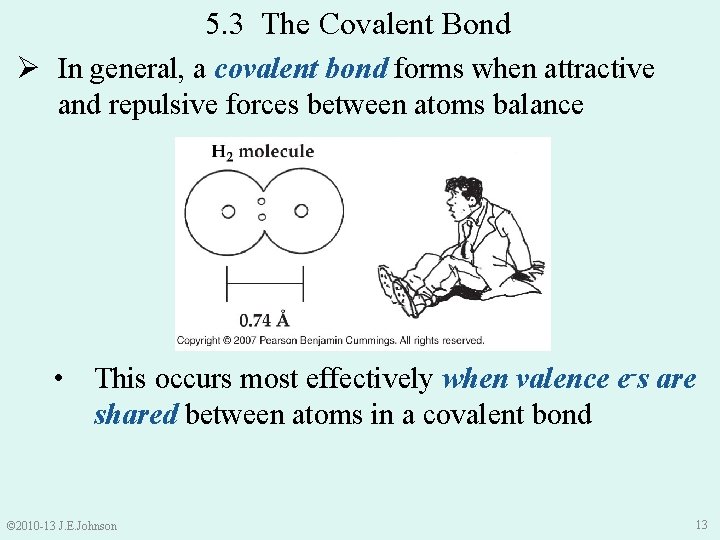
5. 3 The Covalent Bond Ø In general, a covalent bond forms when attractive and repulsive forces between atoms balance • This occurs most effectively when valence e-s are shared between atoms in a covalent bond © 2010 -13 J. E. Johnson 13

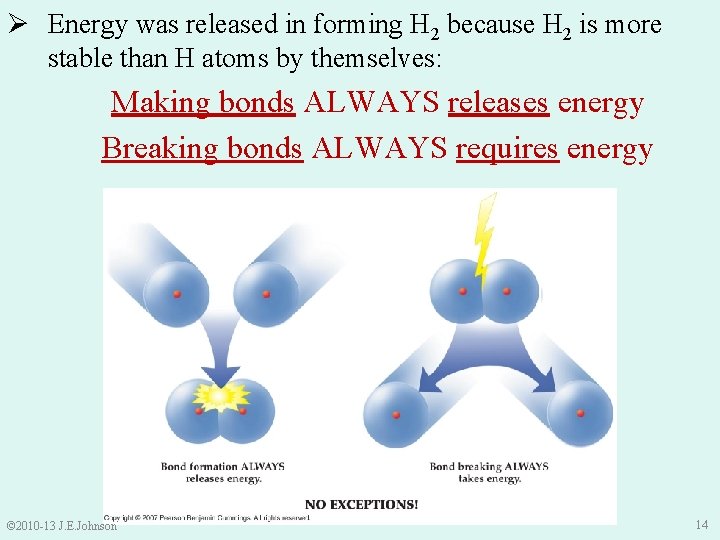
Ø Energy was released in forming H 2 because H 2 is more stable than H atoms by themselves: Making bonds ALWAYS releases energy Breaking bonds ALWAYS requires energy © 2010 -13 J. E. Johnson 14

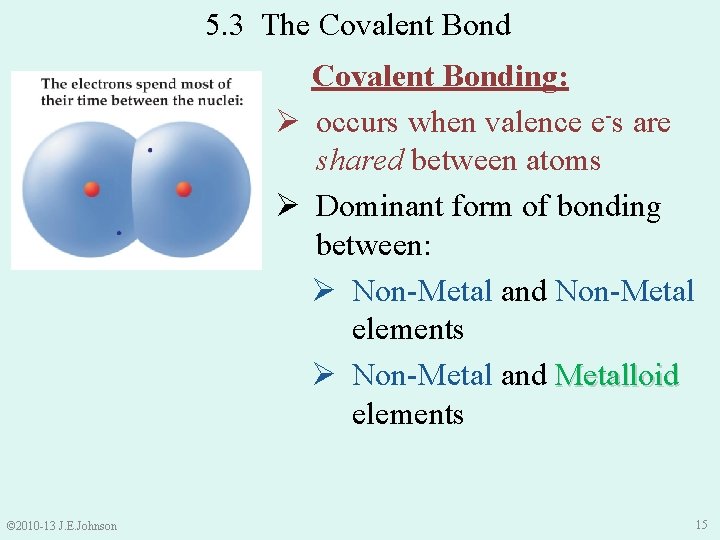
5. 3 The Covalent Bonding: Ø occurs when valence e-s are shared between atoms Ø Dominant form of bonding between: Ø Non-Metal and Non-Metal elements Ø Non-Metal and Metalloid elements © 2010 -13 J. E. Johnson 15

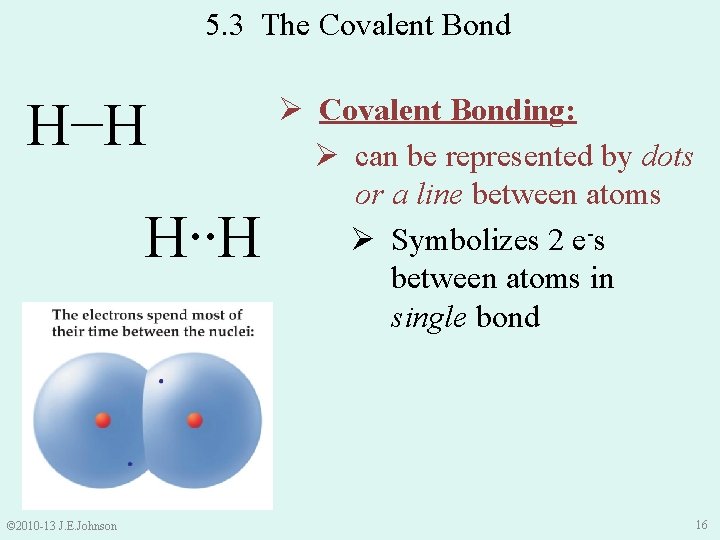
5. 3 The Covalent Bond H−H H∙∙H © 2010 -13 J. E. Johnson Ø Covalent Bonding: Ø can be represented by dots or a line between atoms Ø Symbolizes 2 e-s between atoms in single bond 16

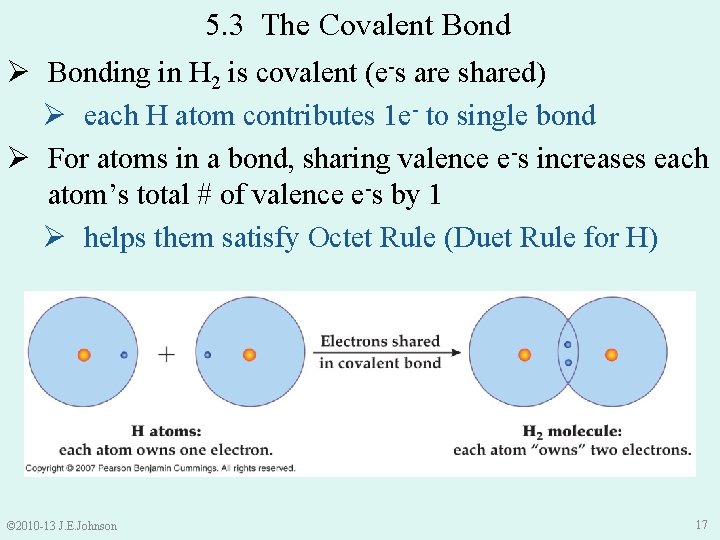
5. 3 The Covalent Bond Ø Bonding in H 2 is covalent (e-s are shared) Ø each H atom contributes 1 e- to single bond Ø For atoms in a bond, sharing valence e-s increases each atom’s total # of valence e-s by 1 Ø helps them satisfy Octet Rule (Duet Rule for H) © 2010 -13 J. E. Johnson 17

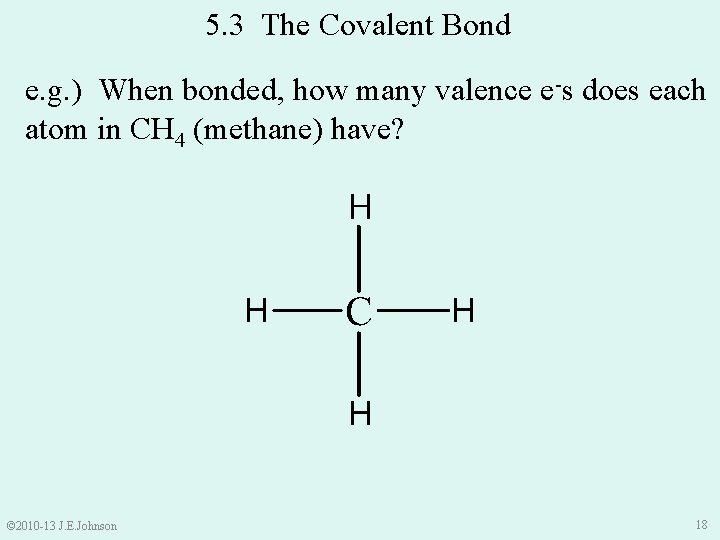
5. 3 The Covalent Bond e. g. ) When bonded, how many valence e-s does each atom in CH 4 (methane) have? © 2010 -13 J. E. Johnson 18

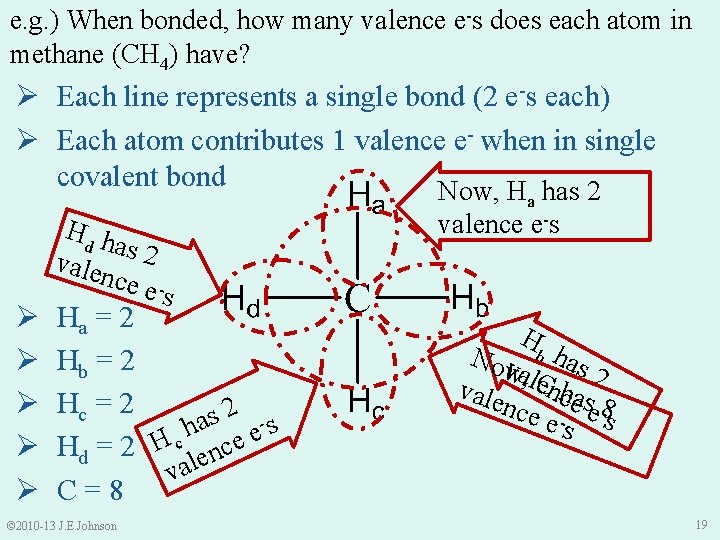
e. g. ) When bonded, how many valence e-s does each atom in methane (CH 4) have? Ø Each line represents a single bond (2 e-s each) Ø Each atom contributes 1 valence e- when in single covalent bond Now, H has 2 a Ø Ø Ø Hd h as 2 vale nce es Ha = 2 Hb = 2 Hc = 2 2 s a s h e Hd = 2 H c lence va C=8 © 2010 -13 J. E. Johnson valence e-s H Now va, lb has 2 ench vale C nce - ease 8 -s es 19

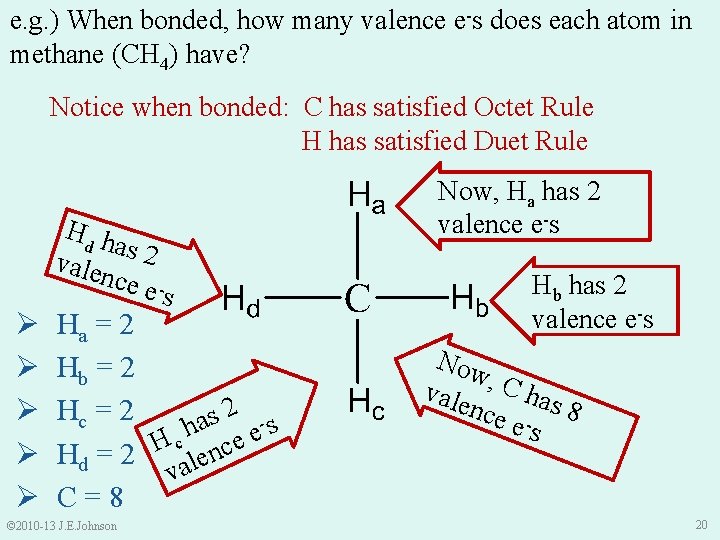
e. g. ) When bonded, how many valence e-s does each atom in methane (CH 4) have? Notice when bonded: C has satisfied Octet Rule H has satisfied Duet Rule Ø Ø Ø Hd h as 2 vale nce es Ha = 2 Hb = 2 2 Hc = 2 s a s h e Hd = 2 H calence v C=8 © 2010 -13 J. E. Johnson Now, Ha has 2 valence e-s Hb has 2 valence e-s Now vale , C has nce - 8 es 20

Ø Main Group number = number of valence e-s Ø 8 – Main Group number = number of bonds the element usually forms (except H and B) © 2010 -13 J. E. Johnson 22

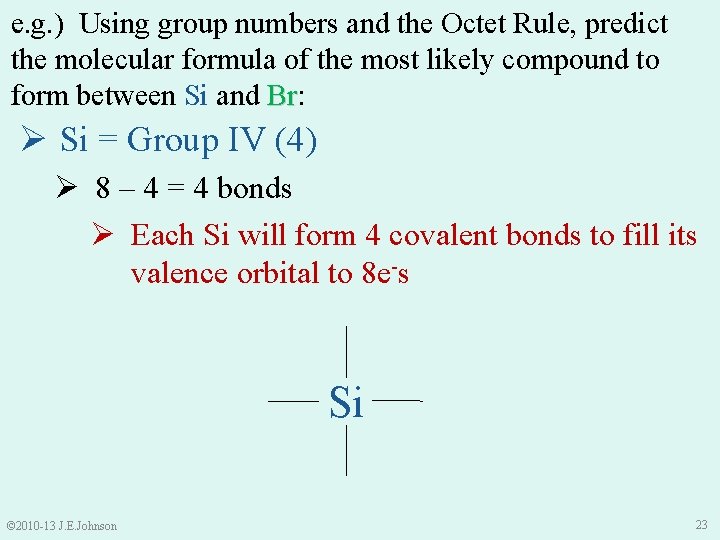
e. g. ) Using group numbers and the Octet Rule, predict the molecular formula of the most likely compound to form between Si and Br: Br Ø Si = Group IV (4) Ø 8 – 4 = 4 bonds Ø Each Si will form 4 covalent bonds to fill its valence orbital to 8 e-s Si © 2010 -13 J. E. Johnson 23

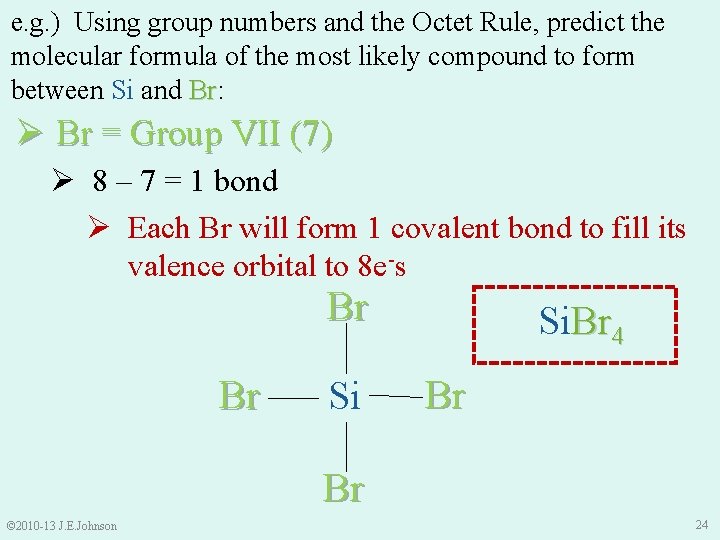
e. g. ) Using group numbers and the Octet Rule, predict the molecular formula of the most likely compound to form between Si and Br: Br Ø Br = Group VII (7) Ø 8 – 7 = 1 bond Ø Each Br will form 1 covalent bond to fill its valence orbital to 8 e-s Br Br Si Si. Br 4 Br Br © 2010 -13 J. E. Johnson 24

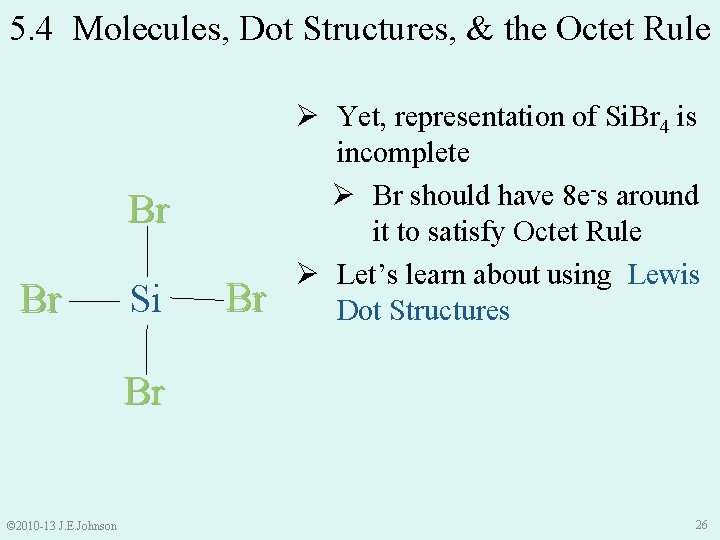
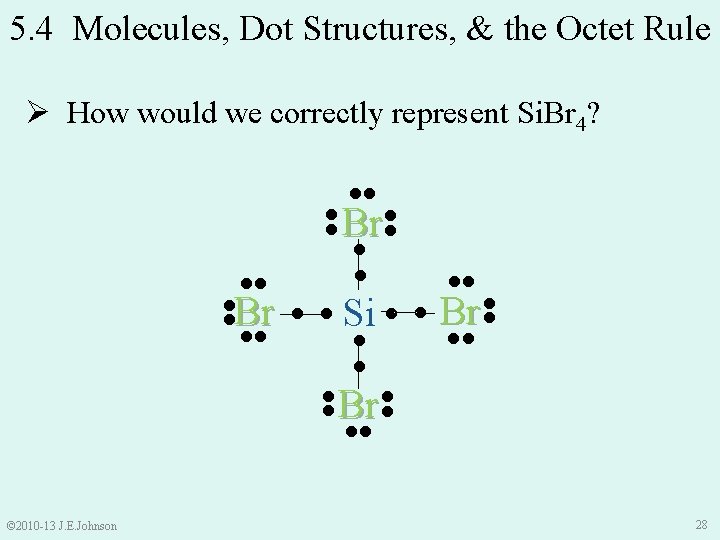
5. 4 Molecules, Dot Structures, & the Octet Rule Br Br Si Br Ø Yet, representation of Si. Br 4 is incomplete Ø Br should have 8 e-s around it to satisfy Octet Rule Ø Let’s learn about using Lewis Dot Structures Br © 2010 -13 J. E. Johnson 26

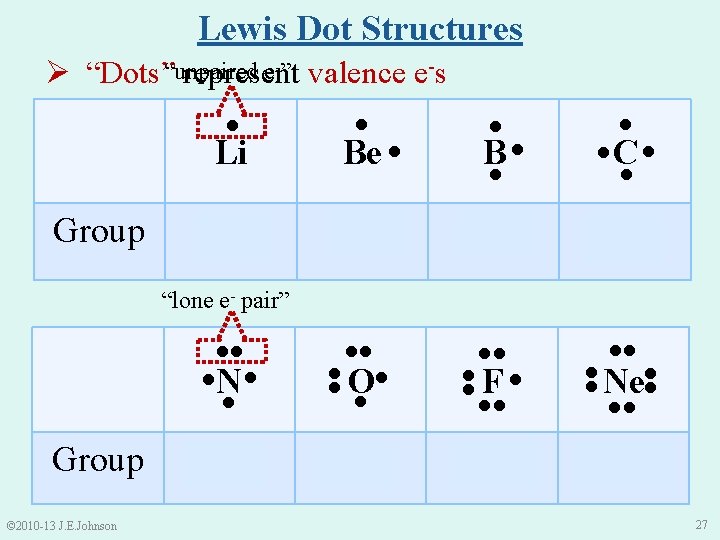
Lewis Dot Structures e-” valence e-s Ø “Dots”“unpaired represent ● Group ● ● ● Li Be ● B● ● ●C ● ● IA IIIA IVA ●● ●● ●● Group © 2010 -13 J. E. Johnson VA VIA ●● VIIA Ne ●● ●● F● ●● ● O ● ●● ●● ● N● ● ●● “lone e- pair” VIIIA 27

5. 4 Molecules, Dot Structures, & the Octet Rule Ø How would we correctly represent Si. Br 4? ●● ●● Br ●● Si ● ● ●● ● Br ●● ●● ●● ●● Br © 2010 -13 J. E. Johnson ●● ●● ●● Br 28

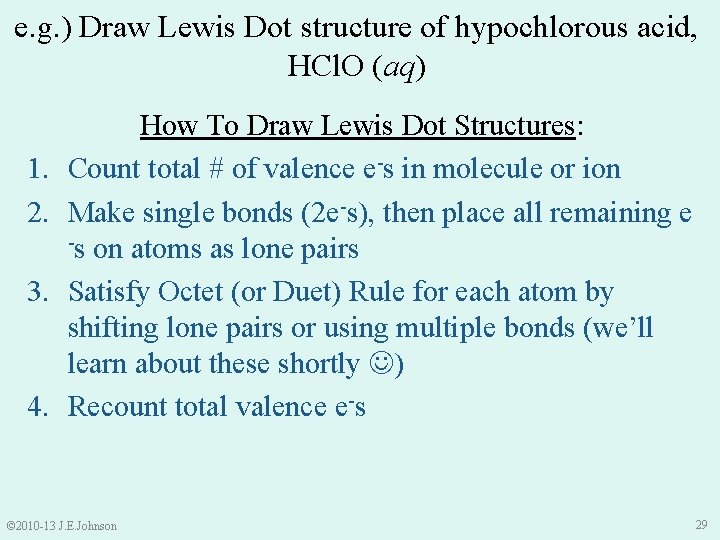
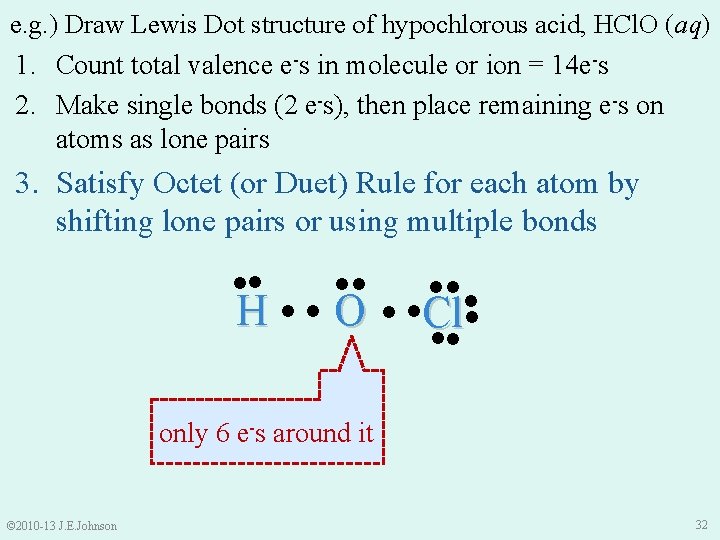
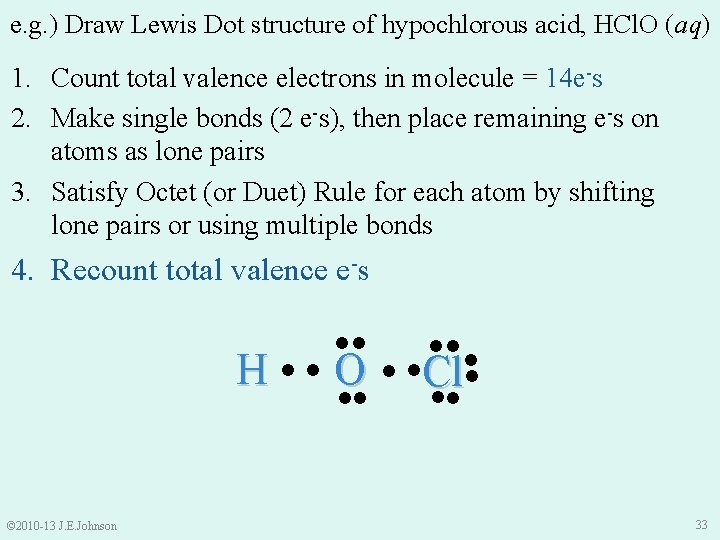
e. g. ) Draw Lewis Dot structure of hypochlorous acid, HCl. O (aq) 1. 2. 3. 4. How To Draw Lewis Dot Structures: Count total # of valence e-s in molecule or ion Make single bonds (2 e-s), then place all remaining e -s on atoms as lone pairs Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds (we’ll learn about these shortly ) Recount total valence e-s © 2010 -13 J. E. Johnson 29

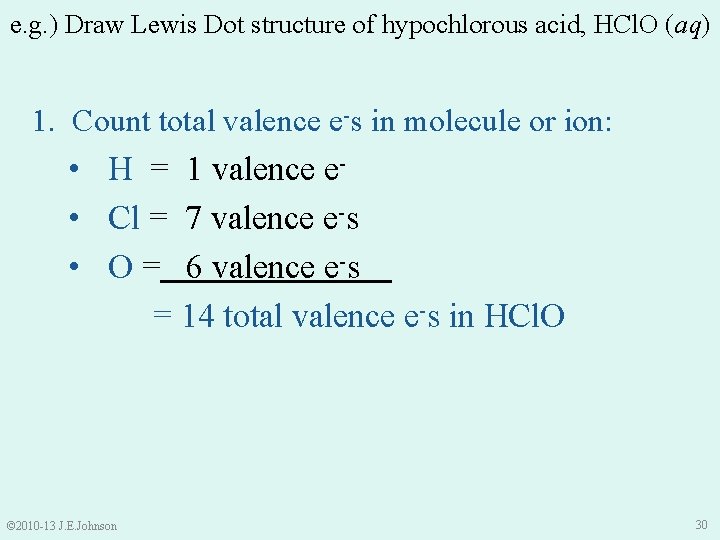
e. g. ) Draw Lewis Dot structure of hypochlorous acid, HCl. O (aq) 1. Count total valence e-s in molecule or ion: • H = 1 valence e- • Cl = 7 valence e-s • O = 6 valence e-s = 14 total valence e-s in HCl. O © 2010 -13 J. E. Johnson 30

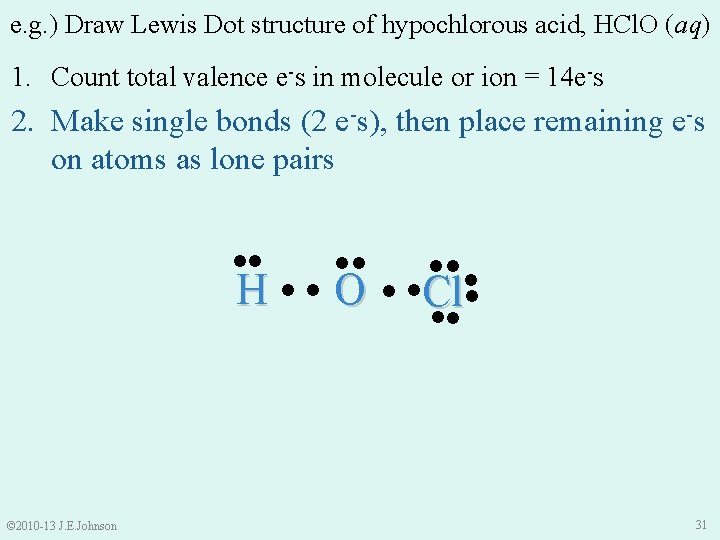
e. g. ) Draw Lewis Dot structure of hypochlorous acid, HCl. O (aq) 1. Count total valence e-s in molecule or ion = 14 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs ●● ●● H ●● O © 2010 -13 J. E. Johnson ●●● ● ●Cl● ●● 31

e. g. ) Draw Lewis Dot structure of hypochlorous acid, HCl. O (aq) 1. Count total valence e-s in molecule or ion = 14 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds ●● ●● H ●● O ●●● ● ●Cl● ●● only 6 e-s around it © 2010 -13 J. E. Johnson 32

e. g. ) Draw Lewis Dot structure of hypochlorous acid, HCl. O (aq) 1. Count total valence electrons in molecule = 14 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds 4. Recount total valence e-s H © 2010 -13 J. E. Johnson ●● ● ● O ● ●Cl● ●● ●● 33

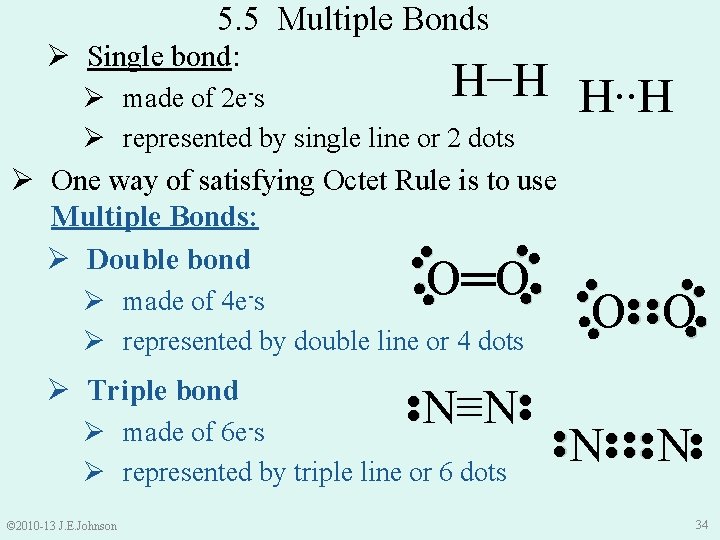
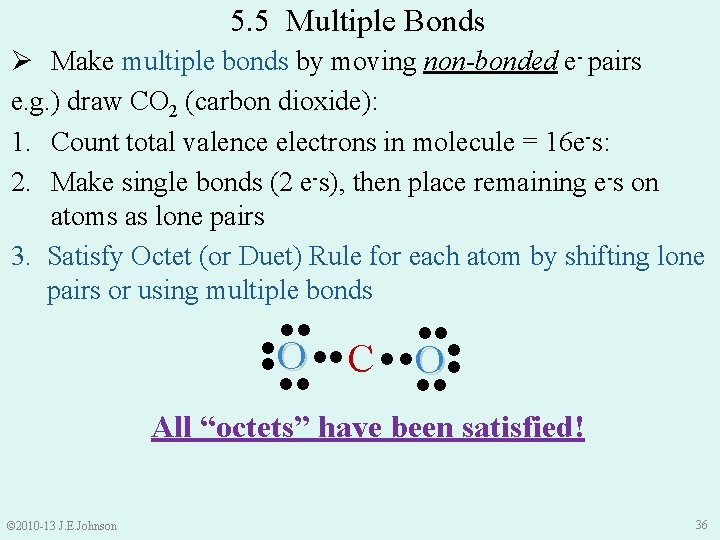
5. 5 Multiple Bonds Ø Single bond: H−H H∙∙H Ø made of 2 e-s Ø represented by single line or 2 dots ●● ●● ●● N N ●● © 2010 -13 J. E. Johnson ●● O O ●● N≡N Ø made of Ø represented by triple line or 6 dots 6 e-s ●● Ø Triple bond ●● Ø made of 4 e-s Ø represented by double line or 4 dots ●● ● ● O═O ●● ●● ● ● Ø One way of satisfying Octet Rule is to use Multiple Bonds: ●● Ø Double bond 34

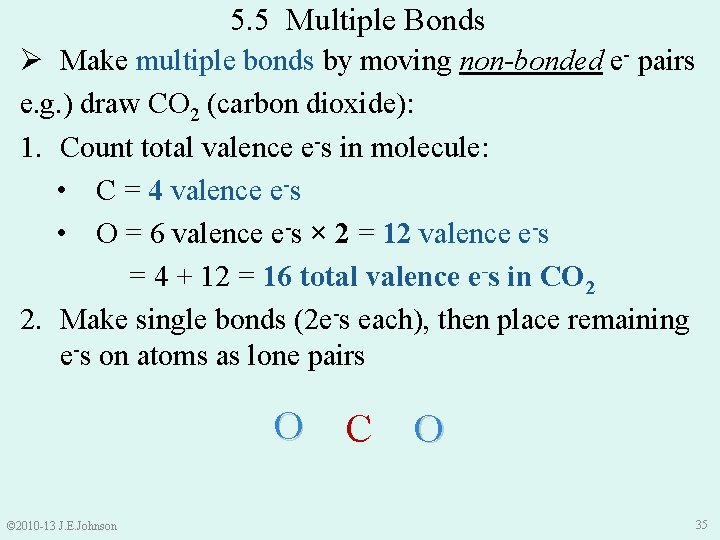
5. 5 Multiple Bonds Ø Make multiple bonds by moving non-bonded e- pairs e. g. ) draw CO 2 (carbon dioxide): 1. Count total valence e-s in molecule: • C = 4 valence e-s • O = 6 valence e-s × 2 = 12 valence e-s = 4 + 12 = 16 total valence e-s in CO 2 2. Make single bonds (2 e-s each), then place remaining e-s on atoms as lone pairs © 2010 -13 J. E. Johnson ●● ●● C ● ●O ● ●● ●● O● ●● ●● 35

5. 5 Multiple Bonds ●● ● ●● Ø Make multiple bonds by moving non-bonded e- pairs e. g. ) draw CO 2 (carbon dioxide): 1. Count total valence electrons in molecule = 16 e-s: 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds ●● ●● O ● C ● ●O ●● ●● All “octets” have been satisfied! © 2010 -13 J. E. Johnson 36

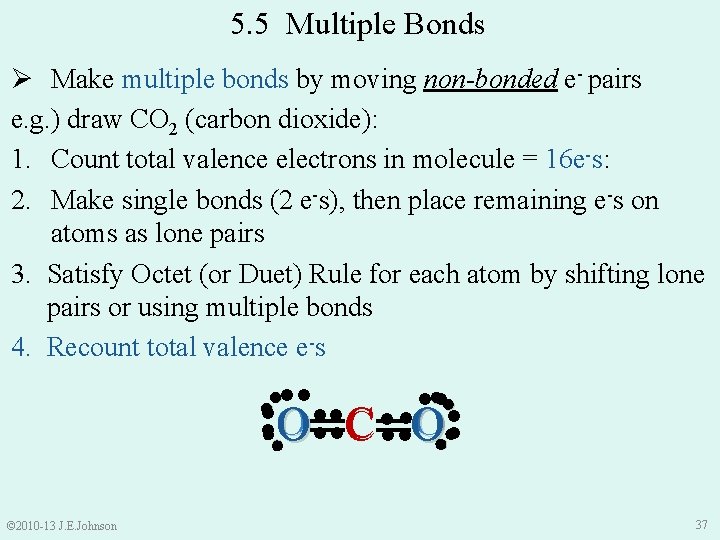
5. 5 Multiple Bonds Ø Make multiple bonds by moving non-bonded e- pairs e. g. ) draw CO 2 (carbon dioxide): 1. Count total valence electrons in molecule = 16 e-s: 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds 4. Recount total valence e-s ●● ●● ●● ●● O ●●● C ●●●●O © 2010 -13 J. E. Johnson ●● ●● ● O═C═O 37

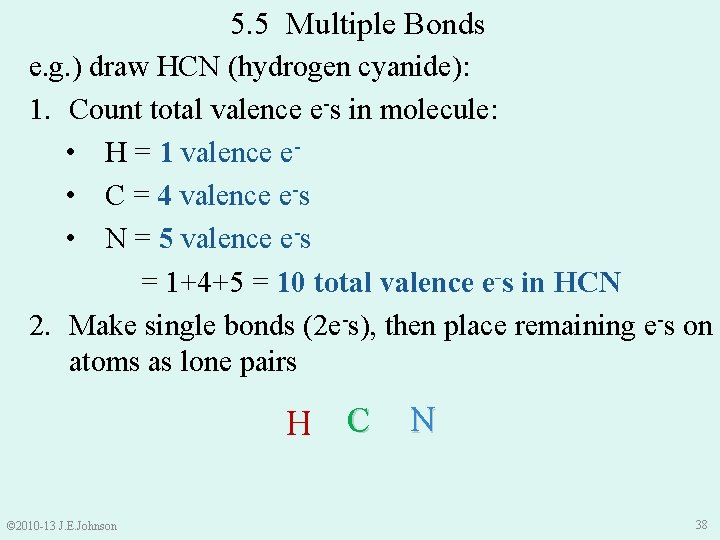
5. 5 Multiple Bonds e. g. ) draw HCN (hydrogen cyanide): 1. Count total valence e-s in molecule: • H = 1 valence e • C = 4 valence e-s • N = 5 valence e-s = 1+4+5 = 10 total valence e-s in HCN 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs © 2010 -13 J. E. Johnson ● ● ●● H● C ●● N ● ●● 38

5. 5 Multiple Bonds e. g. ) draw HCN (hydrogen cyanide): 1. Count total valence e-s in molecule = 10 e-s: 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds 4. Recount total valence e-s ●● ● H●● C ● ● N ●● ● ●● H−C≡N All “rules” have been satisfied! © 2010 -13 J. E. Johnson 39

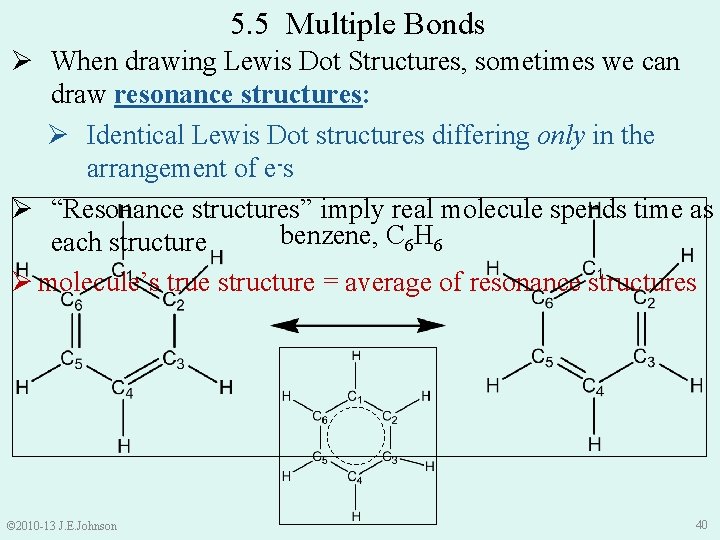
5. 5 Multiple Bonds Ø When drawing Lewis Dot Structures, sometimes we can draw resonance structures: Ø Identical Lewis Dot structures differing only in the arrangement of e-s Ø “Resonance structures” imply real molecule spends time as benzene, C 6 H 6 each structure Ø molecule’s true structure = average of resonance structures © 2010 -13 J. E. Johnson 40

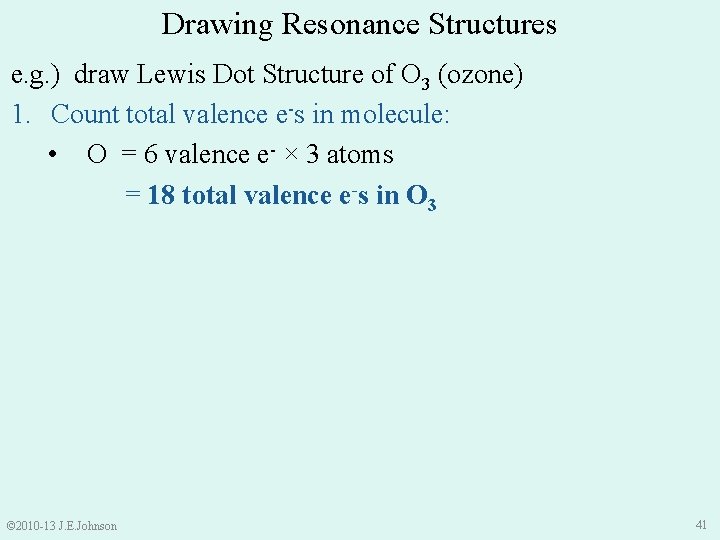
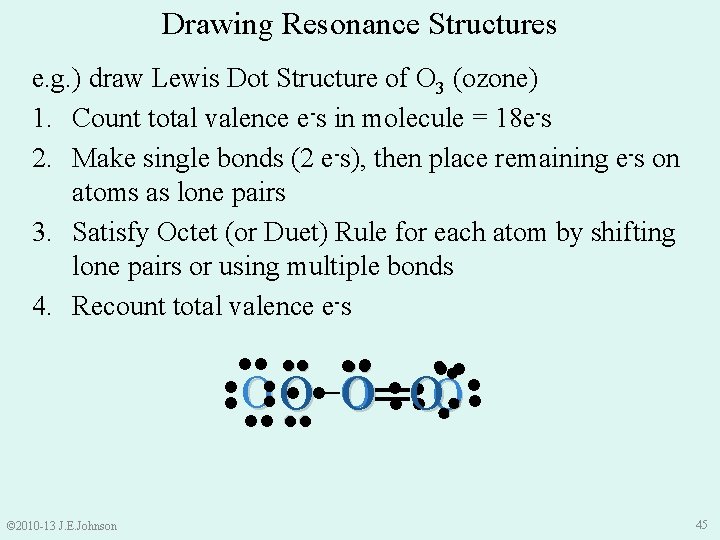
Drawing Resonance Structures e. g. ) draw Lewis Dot Structure of O 3 (ozone) 1. Count total valence e-s in molecule: • O = 6 valence e- × 3 atoms = 18 total valence e-s in O 3 © 2010 -13 J. E. Johnson 41

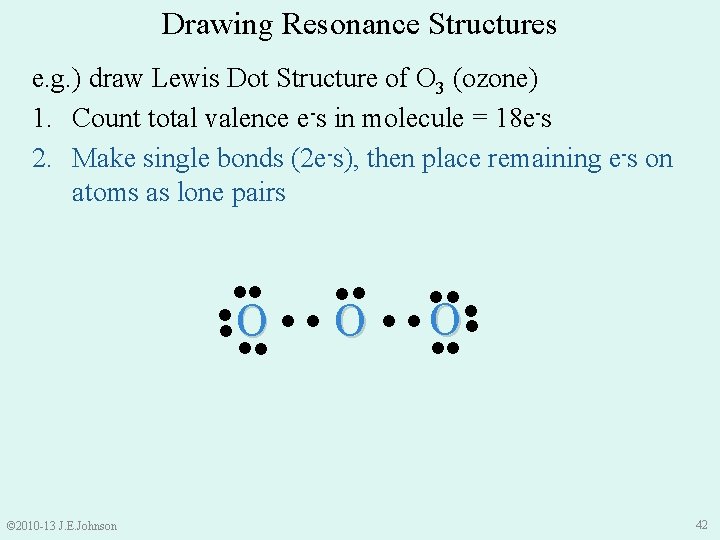
Drawing Resonance Structures e. g. ) draw Lewis Dot Structure of O 3 (ozone) 1. Count total valence e-s in molecule = 18 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs ●● ●● ● O● ● ●● ●● © 2010 -13 J. E. Johnson 42

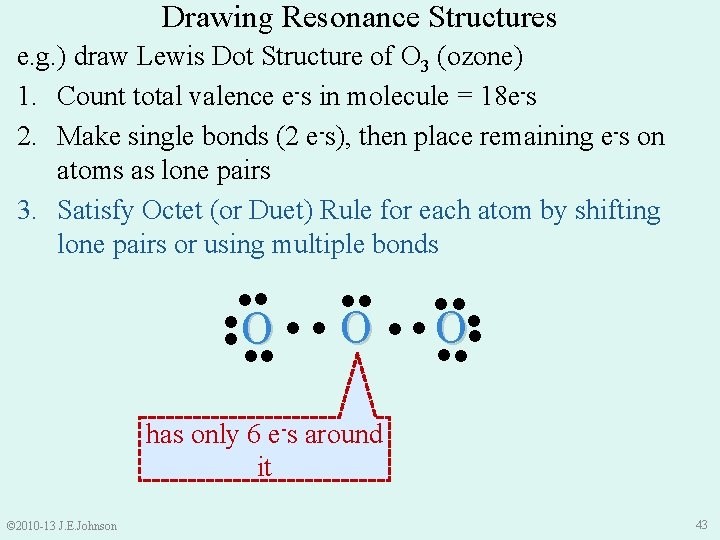
Drawing Resonance Structures e. g. ) draw Lewis Dot Structure of O 3 (ozone) 1. Count total valence e-s in molecule = 18 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds ●● ●● ●● ● O● ● ● ●● ●● has only 6 e-s around it © 2010 -13 J. E. Johnson 43

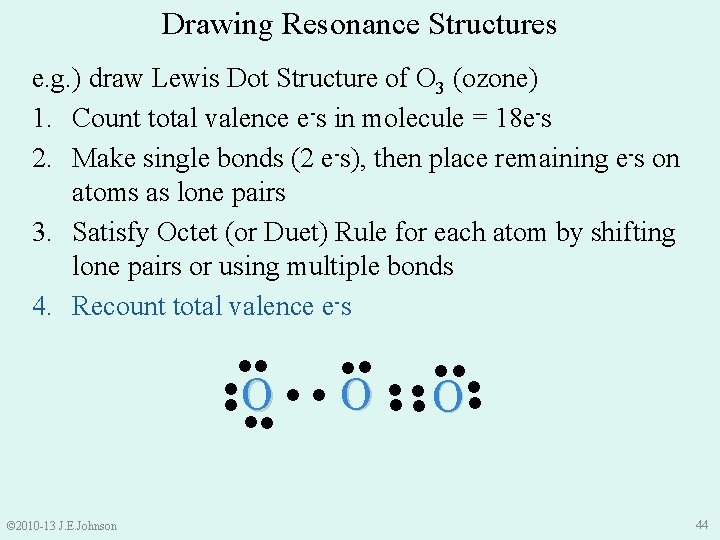
Drawing Resonance Structures e. g. ) draw Lewis Dot Structure of O 3 (ozone) 1. Count total valence e-s in molecule = 18 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds 4. Recount total valence e-s ●● ●● ●● ●O ● ● O● ● ●● © 2010 -13 J. E. Johnson 44

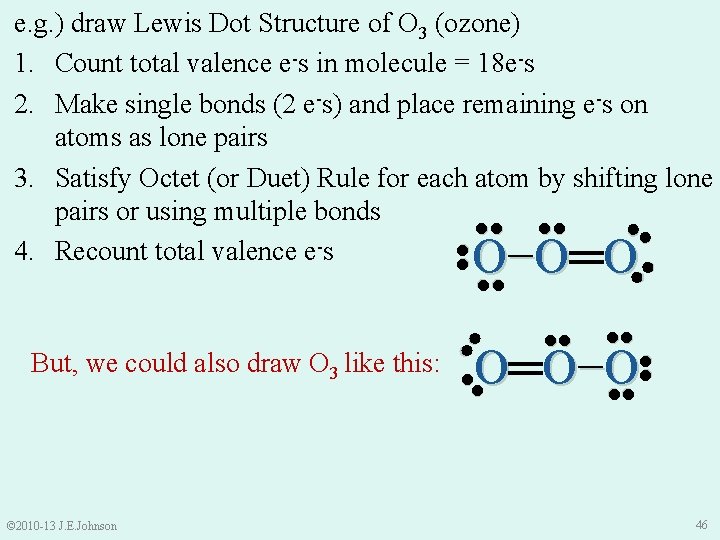
Drawing Resonance Structures e. g. ) draw Lewis Dot Structure of O 3 (ozone) 1. Count total valence e-s in molecule = 18 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds 4. Recount total valence e-s ●●● ●● ●● ● ●O ● ● O● ● ●● ●● © 2010 -13 J. E. Johnson ●● ●● O− O═ O 45

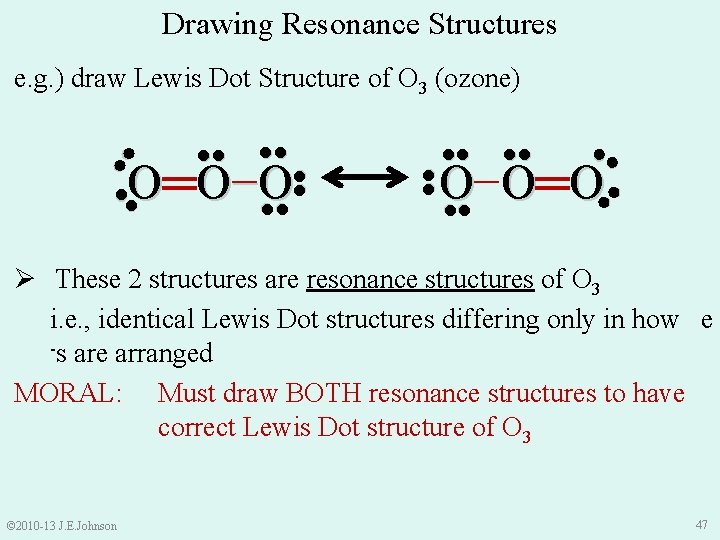
e. g. ) draw Lewis Dot Structure of O 3 (ozone) 1. Count total valence e-s in molecule = 18 e-s 2. Make single bonds (2 e-s) and place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds ●● ●● ●● 4. Recount total valence e-s ●● ●● O− O═ O ●● ●● ●● O═ O− O ●● ●● © 2010 -13 J. E. Johnson ●● But, we could also draw O 3 like this: ●● 46

Drawing Resonance Structures e. g. ) draw Lewis Dot Structure of O 3 (ozone) ●● ●● O− O═ O ●● ●● O═ O− O ●● ●● ●● Ø These 2 structures are resonance structures of O 3 i. e. , identical Lewis Dot structures differing only in how e -s are arranged MORAL: Must draw BOTH resonance structures to have correct Lewis Dot structure of O 3 © 2010 -13 J. E. Johnson 47

Resonance Structures and Polyatomic Ions Ø Many polyatomic ions have resonance structures Ø Polyatomic Ions: ions having more than 1 atom e. g. ) • CO 32 - (carbonate) NO 2 - (nitrite) NH 4+ (ammonium) NOTICE: Within themselves, polyatomic ions have covalent bonds • But, they form ionic bonds with metals or other polyatomic ions. . . Why? © 2010 -13 J. E. Johnson 48

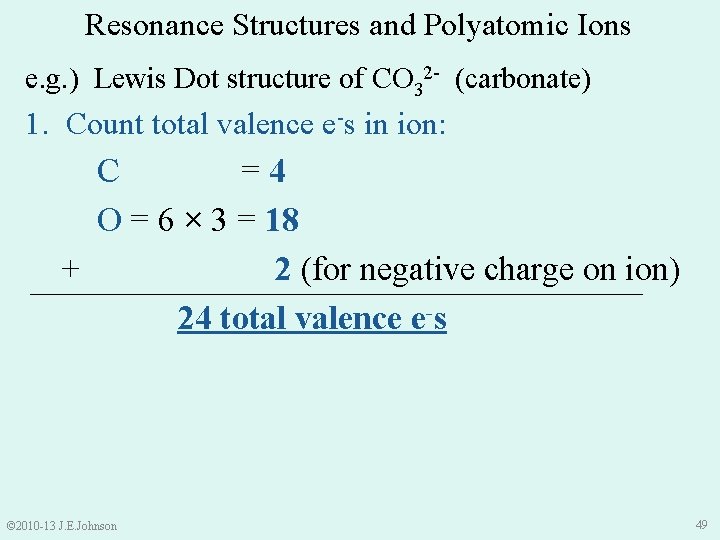
Resonance Structures and Polyatomic Ions e. g. ) Lewis Dot structure of CO 32 - (carbonate) 1. Count total valence e-s in ion: C =4 O = 6 × 3 = 18 + 2 (for negative charge on ion) 24 total valence e-s © 2010 -13 J. E. Johnson 49

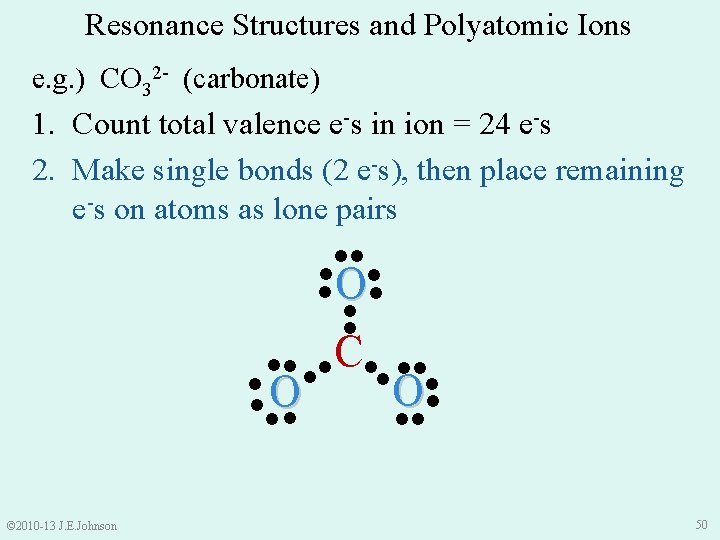
Resonance Structures and Polyatomic Ions e. g. ) CO 32 - (carbonate) 1. Count total valence e-s in ion = 24 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs ●● ● O● ● ● ●● ●● C●● ●● ●O ● ●● © 2010 -13 J. E. Johnson 50

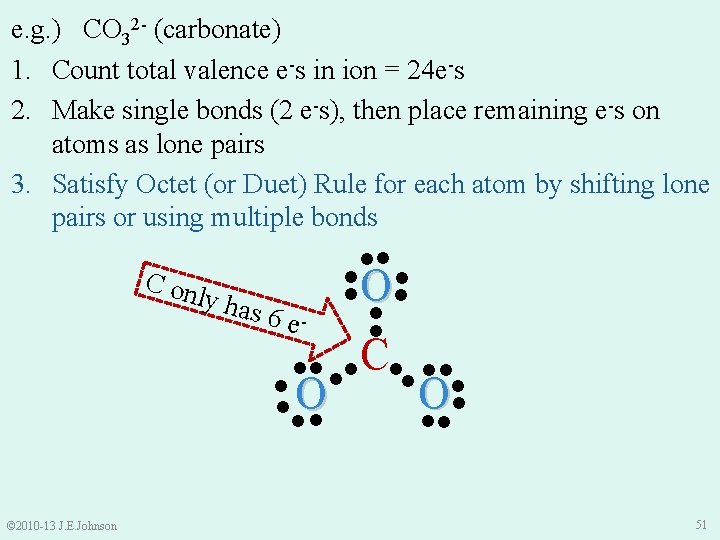
e. g. ) CO 32 - (carbonate) 1. Count total valence e-s in ion = 24 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds ●● ● ● C on ● O● ly ha s 6 e● ● ●● ●● C●● ●● ●O ● ●● © 2010 -13 J. E. Johnson 51

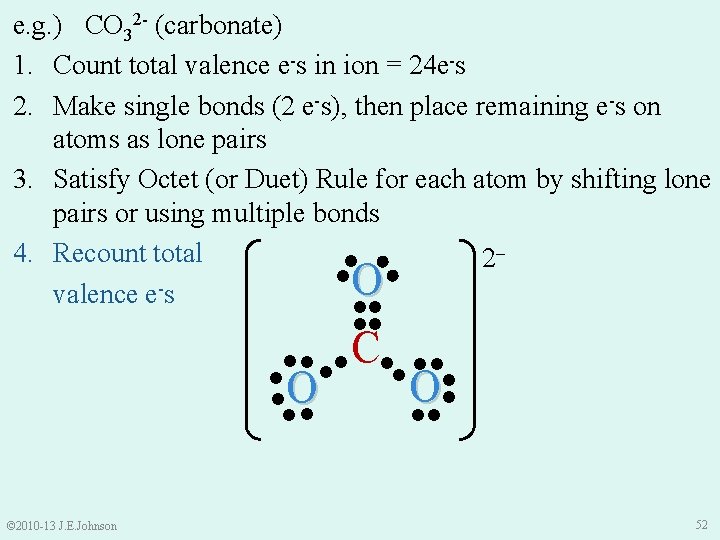
e. g. ) CO 32 - (carbonate) 1. Count total valence e-s in ion = 24 e-s 2. Make single bonds (2 e-s), then place remaining e-s on atoms as lone pairs 3. Satisfy Octet (or Duet) Rule for each atom by shifting lone pairs or using multiple bonds 4. Recount total 2‒ ● ●● ●O valence e s ●● ●● C●● ●● ● ● O O ● ● ●● ●● © 2010 -13 J. E. Johnson 52

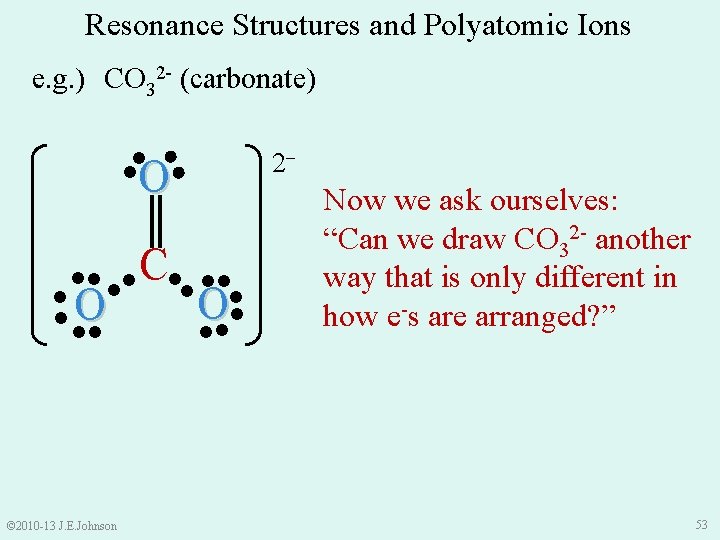
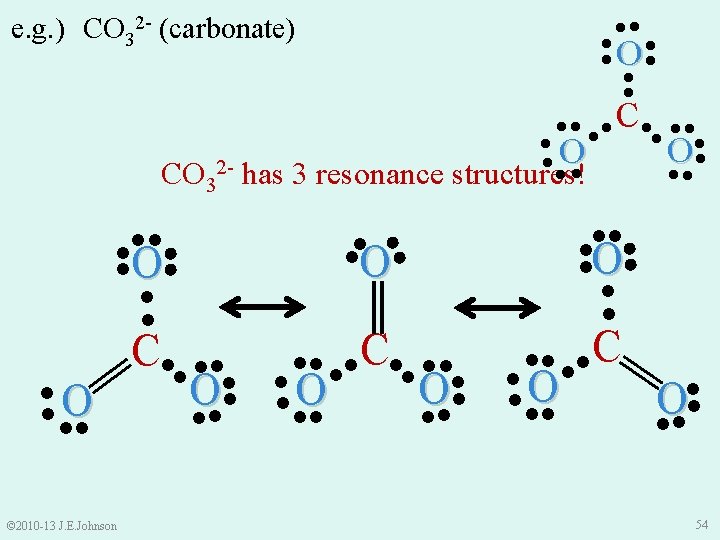
Resonance Structures and Polyatomic Ions e. g. ) CO 32 - (carbonate) ● ● ●O● ║ ●● ●● C●● ●● ●O ● ●● ●● ● © 2010 -13 J. E. Johnson 2‒ Now we ask ourselves: “Can we draw CO 32 - another way that is only different in how e-s are arranged? ” 53


Let’s Practice: Drawing Structures • Draw the NO 2− (nitrite) polyatomic ion along with its resonance structures, if they exist © 2010 -13 J. E. Johnson 55

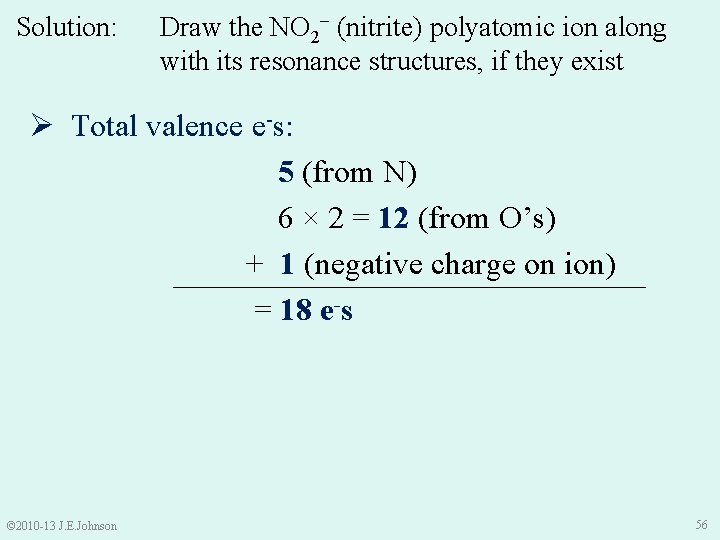
Solution: Draw the NO 2− (nitrite) polyatomic ion along with its resonance structures, if they exist Ø Total valence e-s: 5 (from N) 6 × 2 = 12 (from O’s) + 1 (negative charge on ion) = 18 e-s © 2010 -13 J. E. Johnson 56

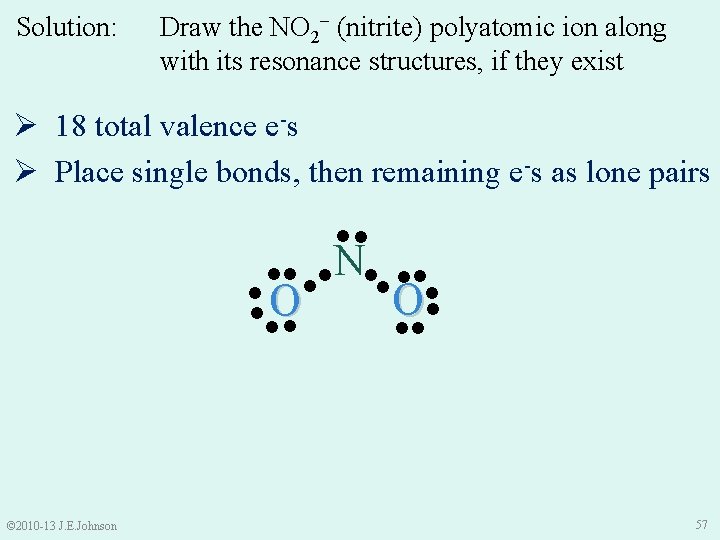
Solution: Draw the NO 2− (nitrite) polyatomic ion along with its resonance structures, if they exist Ø 18 total valence e-s Ø Place single bonds, then remaining e-s as lone pairs ●● ●● ●● N●● ●● ●O ● ●● © 2010 -13 J. E. Johnson 57

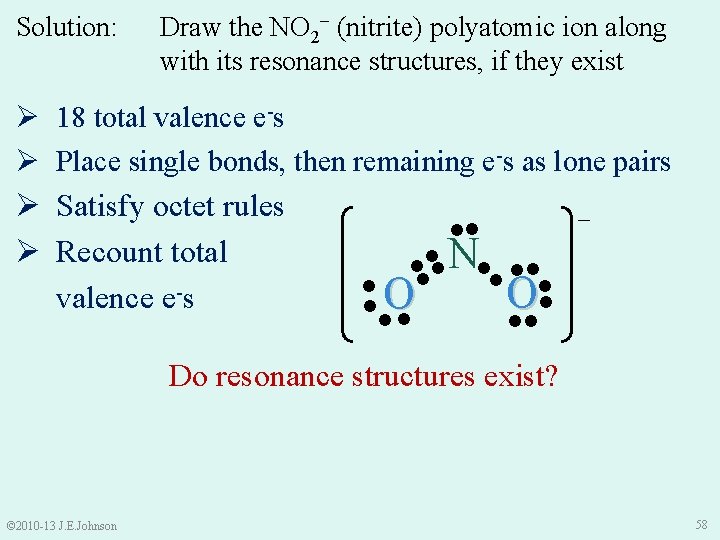
Solution: Draw the NO 2− (nitrite) polyatomic ion along with its resonance structures, if they exist Ø 18 total valence e-s Ø Place single bonds, then remaining e-s as lone pairs Ø Satisfy octet rules ‒ Ø Recount total valence e-s ●● ●●●● N● ●● ● ● ●O O ● ●● Do resonance structures exist? © 2010 -13 J. E. Johnson 58

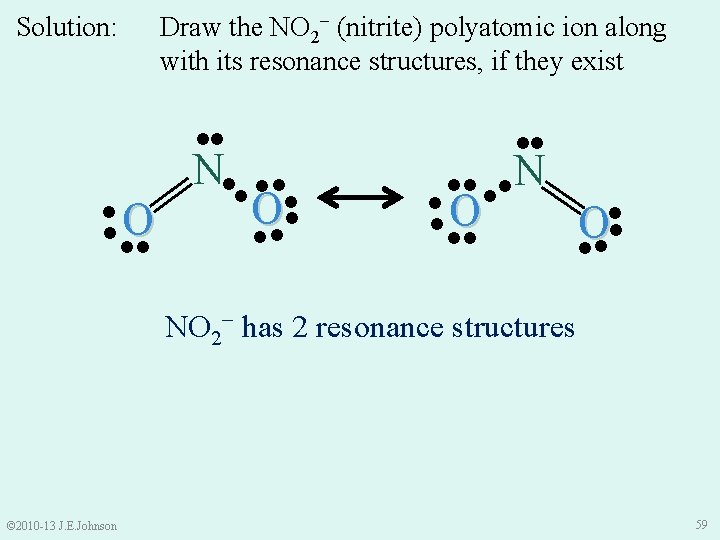
Draw the NO 2− (nitrite) polyatomic ion along with its resonance structures, if they exist ●● ║ N●● ●● ● ●O O ● ●● ●● ●● N ●O ●● ● ║ Solution: ● O ● ●● NO 2− has 2 resonance structures © 2010 -13 J. E. Johnson 59

Let’s learn about 3 Types of Chemical Bonding 1) Ionic bonding 2) Covalent bonding 3) Polar covalent bonding Chemical bonding: o attractive force between atoms in a compound o involves valence e-s © 2010 -13 J. E. Johnson 60

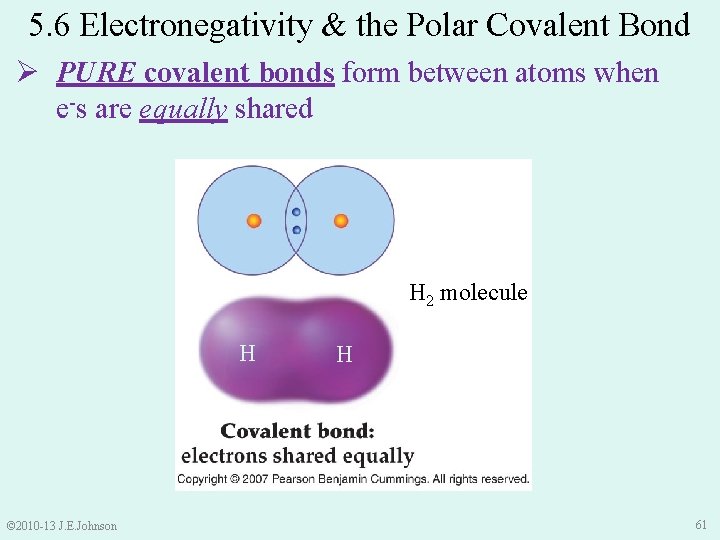
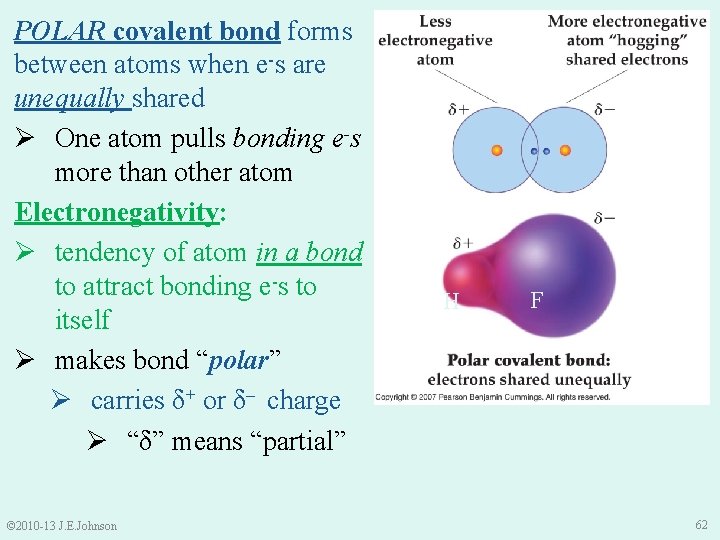
5. 6 Electronegativity & the Polar Covalent Bond Ø PURE covalent bonds form between atoms when e-s are equally shared H 2 molecule H © 2010 -13 J. E. Johnson H 61

POLAR covalent bond forms between atoms when e-s are unequally shared Ø One atom pulls bonding e-s more than other atom Electronegativity: Ø tendency of atom in a bond to attract bonding e-s to itself Ø makes bond “polar” Ø carries δ+ or δ– charge Ø “δ” means “partial” © 2010 -13 J. E. Johnson H F 62

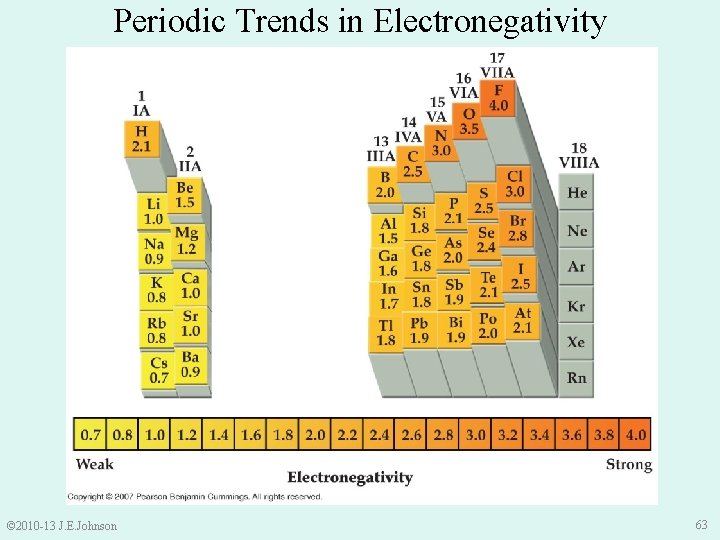
Periodic Trends in Electronegativity © 2010 -13 J. E. Johnson 63
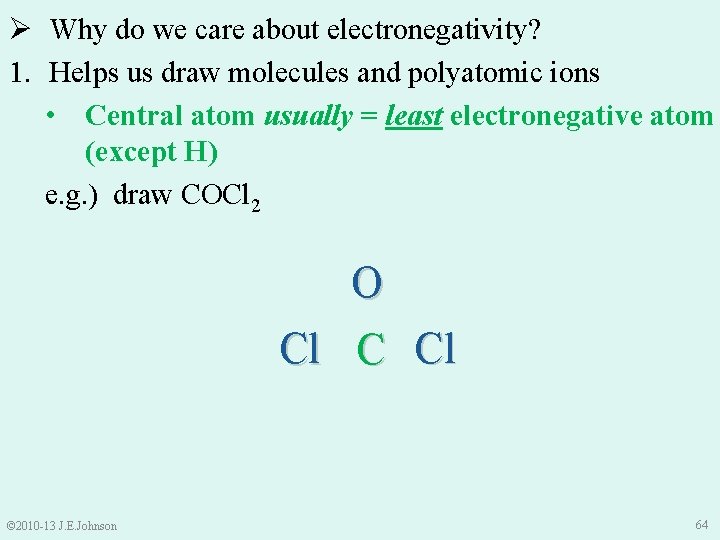
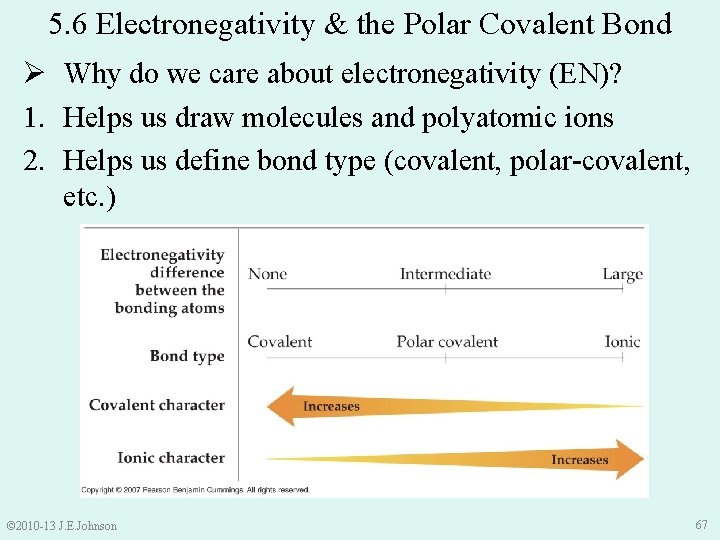
Ø Why do we care about electronegativity? 1. Helps us draw molecules and polyatomic ions • Central atom usually = least electronegative atom (except H) e. g. ) draw COCl 2 O Cl C Cl © 2010 -13 J. E. Johnson 64

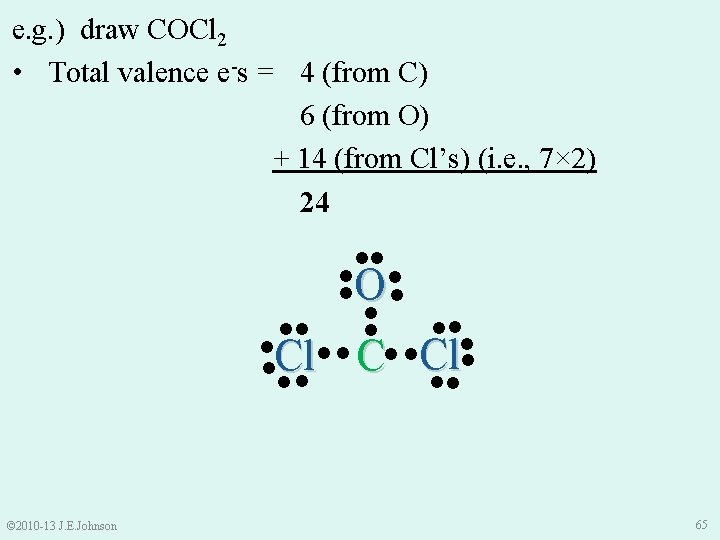
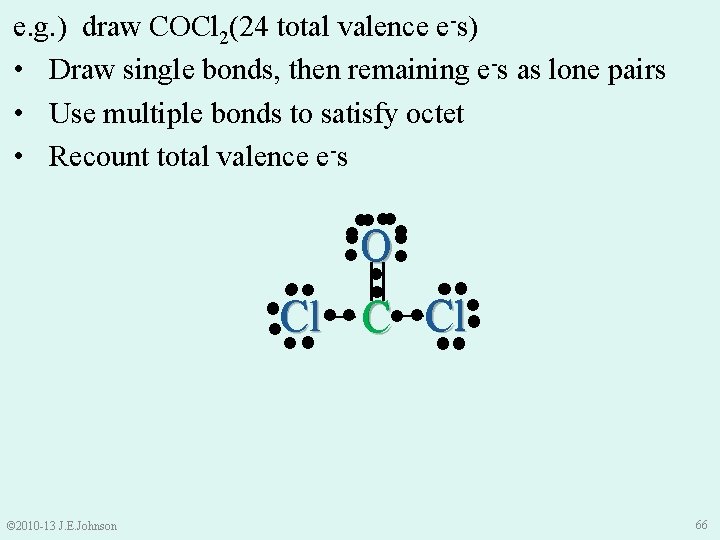
e. g. ) draw COCl 2 • Total valence e-s = 4 (from C) 6 (from O) + 14 (from Cl’s) (i. e. , 7× 2) 24 ●● ● ● ●O ● ● ●● ●● ● ● ●Cl● ● C ●Cl ●● ●● © 2010 -13 J. E. Johnson 65

e. g. ) draw COCl 2(24 total valence e-s) • Draw single bonds, then remaining e-s as lone pairs • Use multiple bonds to satisfy octet • Recount total valence e-s ●● ●● ● ● O● ● ●● ●● ● ● Cl C ●Cl ●● ●● = _ _ © 2010 -13 J. E. Johnson 66

5. 6 Electronegativity & the Polar Covalent Bond Ø Why do we care about electronegativity (EN)? 1. Helps us draw molecules and polyatomic ions 2. Helps us define bond type (covalent, polar-covalent, etc. ) © 2010 -13 J. E. Johnson 67

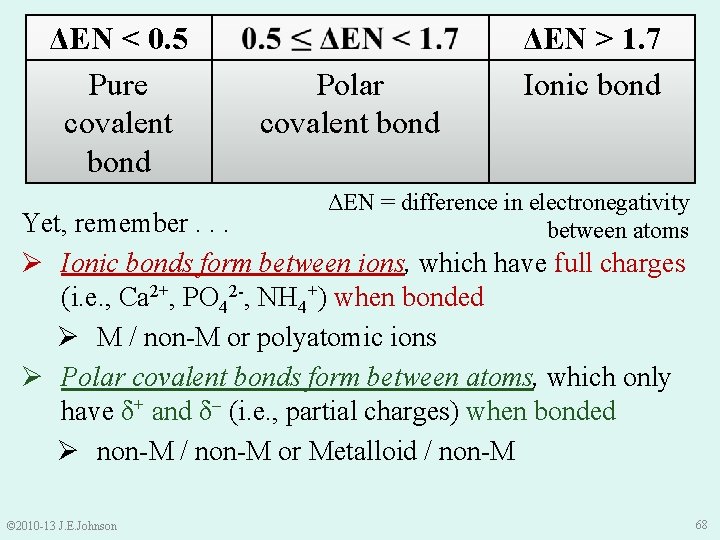
ΔEN < 0. 5 Pure covalent bond Polar covalent bond ΔEN > 1. 7 Ionic bond ΔEN = difference in electronegativity between atoms Yet, remember. . . Ø Ionic bonds form between ions, which have full charges (i. e. , Ca 2+, PO 42 -, NH 4+) when bonded Ø M / non-M or polyatomic ions Ø Polar covalent bonds form between atoms, which only have δ+ and δ– (i. e. , partial charges) when bonded Ø non-M / non-M or Metalloid / non-M © 2010 -13 J. E. Johnson 68

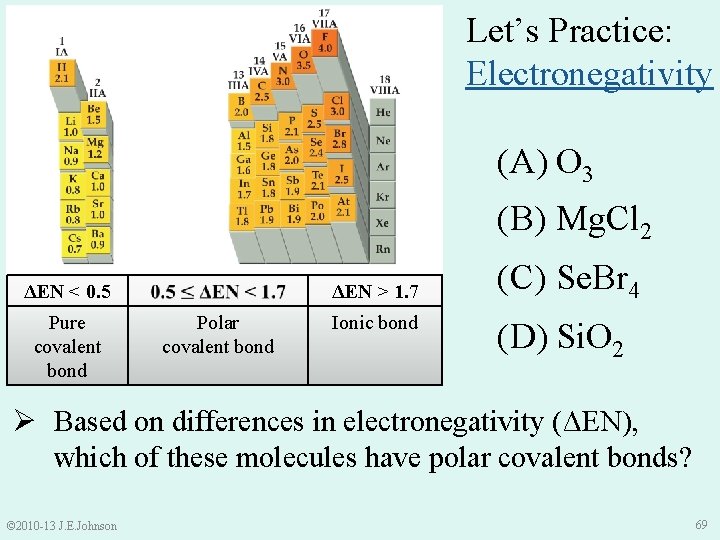
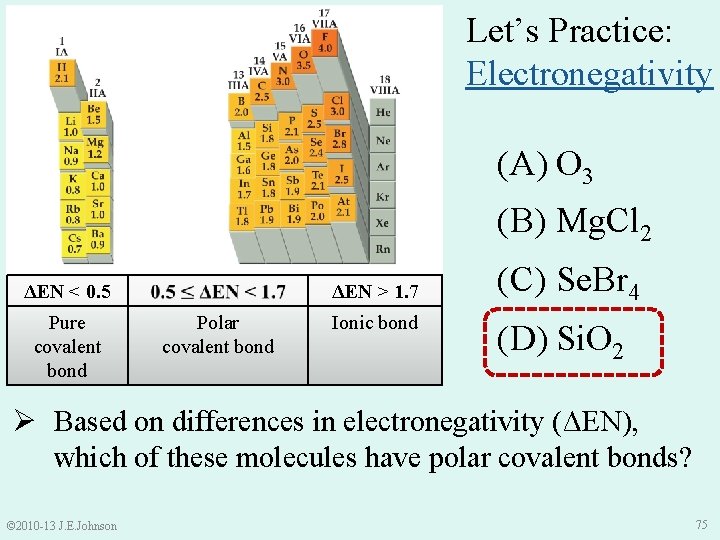
Let’s Practice: Electronegativity (A) O 3 (B) Mg. Cl 2 ΔEN < 0. 5 Pure covalent bond ΔEN > 1. 7 Polar covalent bond Ionic bond (C) Se. Br 4 (D) Si. O 2 Ø Based on differences in electronegativity (ΔEN), which of these molecules have polar covalent bonds? © 2010 -13 J. E. Johnson 69

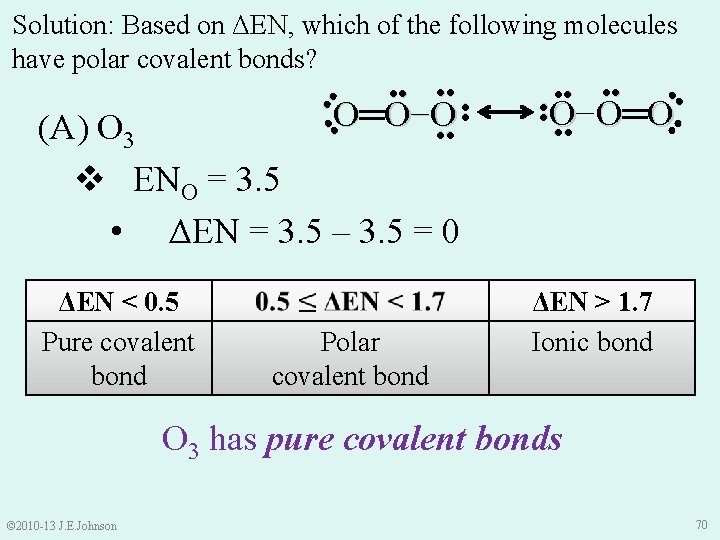
Solution: Based on ΔEN, which of the following molecules have polar covalent bonds? ●● ●● O− O═ O ΔEN > 1. 7 Ionic bond ●● ●● ΔEN < 0. 5 Pure covalent bond ●● ●● O═O−O (A) O 3 v ENO = 3. 5 • ΔEN = 3. 5 – 3. 5 = 0 Polar covalent bond ●● ●● ●● O 3 has pure covalent bonds © 2010 -13 J. E. Johnson 70

Solution: Based on ΔEN, which of the following molecules have polar covalent bonds? (B) Mg. Cl 2 v ENMg = 1. 2 ENCl = 3. 0 • ΔEN = 3. 0 – 1. 2 = 1. 8 ΔEN < 0. 5 Pure covalent bond Polar covalent bond ΔEN > 1. 7 Ionic bond Mg. Cl 2 has ionic bonds © 2010 -13 J. E. Johnson 71

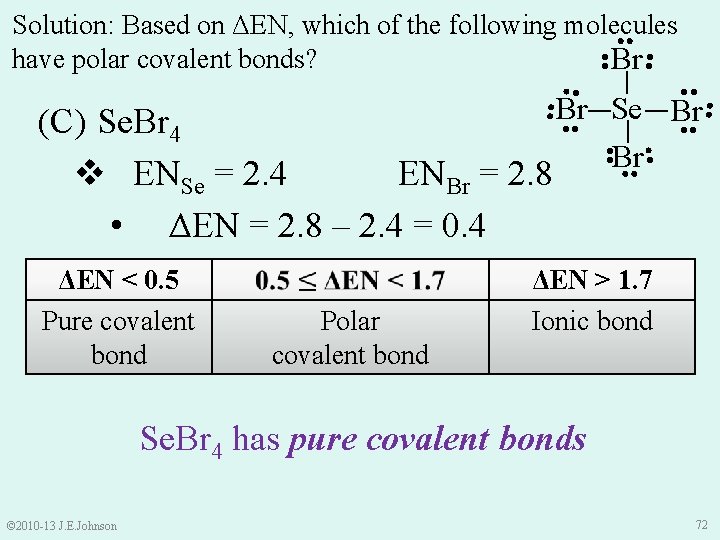
● ● Solution: Based on ΔEN, which of the following molecules ● ● have polar covalent bonds? ● Br ● ● ● _ _ Br Se Br ● ● ● ● Polar covalent bond ● ● ΔEN < 0. 5 Pure covalent bond Br ● ● (C) Se. Br 4 v ENSe = 2. 4 ENBr = 2. 8 • ΔEN = 2. 8 – 2. 4 = 0. 4 ● ● ΔEN > 1. 7 Ionic bond Se. Br 4 has pure covalent bonds © 2010 -13 J. E. Johnson 72

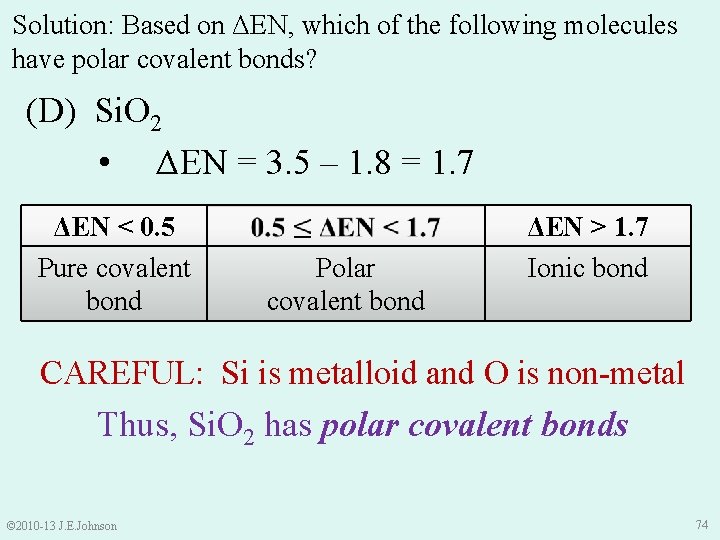
Solution: Based on ΔEN, which of the following molecules have polar covalent bonds? (D) Si. O 2 v ENSi = 1. 8 ENO = 3. 5 • ΔEN = 3. 5 – 1. 8 = 1. 7 ΔEN < 0. 5 Pure covalent bond Polar covalent bond ΔEN > 1. 7 Ionic bond Si. O 2 has polar covalent or ionic bonds? ? © 2010 -13 J. E. Johnson 73

Solution: Based on ΔEN, which of the following molecules have polar covalent bonds? (D) Si. O 2 • ΔEN = 3. 5 – 1. 8 = 1. 7 ΔEN < 0. 5 Pure covalent bond Polar covalent bond ΔEN > 1. 7 Ionic bond CAREFUL: Si is metalloid and O is non-metal Thus, Si. O 2 has polar covalent bonds © 2010 -13 J. E. Johnson 74

Let’s Practice: Electronegativity (A) O 3 (B) Mg. Cl 2 ΔEN < 0. 5 Pure covalent bond ΔEN > 1. 7 Polar covalent bond Ionic bond (C) Se. Br 4 (D) Si. O 2 Ø Based on differences in electronegativity (ΔEN), which of these molecules have polar covalent bonds? © 2010 -13 J. E. Johnson 75

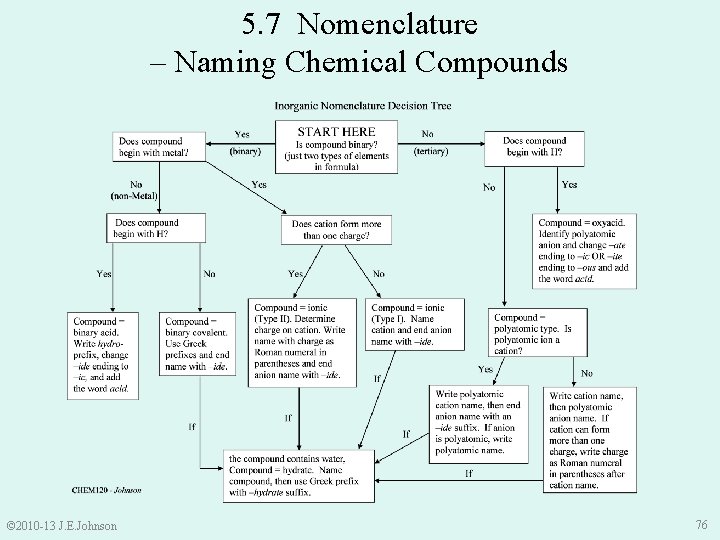
5. 7 Nomenclature – Naming Chemical Compounds © 2010 -13 J. E. Johnson 76

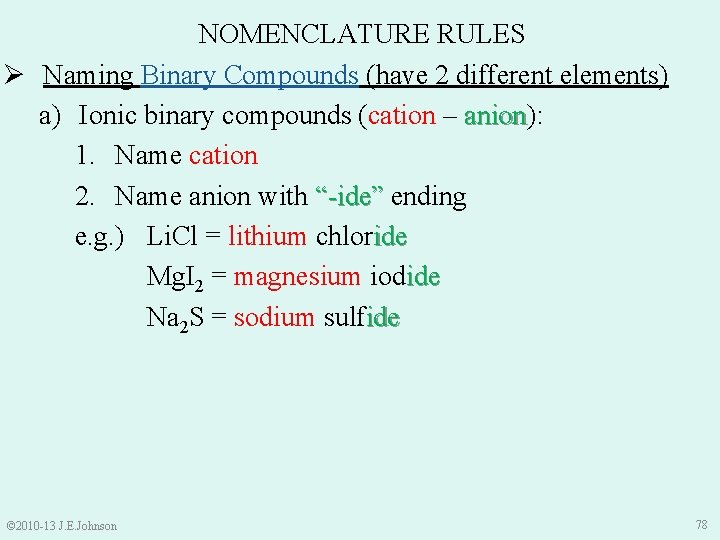
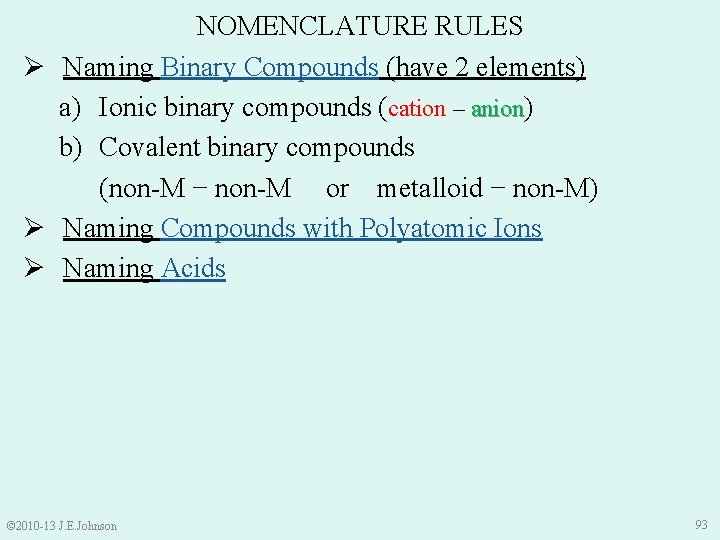
NOMENCLATURE RULES Ø Naming Binary Compounds (have 2 different elements) a) Ionic binary compounds (cation – anion): anion 1. Name cation 2. Name anion with “-ide” ending e. g. ) Li. Cl = lithium chloride Mg. I 2 = magnesium iodide Na 2 S = sodium sulfide © 2010 -13 J. E. Johnson 78

NOMENCLATURE RULES Ø Naming Binary Compounds (have 2 different elements) a) Ionic binary compounds (cation – anion): anion 1. Name cation • If cation is transition, lanthanide, or actinide metal • use Roman numeral to indicate “charge” 2. Name anion with “-ide” ending e. g. ) Fe. Cl 3 = iron(III) chloride © 2010 -13 J. E. Johnson 80

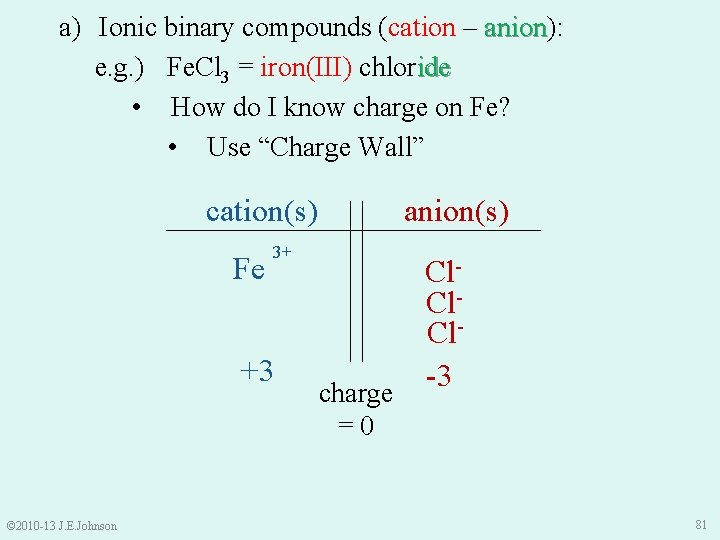
a) Ionic binary compounds (cation – anion): anion e. g. ) Fe. Cl 3 = iron(III) chloride • How do I know charge on Fe? • Use “Charge Wall” anion(s) cation(s) 3+ Fe? +3 © 2010 -13 J. E. Johnson charge =0 Cl. Cl-3 81

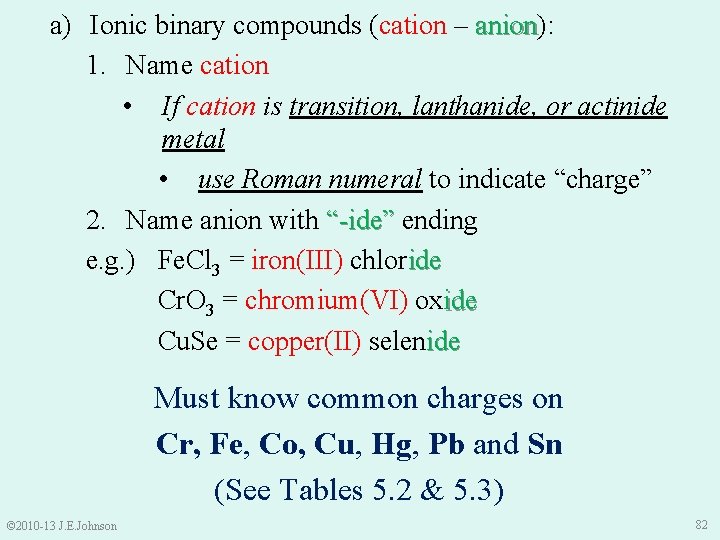
a) Ionic binary compounds (cation – anion): anion 1. Name cation • If cation is transition, lanthanide, or actinide metal • use Roman numeral to indicate “charge” 2. Name anion with “-ide” ending e. g. ) Fe. Cl 3 = iron(III) chloride Cr. O 3 = chromium(VI) oxide Cu. Se = copper(II) selenide Must know common charges on Cr, Fe, Co, Cu, Hg, Pb and Sn (See Tables 5. 2 & 5. 3) © 2010 -13 J. E. Johnson 82

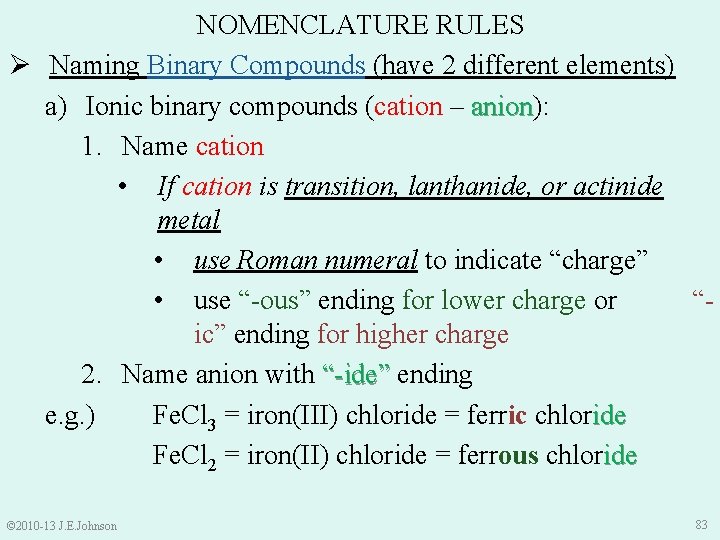
NOMENCLATURE RULES Ø Naming Binary Compounds (have 2 different elements) a) Ionic binary compounds (cation – anion): anion 1. Name cation • If cation is transition, lanthanide, or actinide metal • use Roman numeral to indicate “charge” • use “-ous” ending for lower charge or “ic” ending for higher charge 2. Name anion with “-ide” ending e. g. ) Fe. Cl 3 = iron(III) chloride = ferric chloride Fe. Cl 2 = iron(II) chloride = ferrous chloride © 2010 -13 J. E. Johnson 83

a) Ionic binary compounds (cation – anion): anion e. g. ) • Fe. Cl 3 = iron(III) chloride = ferric chloride • Fe. Cl 2 = iron(II) chloride = ferrous chloride • Sn. O 2 = tin(IV) oxide = stannic oxide • Sn. O = tin(II) oxide = stannous oxide • Cu. Se = copper(II) selenide = cupric selenide • Cu 2 Se = copper(I) selenide = cuprous selenide © 2010 -13 J. E. Johnson 84

Ø Naming Binary Compounds (have 2 different elements) a) Ionic binary compounds (cation – anion) b) Covalent binary compounds (non-M − non-M or Metalloid − non-M) • Require using Greek prefixes: © 2010 -13 J. E. Johnson 85

NOMENCLATURE RULES Ø Naming Binary Compounds (have 2 different elements) a) Ionic binary compounds (cation – anion) anion b) Covalent binary compounds (non -M − non-M or metalloid − non-M) 1. Name 1 st non-M using Greek prefix (except if only 1 atom) 2. Name 2 nd non-M using Greek prefix and “-ide” ending © 2010 -13 J. E. Johnson 86

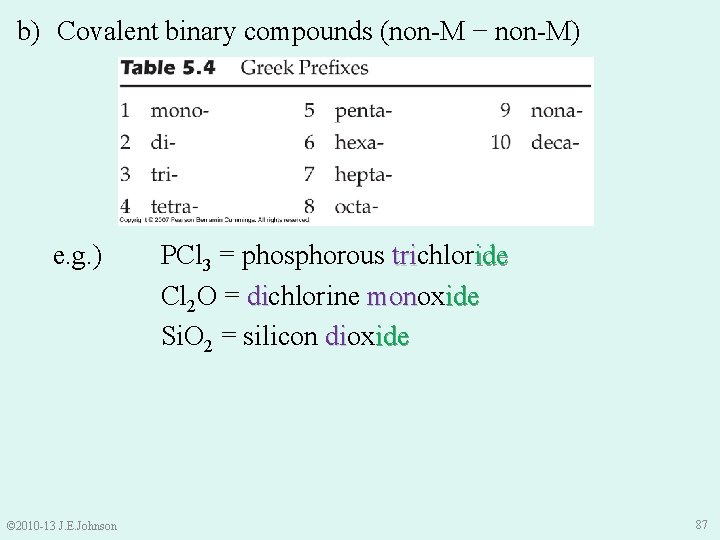
b) Covalent binary compounds (non-M − non-M) e. g. ) © 2010 -13 J. E. Johnson PCl 3 = phosphorous trichlor ide tri Cl 2 O = dichlorine monox di mon ide Si. O 2 = silicon diox di ide 87

Let’s Practice together: Nomenclature Ø Name the following compounds: (A) SO 3 (B) H 2 O (C) Al 2 S 3 (D) N 3 P 6 (E) Ca. C 2 © 2010 -13 J. E. Johnson 88

Solution: Name the following compounds: (A) • (B) • (C) • (D) • (E) • SO 3 Non-M – Non-M → sulfur trioxide H 2 O Non-M – Non-M → dihydrogen monoxide Al 2 S 3 cation – anion → aluminum sulfide N 3 P 6 Non-M – Non-M → trinitrogen hexaphosphide Ca. C 2 cation – anion → calcium carbide © 2010 -13 J. E. Johnson 89

NOMENCLATURE RULES Ø Naming Binary Compounds (have 2 elements) a) Ionic binary compounds (cation – anion) b) Covalent binary compounds (non-M − non-M or metalloid − non-M) Ø Naming Compounds with Polyatomic Ions 1. Name cation or polyatomic cation name 2. Name anion or polyatomic anion name © 2010 -13 J. E. Johnson 90

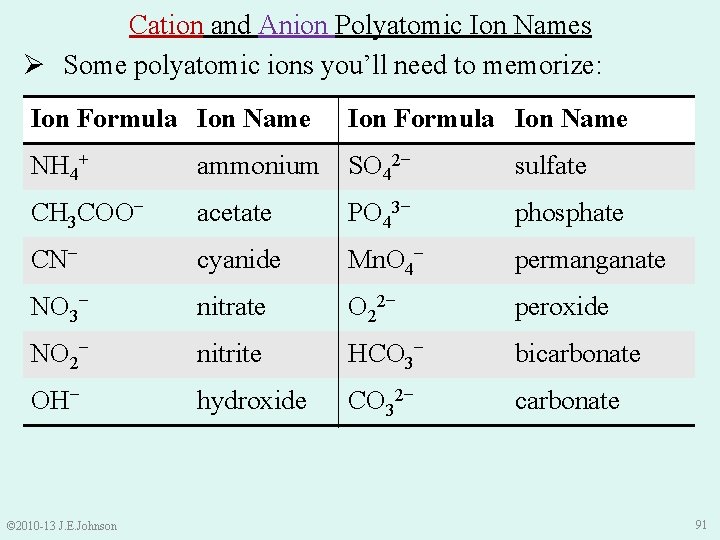
Cation and Anion Polyatomic Ion Names Ø Some polyatomic ions you’ll need to memorize: Ion Formula Ion Name NH 4+ ammonium SO 42− sulfate CH 3 COO− acetate PO 43− phosphate CN− cyanide Mn. O 4− permanganate NO 3− nitrate O 22− peroxide NO 2− nitrite HCO 3− bicarbonate OH− hydroxide CO 32− carbonate © 2010 -13 J. E. Johnson 91

Ø Naming Compounds with Polyatomic Ions 1. Name cation or use cation name 2. Name anion or use anion name e. g. ) KCN = potassium cyanide Fe. PO 4 = iron(III) phosphate NH 4 OH = ammonium hydroxide © 2010 -13 J. E. Johnson 92

NOMENCLATURE RULES Ø Naming Binary Compounds (have 2 elements) a) Ionic binary compounds (cation – anion) b) Covalent binary compounds (non-M − non-M or metalloid − non-M) Ø Naming Compounds with Polyatomic Ions Ø Naming Acids © 2010 -13 J. E. Johnson 93

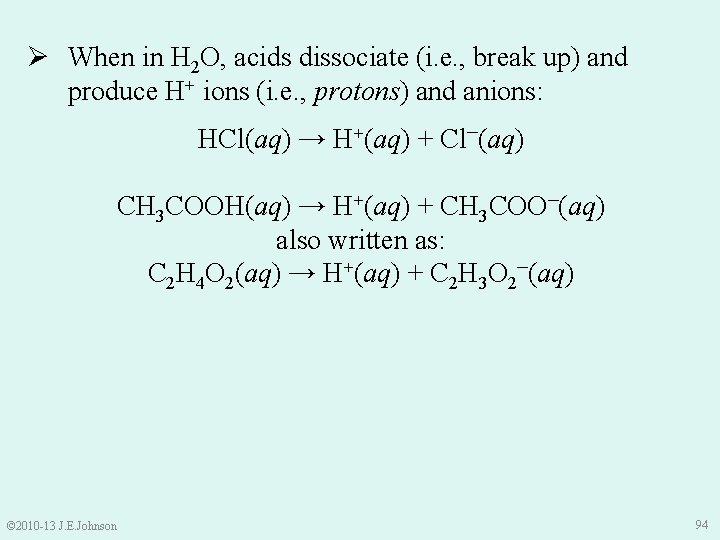
Ø When in H 2 O, acids dissociate (i. e. , break up) and produce H+ ions (i. e. , protons) and anions: HCl(aq) → H+(aq) + Cl–(aq) CH 3 COOH(aq) → H+(aq) + CH 3 COO–(aq) also written as: C 2 H 4 O 2(aq) → H+(aq) + C 2 H 3 O 2–(aq) © 2010 -13 J. E. Johnson 94

Ø Naming Acids a) Without oxygen 1. Use “hydro-” prefix 2. Name anion using “-ic” ending 3. Put “acid” at end of whole name e. g. ) © 2010 -13 J. E. Johnson HCl (aq) = hydrochloric acid HCN (aq) = hydrocyanic acid H 2 S (aq) = hydrosulfuric acid 95

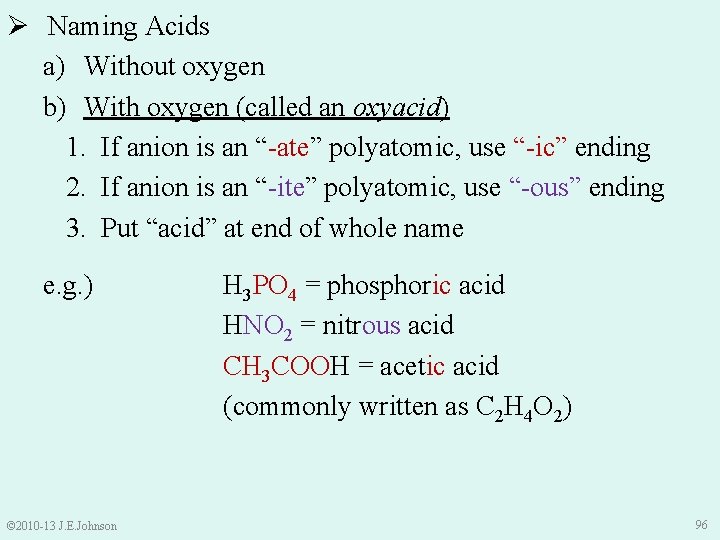
Ø Naming Acids a) Without oxygen b) With oxygen (called an oxyacid) 1. If anion is an “-ate” polyatomic, use “-ic” ending 2. If anion is an “-ite” polyatomic, use “-ous” ending 3. Put “acid” at end of whole name e. g. ) © 2010 -13 J. E. Johnson H 3 PO 4 = phosphoric acid HNO 2 = nitrous acid CH 3 COOH = acetic acid (commonly written as C 2 H 4 O 2) 96

Let’s Practice together: Nomenclature Ø Name the following acids: (A) H 2 SO 4(aq) (B) H 2 S(aq) (C) H 2 CO 3(aq) (D) HF(aq) © 2010 -13 J. E. Johnson aq = aqueous (i. e. , in H 2 O) 97

Solution: Name the following acids: (A) H 2 SO 4(aq) • • © 2010 -13 J. E. Johnson O present – is an oxyacid SO 42 - is “sulfate” anion = “-ic” ending → sulfuric acid 98

Solution: Name the following acids: (A) H 2 SO 4(aq) → sulfuric acid (B) H 2 S(aq) • No O present – Not an oxyacid • Use “hydro” prefix → hydrosulfuric acid © 2010 -13 J. E. Johnson 99

Solution: Name the following acids: (A) H 2 SO 4(aq) → sulfuric acid (B) H 2 S(aq) → hydrosulfuric acid (C) H 2 CO 3(aq) • O present – Is an oxyacid • CO 32 - is “carbonate” anion = “-ic” ending → carbonic acid © 2010 -13 J. E. Johnson 100

Solution: Name the following acids: (A) (B) (C) (D) • H 2 SO 4(aq) → sulfuric acid H 2 S(aq) → hydrosulfuric acid H 2 CO 3(aq) → carbonic acid HF(aq) No O present – Not an oxyacid → hydrofluoric acid © 2010 -13 J. E. Johnson 101

** DON’T FORGET ** Take the Chapter 5 Quiz - it’s available on Web. CT after 7 pm tonight! © 2010 -13 J. E. Johnson 102