Please turn cell phones off http www snnrdr






















- Slides: 102

Please turn cell phones off http: //www. snn-rdr. ca/old/nov 99/moleday. html Chapter 9 – Stoichiometry & the Mole www. nisdtx. org/120820731141731840/ (Worksheets available on Web. CT) © 2010 -13 J. E. Johnson 1

9. 1 Stoichiometry – What is it? Stoichiometry: Ø relative relationships (ratios) of reactants and products in reactions (rxns) Ø “how much” reactants will generate “that much” products Ø can use it to predict quantities of reactants and products! © 2010 -13 J. E. Johnson 2

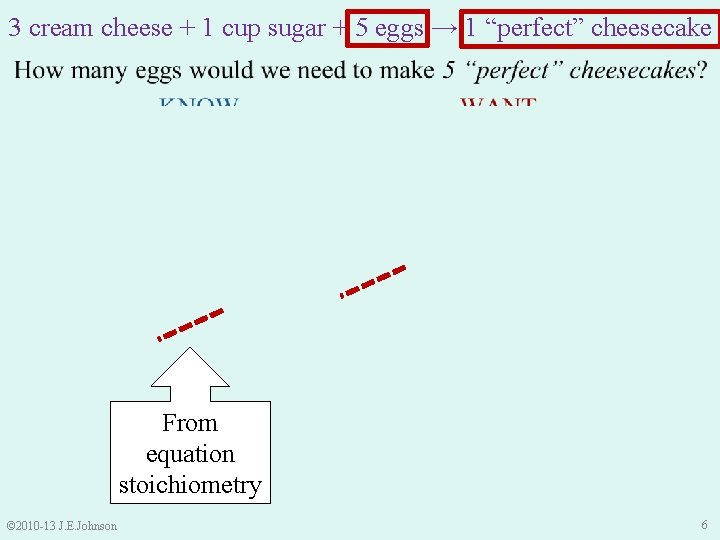
e. g. ) Let’s write an reaction equation for one “perfect” cheesecake: 3 cream cheese + 1 cup sugar + 5 eggs → 1 “perfect” cheesecake Ø What if we wanted to make 2 “perfect” cheesecakes? • Multiply all coefficients by “ 2”: 6 cream cheese + 2 cup sugar + 10 eggs → 2 “perfect” cheesecakes Ø How many eggs would we need to make 3 “perfect” cheesecakes? • Multiply all coefficients by “ 3”: 9 cream cheese + 3 cup sugar + 15 eggs → 3 “perfect” cheesecakes © 2010 -13 J. E. Johnson 4

9. 1 Stoichiometry – What is it? 3 cream cheese + 1 cup sugar + 5 eggs → 1 “perfect” cheesecake 6 cream cheese + 2 cup sugar + 10 eggs → 2 “perfect” cheesecakes 9 cream cheese + 3 cup sugar + 15 eggs → 3 “perfect” cheesecake • Multiply by coefficients to convert from 1 to 3 cheesecakes • Instead, let’s set this up like a unit conversion. . . © 2010 -13 J. E. Johnson 5

3 cream cheese + 1 cup sugar + 5 eggs → 1 “perfect” cheesecake From equation stoichiometry © 2010 -13 J. E. Johnson 6

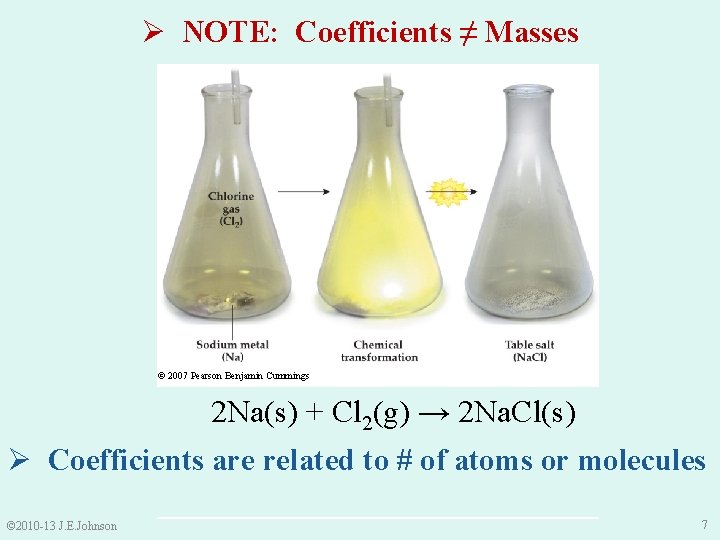
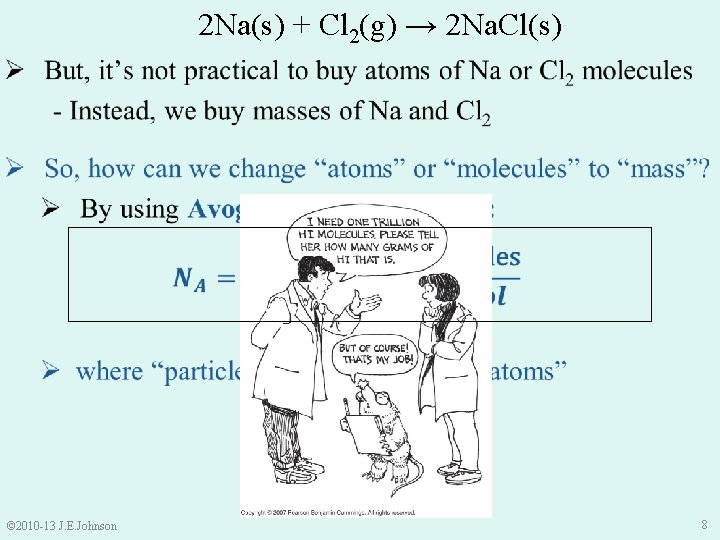
Ø NOTE: Coefficients ≠ Masses © 2007 Pearson Benjamin Cummings 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) Ø Coefficients are related to # of atoms or molecules © 2010 -13 J. E. Johnson 7

© 2010 -13 J. E. Johnson 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) 8

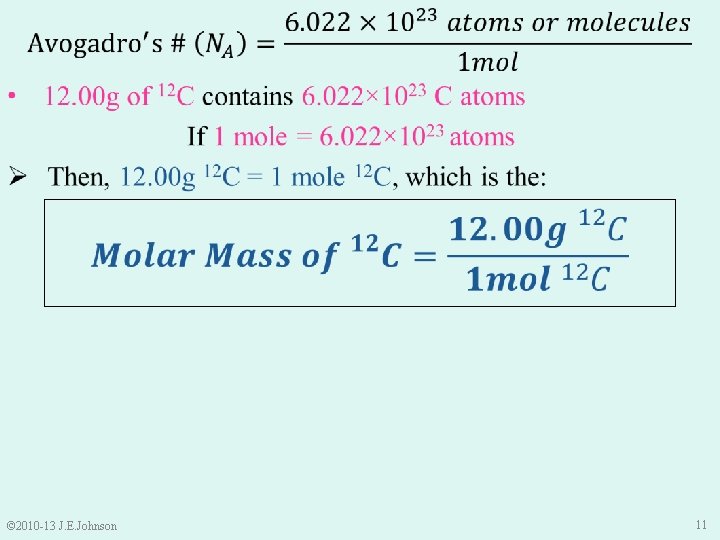
9. 2 The Mole (n) I came up with the “mol”! http: //www. snnrdr. ca/old/nov 99/moleday. html © 2010 -13 J. E. Johnson Wilhelm Ostwald 9

© 2010 -13 J. E. Johnson 10

© 2010 -13 J. E. Johnson 11

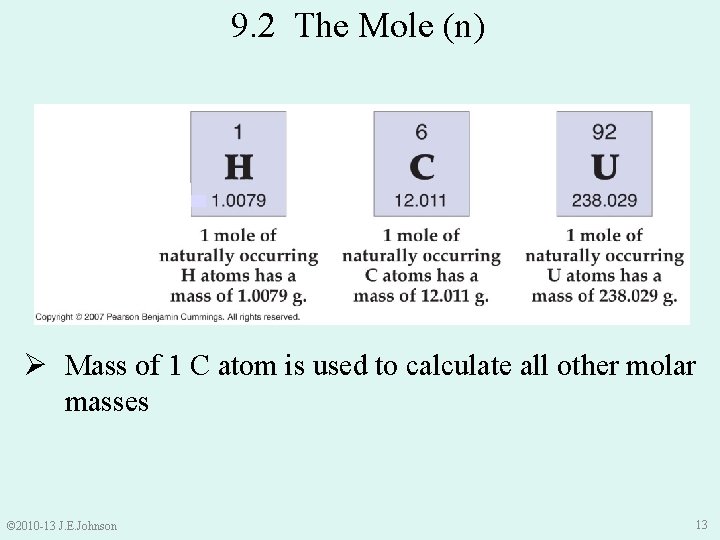
9. 2 The Mole (n) Ø Mass of 1 C atom is used to calculate all other molar masses © 2010 -13 J. E. Johnson 13

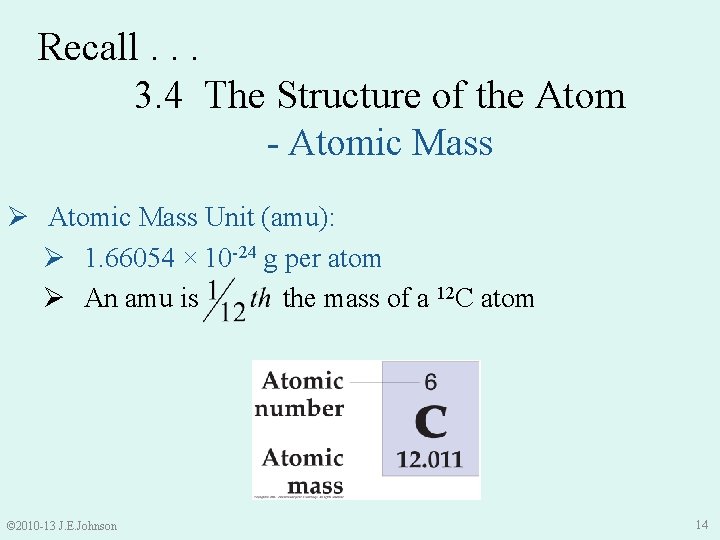
Recall. . . 3. 4 The Structure of the Atom - Atomic Mass Ø Atomic Mass Unit (amu): Ø 1. 66054 × 10 -24 g per atom Ø An amu is the mass of a 12 C atom © 2010 -13 J. E. Johnson 14

© 2010 -13 J. E. Johnson 15

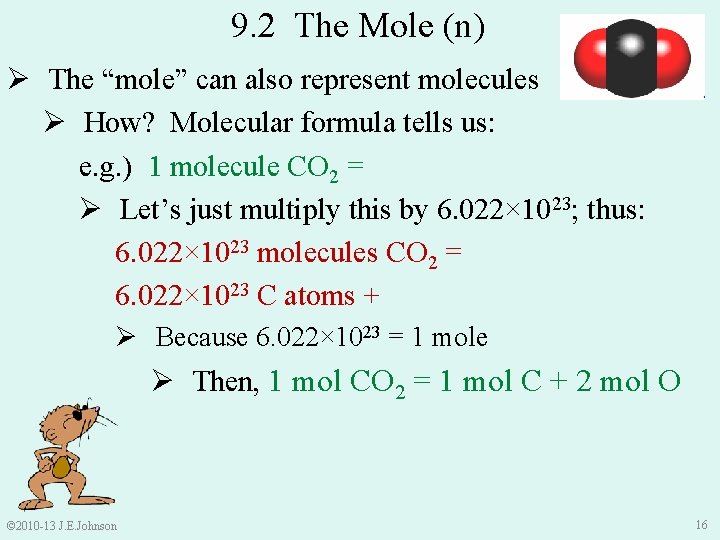
9. 2 The Mole (n) Ø The “mole” can also represent molecules Ø How? Molecular formula tells us: e. g. ) 1 molecule CO 2 = 1 C atom + 2 O atoms Ø Let’s just multiply this by 6. 022× 1023; thus: 6. 022× 1023 molecules CO 2 = 6. 022× 1023 C atoms + 2(6. 022× 1023) O atoms Ø Because 6. 022× 1023 = 1 mole Ø Then, 1 mol CO 2 = 1 mol C + 2 mol O © 2010 -13 J. E. Johnson 16

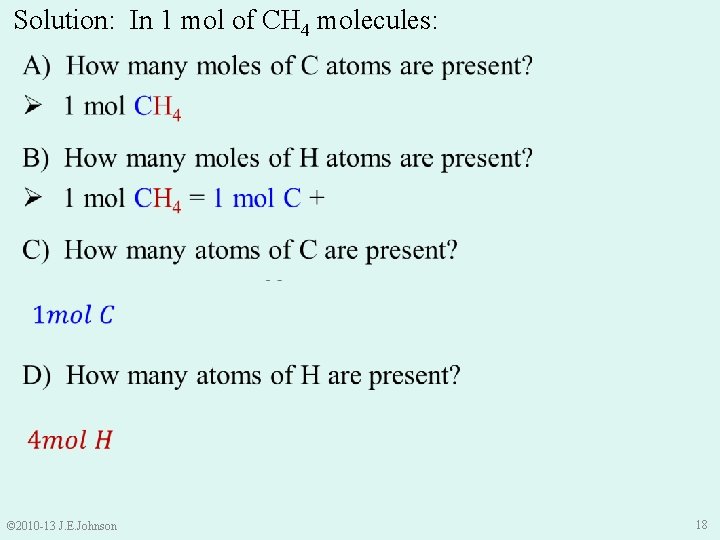
Let’s Practice: The Mole Ø In 1 mole of CH 4 molecules: A) How many moles of C atoms are present? B) How many moles of H atoms are present? C) How many atoms of C are present? D) How many atoms of H are present? © 2010 -13 J. E. Johnson 17

Solution: In 1 mol of CH 4 molecules: © 2010 -13 J. E. Johnson 18

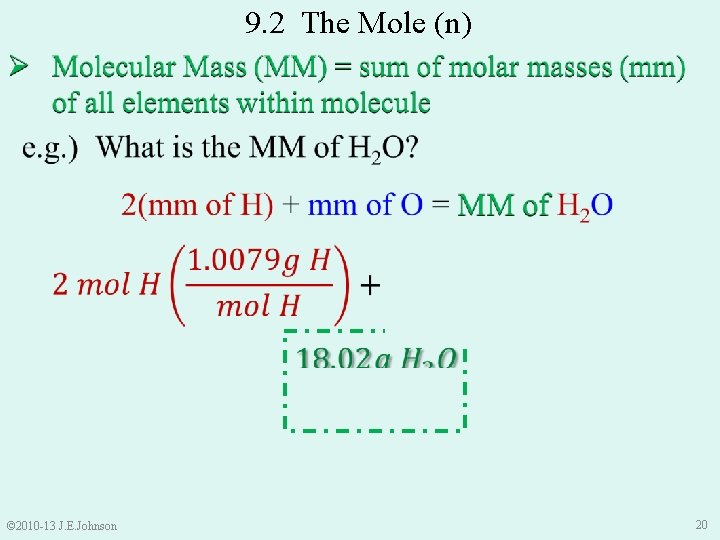
9. 2 The Mole (n) Ø Earlier, 1 mole of an element = Molar Mass (mm) of that element (i. e. , 1 mol C = 12. 011 g C) Ø Same idea for molecules: Ø 1 mole of a molecule = Molar Mass of that molecule (Molecular Mass) Molecular Mass (MM) = sum of all molar masses of elements within molecule © 2010 -13 J. E. Johnson 19

9. 2 The Mole (n) © 2010 -13 J. E. Johnson 20

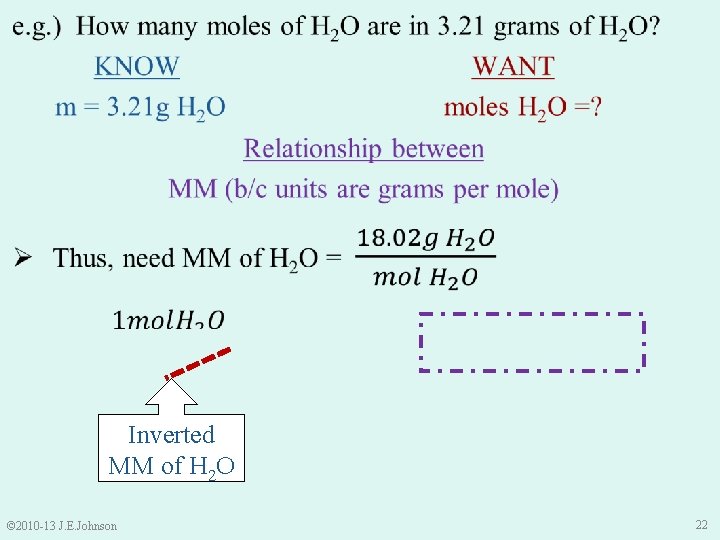
Inverted MM of H 2 O © 2010 -13 J. E. Johnson 22

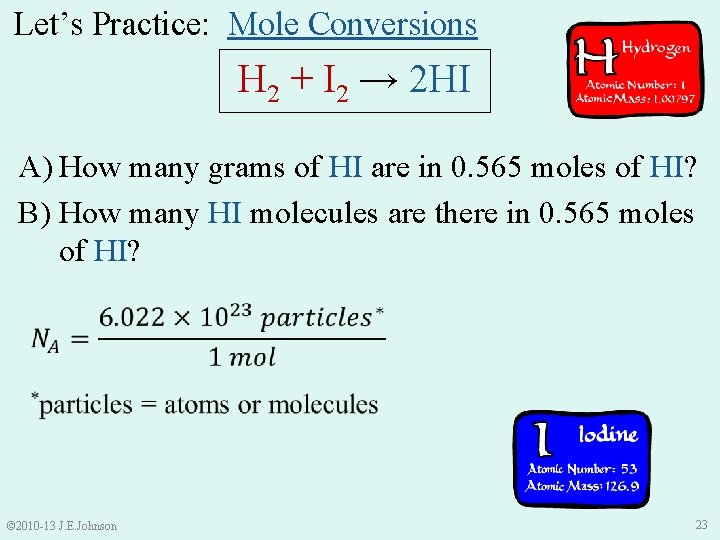
Let’s Practice: Mole Conversions H 2 + I 2 → 2 HI A) How many grams of HI are in 0. 565 moles of HI? B) How many HI molecules are there in 0. 565 moles of HI? © 2010 -13 J. E. Johnson 23

Solution: A) How many grams are in 0. 565 moles of HI? KNOW n = 0. 565 mol HI WANT grams HI = ? Relationship between MM of HI (b/c units are grams per mole) Ø Thus, need MM of HI: • MM of HI = mm of H + mm of I = 1. 0079 g/mol + 126. 904 g/mol = 127. 912 g/mol © 2010 -13 J. E. Johnson 24

Solution: A) How many grams are in 0. 565 moles of HI? © 2010 -13 J. E. Johnson 25

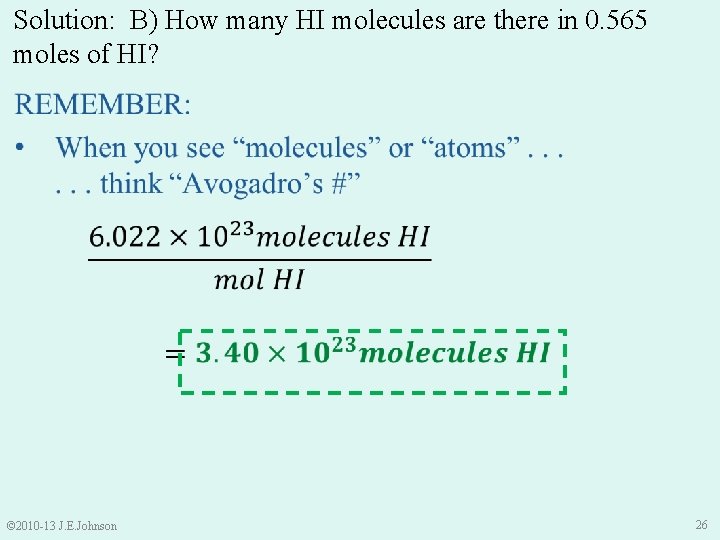
Solution: B) How many HI molecules are there in 0. 565 moles of HI? © 2010 -13 J. E. Johnson 26

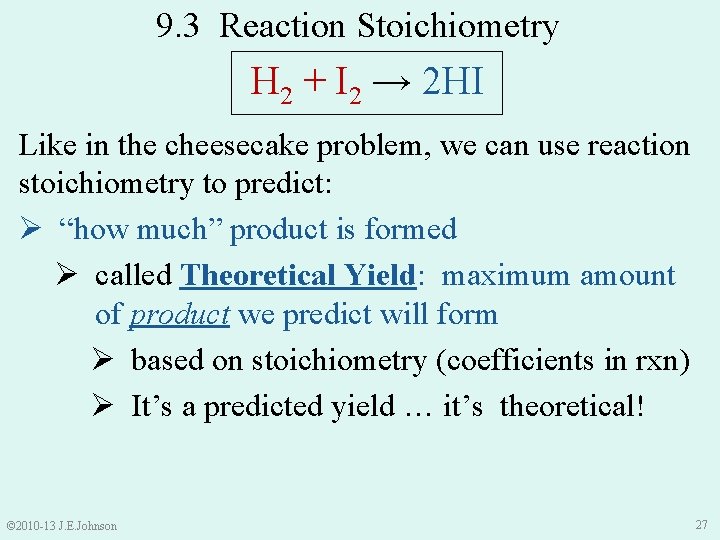
9. 3 Reaction Stoichiometry H 2 + I 2 → 2 HI Like in the cheesecake problem, we can use reaction stoichiometry to predict: Ø “how much” product is formed Ø called Theoretical Yield: maximum amount of product we predict will form Ø based on stoichiometry (coefficients in rxn) Ø It’s a predicted yield … it’s theoretical! © 2010 -13 J. E. Johnson 27

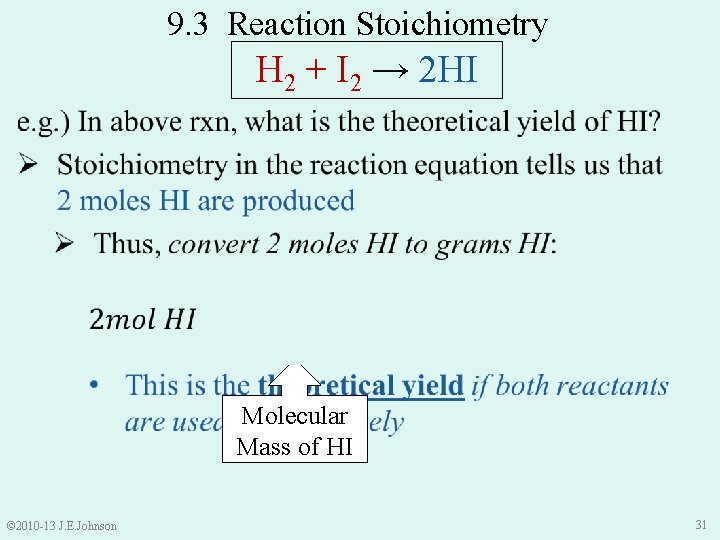
9. 3 Reaction Stoichiometry H 2 + I 2 → 2 HI Molecular Mass of HI © 2010 -13 J. E. Johnson 31

9. 3 Reaction Stoichiometry H 2 + I 2 → 2 HI Ø. . . again, as in the cheesecake problem, reaction stoichiometry helps us predict: Ø “how much” product is formed (Theoretical Yield) Ø “how much” reactant is needed Stoichiometry is so useful! © 2010 -13 J. E. Johnson 32

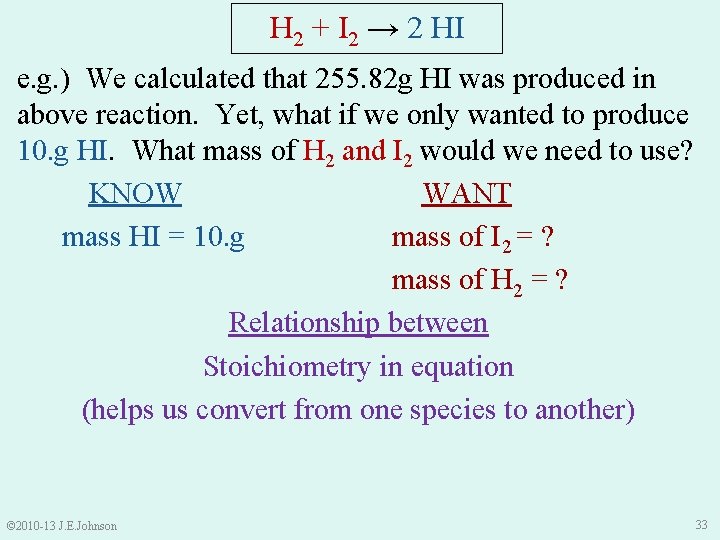
H 2 + I 2 → 2 HI e. g. ) We calculated that 255. 82 g HI was produced in above reaction. Yet, what if we only wanted to produce 10. g HI. What mass of H 2 and I 2 would we need to use? KNOW WANT mass HI = 10. g mass of I 2 = ? mass of H 2 = ? Relationship between Stoichiometry in equation (helps us convert from one species to another) © 2010 -13 J. E. Johnson 33

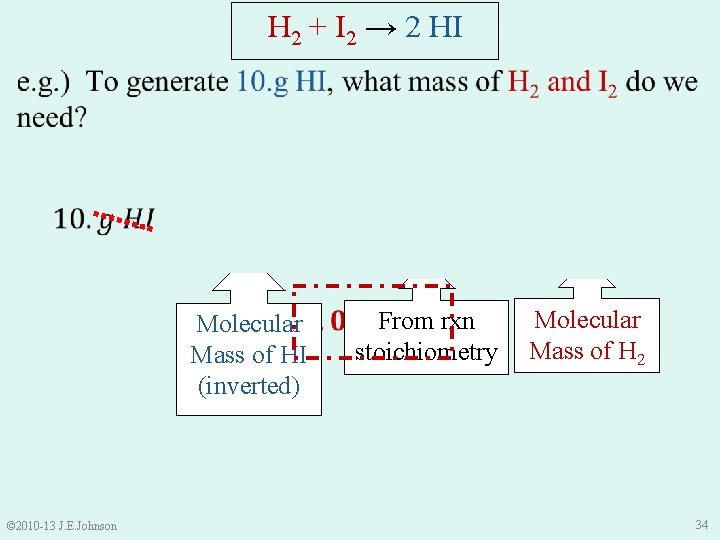
H 2 + I 2 → 2 HI Molecular Mass of HI (inverted) © 2010 -13 J. E. Johnson From rxn stoichiometry Molecular Mass of H 2 34

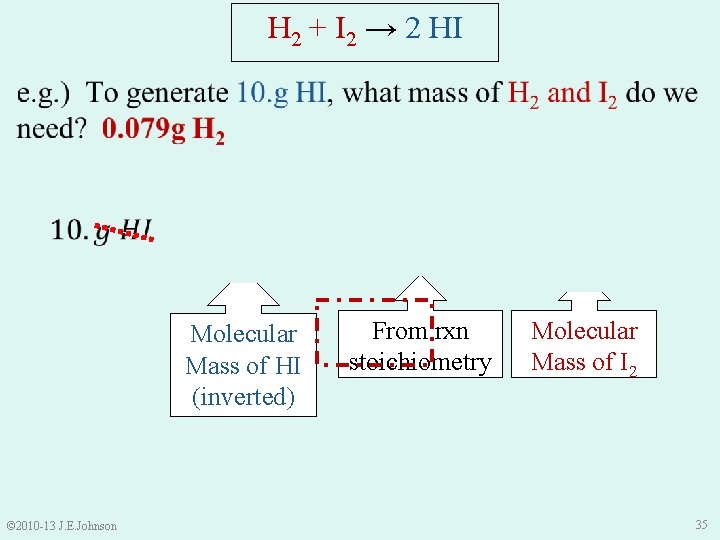
H 2 + I 2 → 2 HI Molecular Mass of HI (inverted) © 2010 -13 J. E. Johnson From rxn stoichiometry Molecular Mass of I 2 35

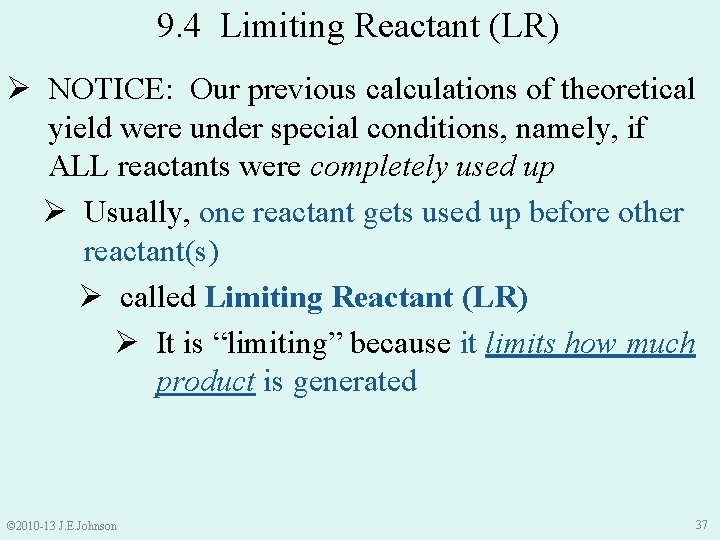
9. 4 Limiting Reactant (LR) Ø NOTICE: Our previous calculations of theoretical yield were under special conditions, namely, if ALL reactants were completely used up Ø Usually, one reactant gets used up before other reactant(s) Ø called Limiting Reactant (LR) Ø It is “limiting” because it limits how much product is generated © 2010 -13 J. E. Johnson 37

9. 4 Limiting Reactant (LR) e. g. ) How many bicycles can this man construct? Frame + 2 Tires → Bicycle Ø 3 (only has 3 bicycle frames) Ø Frame is the LR – it limits how many bikes can be made Ø Extra tires are excess reactant (what’s left over) © 2010 -13 J. E. Johnson 38

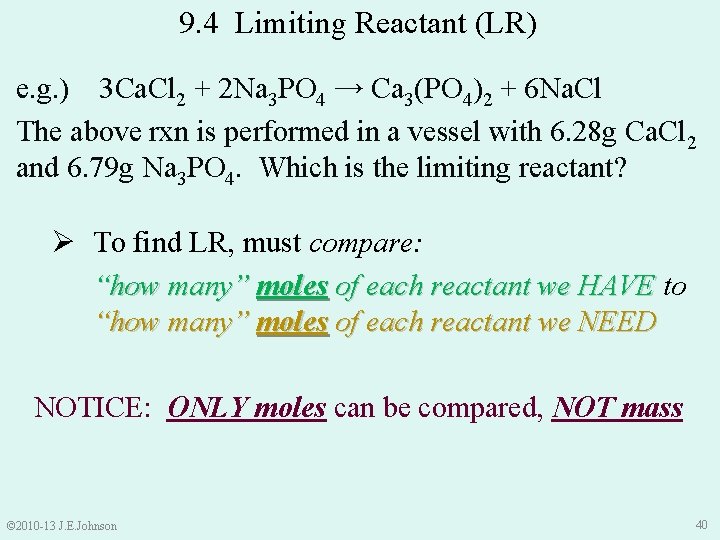
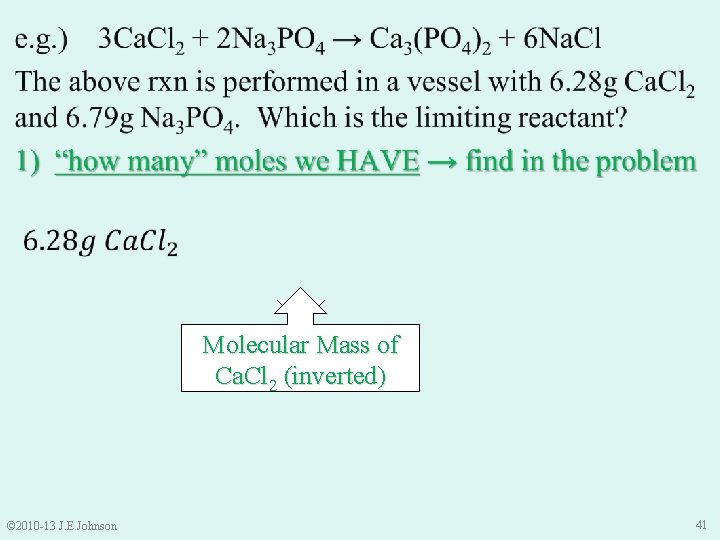
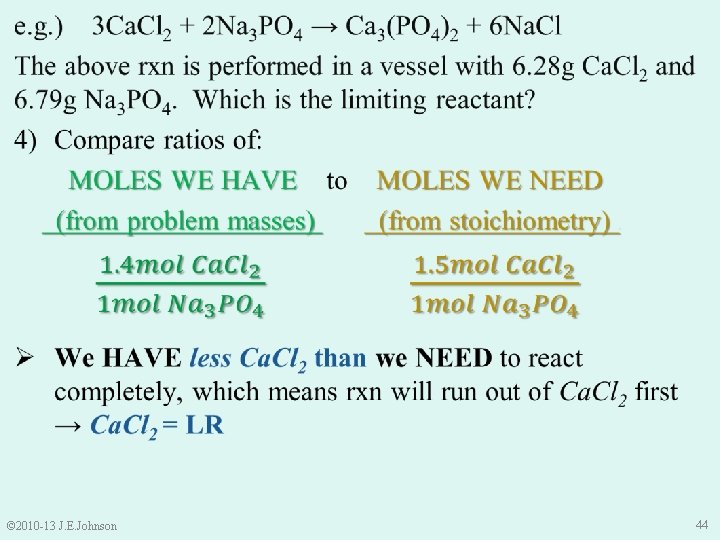
9. 4 Limiting Reactant (LR) e. g. ) 3 Ca. Cl 2 + 2 Na 3 PO 4 → Ca 3(PO 4)2 + 6 Na. Cl The above rxn is performed in a vessel with 6. 28 g Ca. Cl 2 and 6. 79 g Na 3 PO 4. Which is the limiting reactant? Ø Unlike the bicycles, cannot predict this by inspection Ø Excess reactant? Do not know Ø LR? Do not know © 2010 -13 J. E. Johnson 39

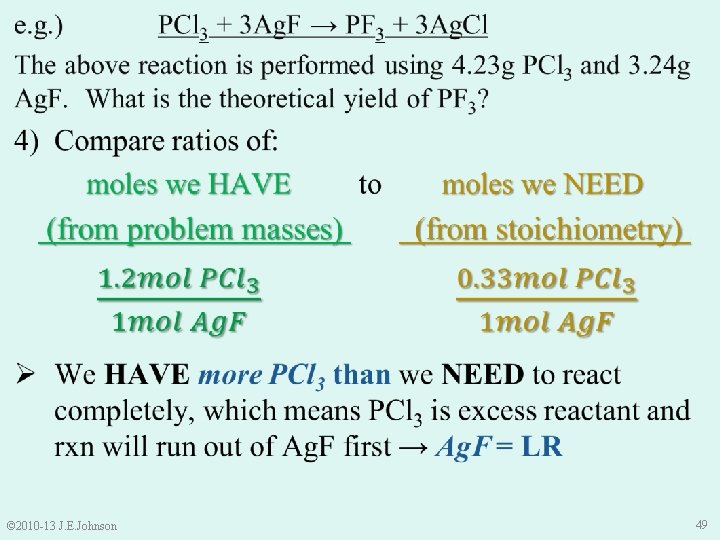
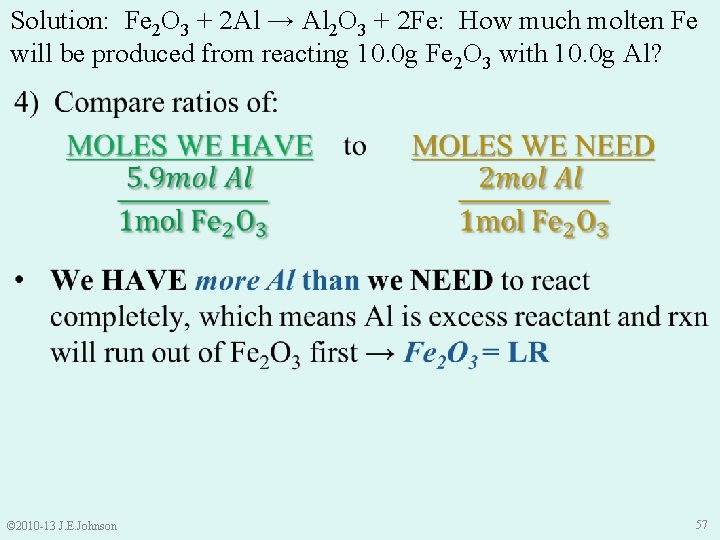
9. 4 Limiting Reactant (LR) e. g. ) 3 Ca. Cl 2 + 2 Na 3 PO 4 → Ca 3(PO 4)2 + 6 Na. Cl The above rxn is performed in a vessel with 6. 28 g Ca. Cl 2 and 6. 79 g Na 3 PO 4. Which is the limiting reactant? Ø To find LR, must compare: “how many” moles of each reactant we HAVE to “how many” moles of each reactant we NEED NOTICE: ONLY moles can be compared, NOT mass © 2010 -13 J. E. Johnson 40

Molecular Mass of Na 3 PO 4 (inverted) Molecular Mass of Ca. Cl 2 (inverted) © 2010 -13 J. E. Johnson 41

© 2010 -13 J. E. Johnson 42

© 2010 -13 J. E. Johnson 43

© 2010 -13 J. E. Johnson 44

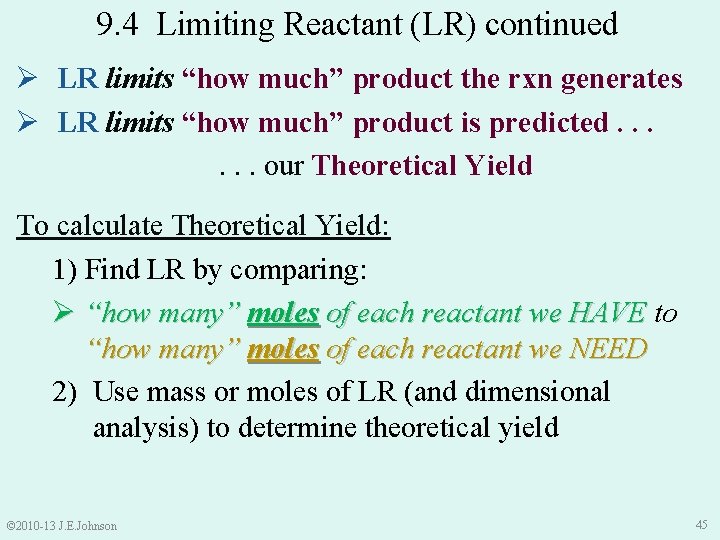
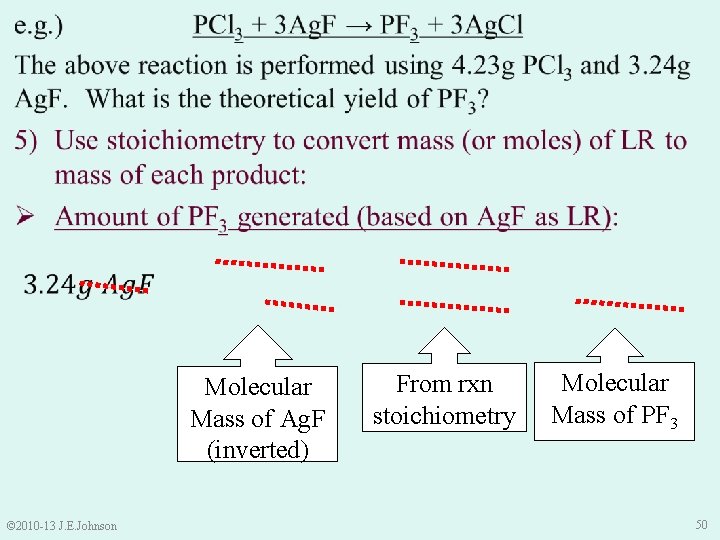
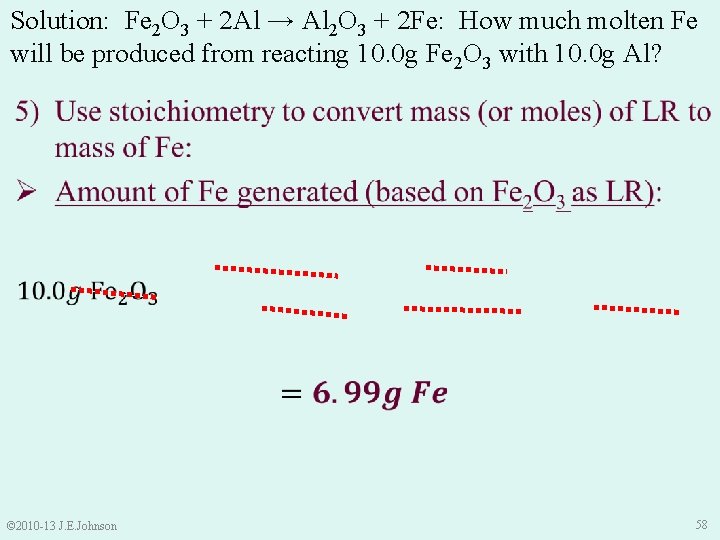
9. 4 Limiting Reactant (LR) continued Ø LR limits “how much” product the rxn generates Ø LR limits “how much” product is predicted. . . our Theoretical Yield To calculate Theoretical Yield: 1) Find LR by comparing: Ø “how many” moles of each reactant we HAVE to “how many” moles of each reactant we NEED 2) Use mass or moles of LR (and dimensional analysis) to determine theoretical yield © 2010 -13 J. E. Johnson 45

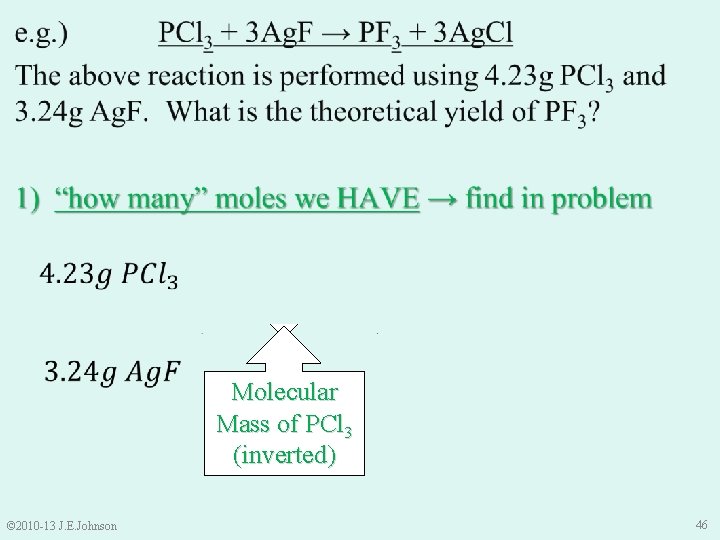
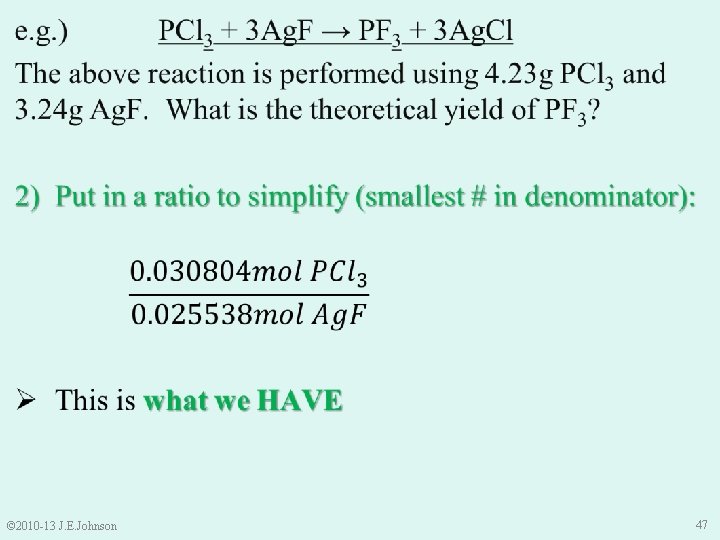
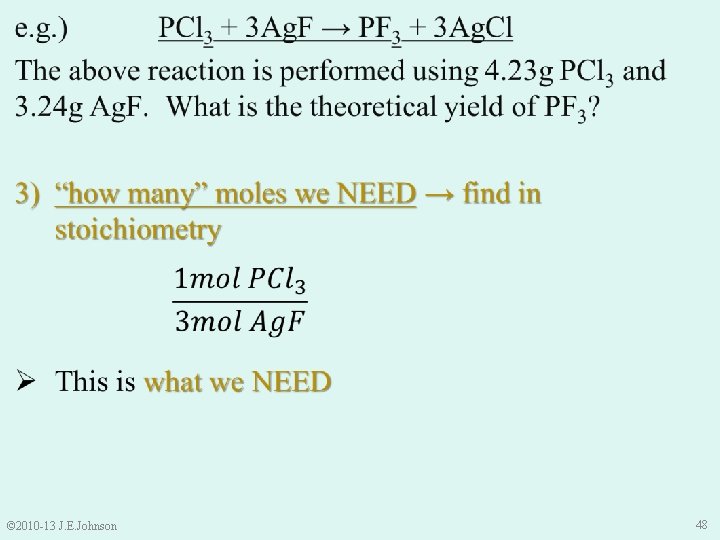
Molecular Mass of Ag. F (inverted) Molecular Mass of PCl 3 (inverted) © 2010 -13 J. E. Johnson 46

© 2010 -13 J. E. Johnson 47

© 2010 -13 J. E. Johnson 48

© 2010 -13 J. E. Johnson 49

Molecular Mass of Ag. F (inverted) © 2010 -13 J. E. Johnson From rxn stoichiometry Molecular Mass of PF 3 50

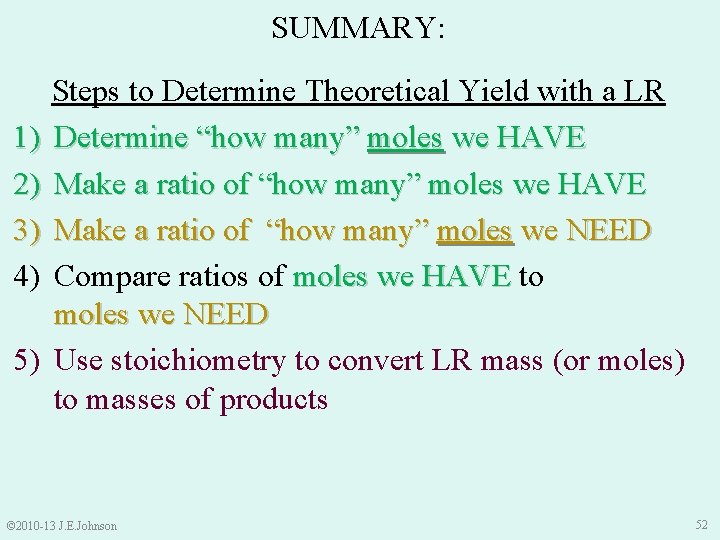
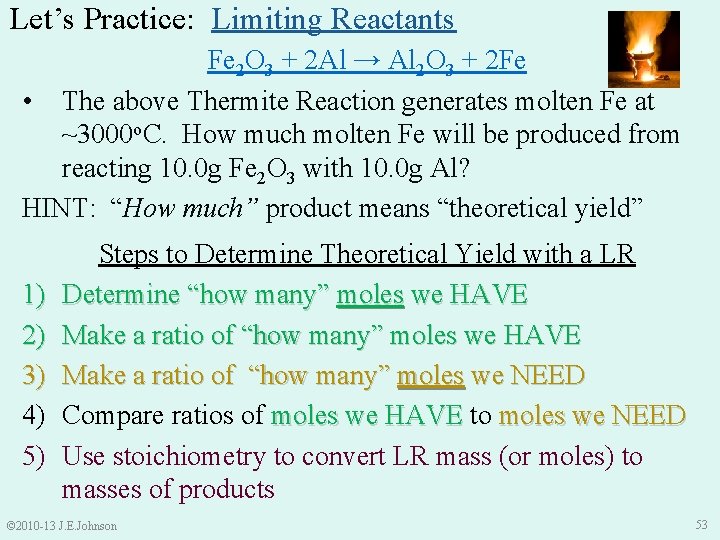
SUMMARY: 1) 2) 3) 4) 5) Steps to Determine Theoretical Yield with a LR Determine “how many” moles we HAVE Make a ratio of “how many” moles we NEED Compare ratios of moles we HAVE to moles we HAVE moles we NEED Use stoichiometry to convert LR mass (or moles) to masses of products © 2010 -13 J. E. Johnson 52

Let’s Practice: Limiting Reactants Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe • The above Thermite Reaction generates molten Fe at ~3000 o. C. How much molten Fe will be produced from reacting 10. 0 g Fe 2 O 3 with 10. 0 g Al? HINT: “How much” product means “theoretical yield” 1) 2) 3) 4) 5) Steps to Determine Theoretical Yield with a LR Determine “how many” moles we HAVE Make a ratio of “how many” moles we NEED Compare ratios of moles we HAVE to moles we HAVE moles we NEED Use stoichiometry to convert LR mass (or moles) to masses of products © 2010 -13 J. E. Johnson 53

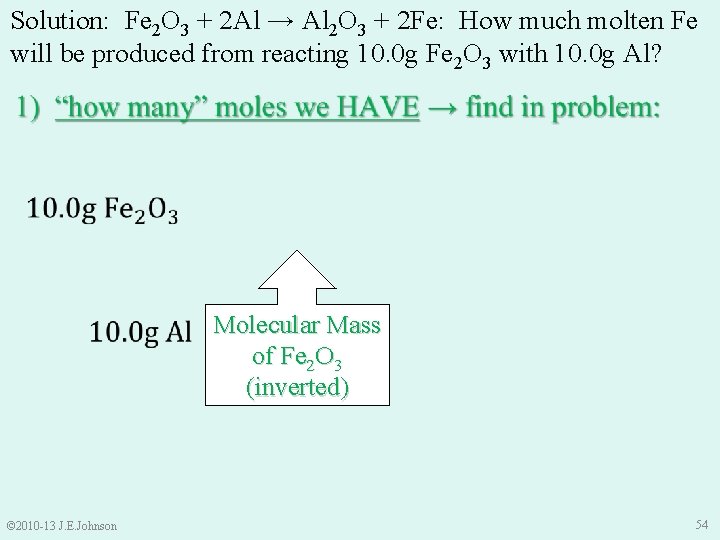
Solution: Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe: How much molten Fe will be produced from reacting 10. 0 g Fe 2 O 3 with 10. 0 g Al? Molar Mass of Al (inverted) Molecular Mass of Fe 2 O 3 (inverted) © 2010 -13 J. E. Johnson 54

Solution: Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe: How much molten Fe will be produced from reacting 10. 0 g Fe 2 O 3 with 10. 0 g Al? © 2010 -13 J. E. Johnson 55

Solution: Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe: How much molten Fe will be produced from reacting 10. 0 g Fe 2 O 3 with 10. 0 g Al? © 2010 -13 J. E. Johnson 56

Solution: Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe: How much molten Fe will be produced from reacting 10. 0 g Fe 2 O 3 with 10. 0 g Al? © 2010 -13 J. E. Johnson 57

Solution: Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe: How much molten Fe will be produced from reacting 10. 0 g Fe 2 O 3 with 10. 0 g Al? © 2010 -13 J. E. Johnson 58

© 2010 -13 J. E. Johnson 59

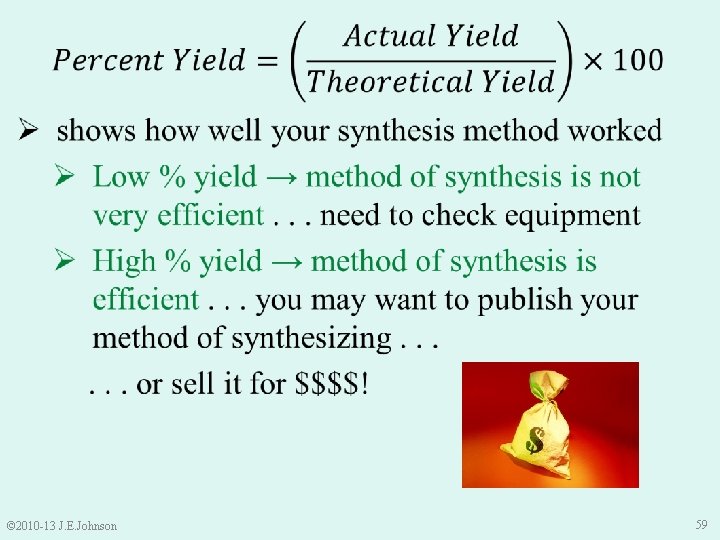
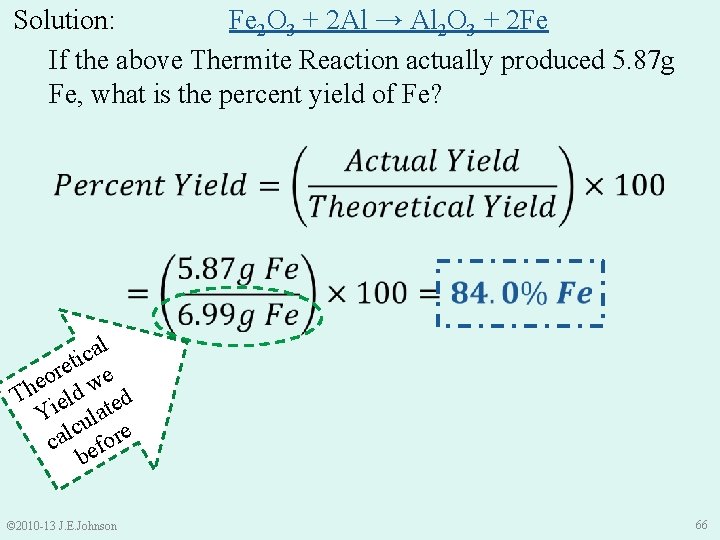
Let’s Practice together: Limiting Reactants Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe • If the above Thermite Reaction actually produced 5. 87 g Fe, what is the percent yield of Fe? • % Yield is where it’s “AT” © 2010 -13 J. E. Johnson 65

Solution: Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe If the above Thermite Reaction actually produced 5. 87 g Fe, what is the percent yield of Fe? l a c ti e r e o e w Th ield ted Y ula c cal efore b © 2010 -13 J. E. Johnson 66

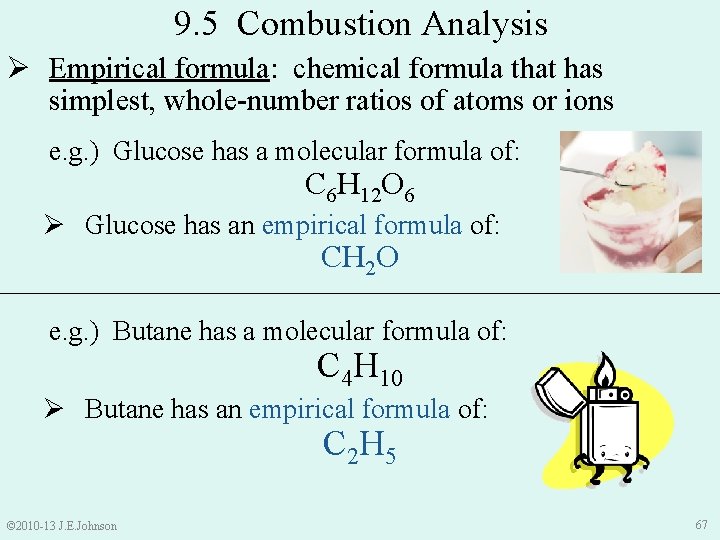
9. 5 Combustion Analysis Ø Empirical formula: chemical formula that has simplest, whole-number ratios of atoms or ions e. g. ) Glucose has a molecular formula of: C 6 H 12 O 6 Ø Glucose has an empirical formula of: CH 2 O e. g. ) Butane has a molecular formula of: C 4 H 10 Ø Butane has an empirical formula of: C 2 H 5 © 2010 -13 J. E. Johnson 67

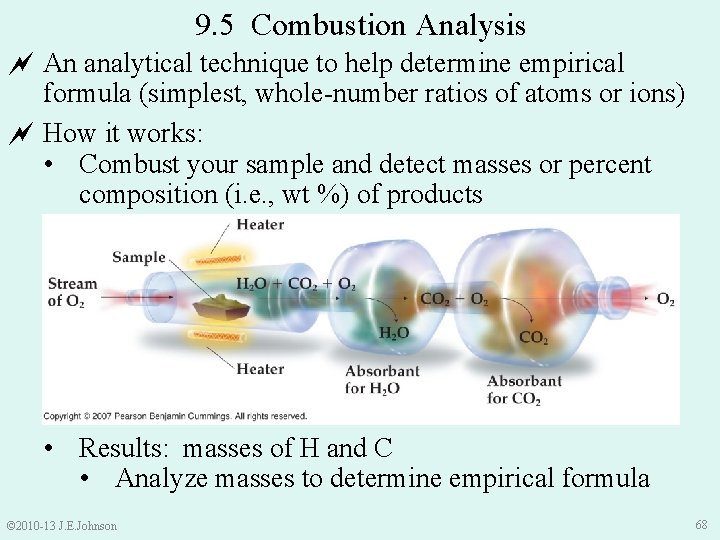
9. 5 Combustion Analysis An analytical technique to help determine empirical formula (simplest, whole-number ratios of atoms or ions) How it works: • Combust your sample and detect masses or percent composition (i. e. , wt %) of products • Results: masses of H and C • Analyze masses to determine empirical formula © 2010 -13 J. E. Johnson 68

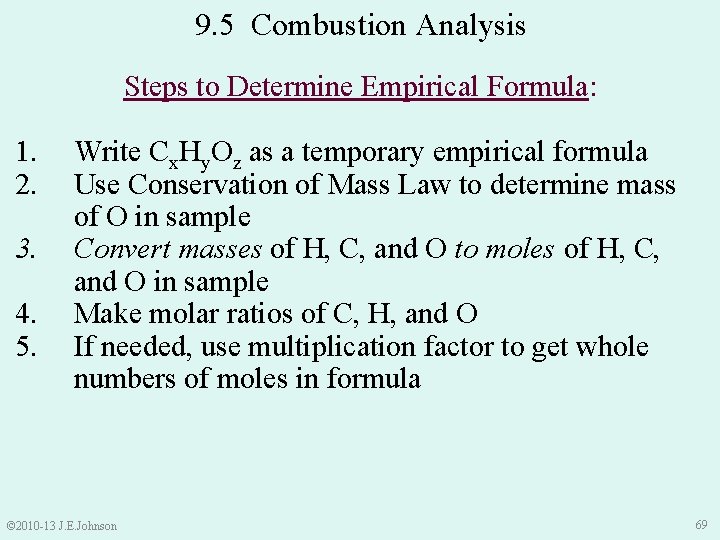
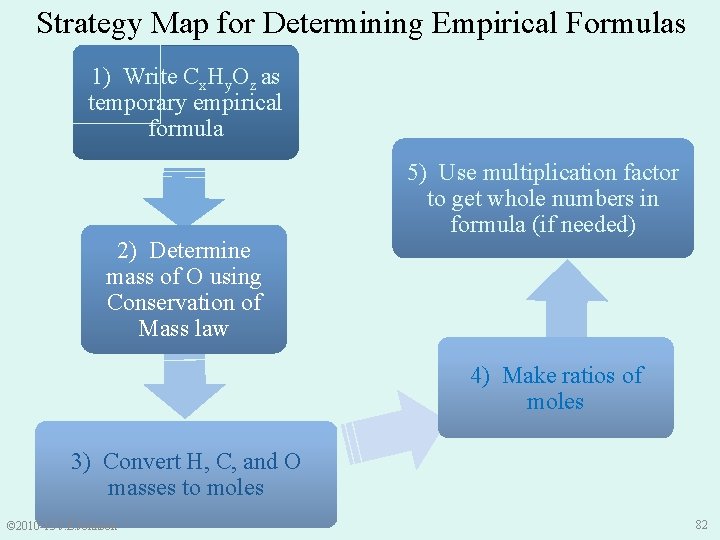
9. 5 Combustion Analysis Steps to Determine Empirical Formula: 1. 2. 3. 4. 5. Write Cx. Hy. Oz as a temporary empirical formula Use Conservation of Mass Law to determine mass of O in sample Convert masses of H, C, and O to moles of H, C, and O in sample Make molar ratios of C, H, and O If needed, use multiplication factor to get whole numbers of moles in formula © 2010 -13 J. E. Johnson 69

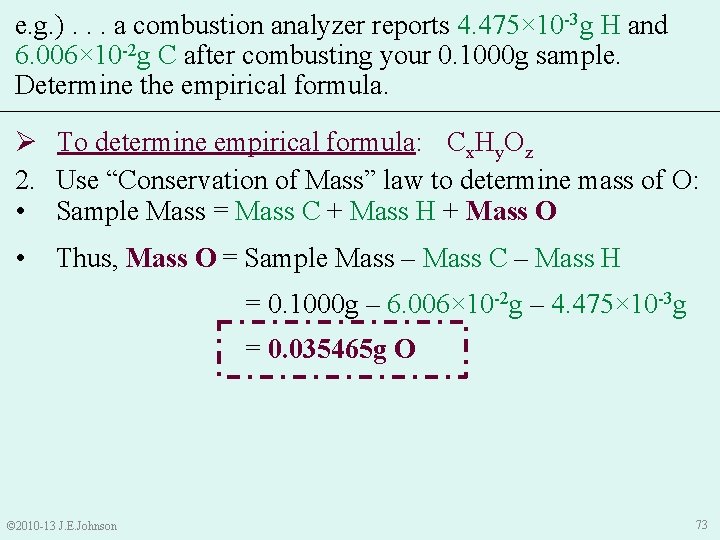
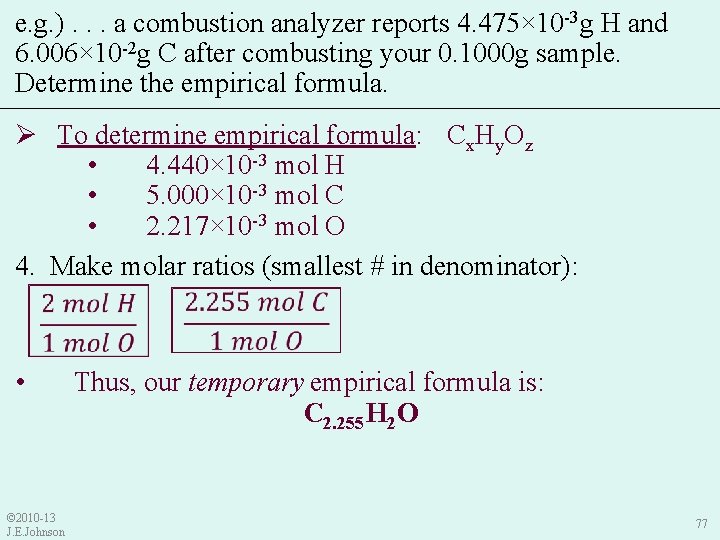
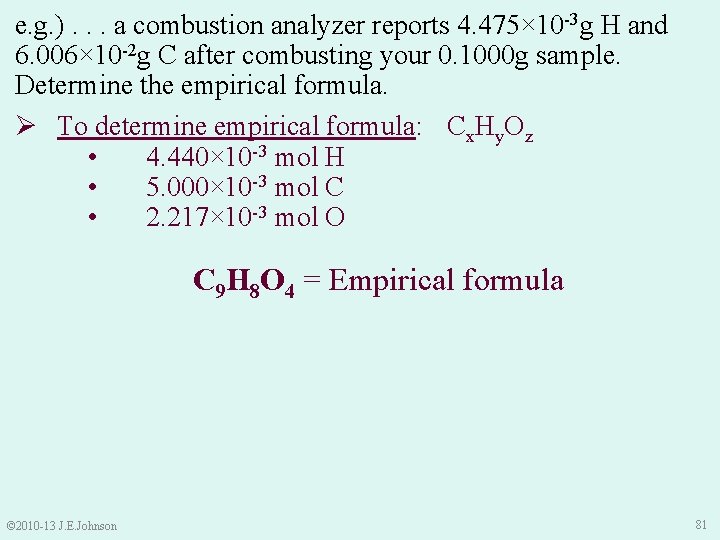
9. 5 Combustion Analysis e. g. ) In lab, you synthesized what you think is aspirin that contains C, H, and O. After combusting your 0. 1000 g sample in a combustion analyzer, it reports that your sample -3 g H and 6. 006× 10 -2 g C. Determine the contained 4. 475× 10 empirical formula of your sample. Ø To determine empirical formula: 1. Write Cx. Hy. Oz as a temporary empirical formula: Cx. Hy. Oz • Helps us remember our goal: → GOAL: to get mole relationships (molar ratios) between each element in empirical formula © 2010 -13 J. E. Johnson 70

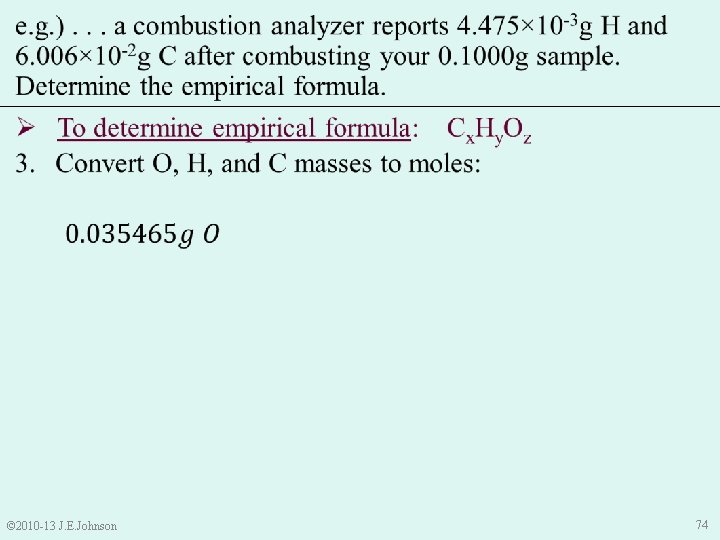
e. g. ). . . a combustion analyzer reports 4. 475× 10 -3 g H and 6. 006× 10 -2 g C after combusting your 0. 1000 g sample. Determine the empirical formula. Ø To determine empirical formula: Cx. Hy. Oz 2. Use “Conservation of Mass” law to determine mass of O: • Sample Mass = Mass C + Mass H + Mass O • Thus, Mass O = Sample Mass – Mass C – Mass H = 0. 1000 g – 6. 006× 10 -2 g – 4. 475× 10 -3 g = 0. 035465 g O © 2010 -13 J. E. Johnson 73

© 2010 -13 J. E. Johnson 74

© 2010 -13 J. E. Johnson 75

© 2010 -13 J. E. Johnson 76

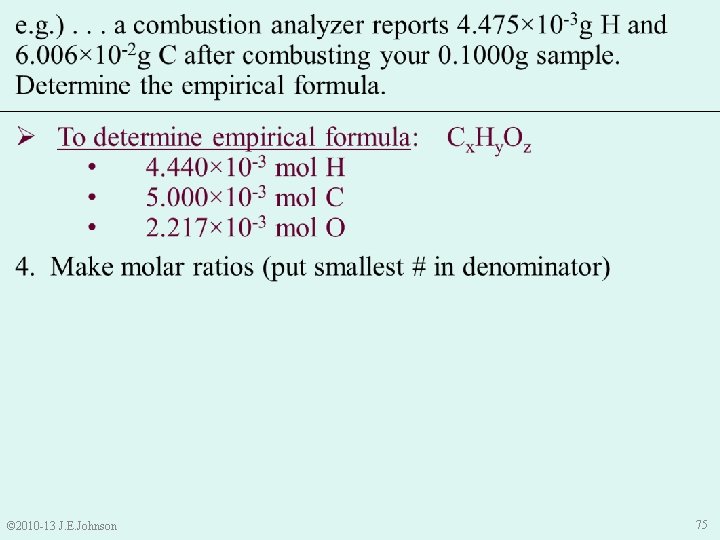
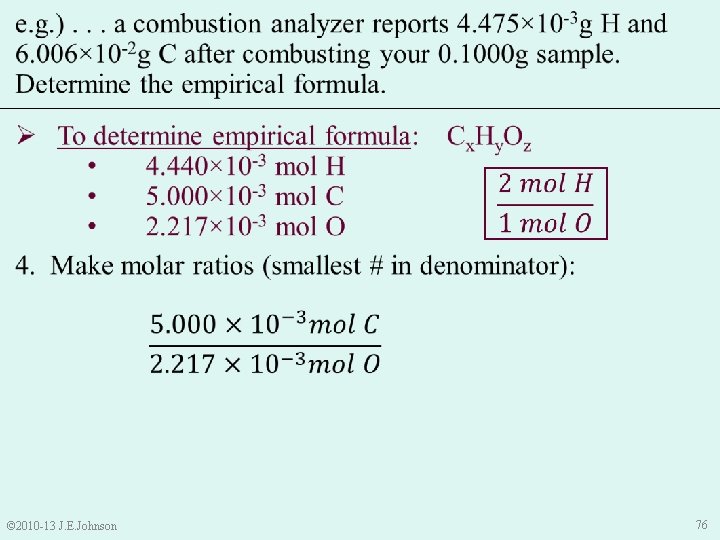
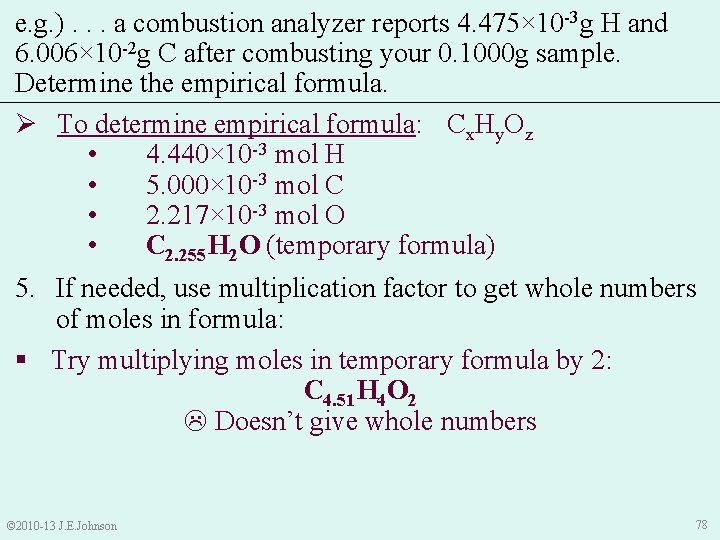
e. g. ). . . a combustion analyzer reports 4. 475× 10 -3 g H and 6. 006× 10 -2 g C after combusting your 0. 1000 g sample. Determine the empirical formula. Ø To determine empirical formula: Cx. Hy. Oz • 4. 440× 10 -3 mol H • 5. 000× 10 -3 mol C • 2. 217× 10 -3 mol O 4. Make molar ratios (smallest # in denominator): • © 2010 -13 J. E. Johnson Thus, our temporary empirical formula is: C 2. 255 H 2 O 77

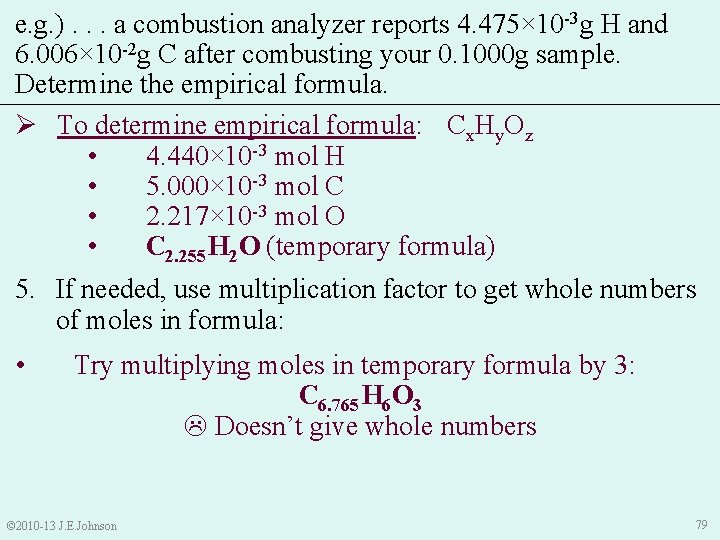
e. g. ). . . a combustion analyzer reports 4. 475× 10 -3 g H and 6. 006× 10 -2 g C after combusting your 0. 1000 g sample. Determine the empirical formula. Ø To determine empirical formula: Cx. Hy. Oz • 4. 440× 10 -3 mol H • 5. 000× 10 -3 mol C • 2. 217× 10 -3 mol O • C 2. 255 H 2 O (temporary formula) 5. If needed, use multiplication factor to get whole numbers of moles in formula: § Try multiplying moles in temporary formula by 2: C 4. 51 H 4 O 2 Doesn’t give whole numbers © 2010 -13 J. E. Johnson 78

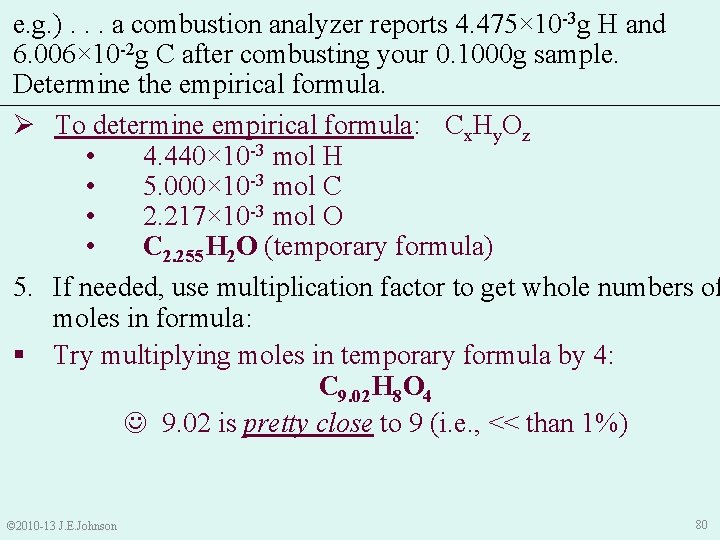
e. g. ). . . a combustion analyzer reports 4. 475× 10 -3 g H and 6. 006× 10 -2 g C after combusting your 0. 1000 g sample. Determine the empirical formula. Ø To determine empirical formula: Cx. Hy. Oz • 4. 440× 10 -3 mol H • 5. 000× 10 -3 mol C • 2. 217× 10 -3 mol O • C 2. 255 H 2 O (temporary formula) 5. If needed, use multiplication factor to get whole numbers of moles in formula: • Try multiplying moles in temporary formula by 3: C 6. 765 H 6 O 3 Doesn’t give whole numbers © 2010 -13 J. E. Johnson 79

e. g. ). . . a combustion analyzer reports 4. 475× 10 -3 g H and 6. 006× 10 -2 g C after combusting your 0. 1000 g sample. Determine the empirical formula. Ø To determine empirical formula: Cx. Hy. Oz • 4. 440× 10 -3 mol H • 5. 000× 10 -3 mol C • 2. 217× 10 -3 mol O • C 2. 255 H 2 O (temporary formula) 5. If needed, use multiplication factor to get whole numbers of moles in formula: § Try multiplying moles in temporary formula by 4: C 9. 02 H 8 O 4 9. 02 is pretty close to 9 (i. e. , << than 1%) © 2010 -13 J. E. Johnson 80

e. g. ). . . a combustion analyzer reports 4. 475× 10 -3 g H and 6. 006× 10 -2 g C after combusting your 0. 1000 g sample. Determine the empirical formula. Ø To determine empirical formula: Cx. Hy. Oz • 4. 440× 10 -3 mol H • 5. 000× 10 -3 mol C • 2. 217× 10 -3 mol O C 9 H 8 O 4 = Empirical formula © 2010 -13 J. E. Johnson 81

Strategy Map for Determining Empirical Formulas 1) Write Cx. Hy. Oz as temporary empirical formula 2) Determine mass of O using Conservation of Mass law 5) Use multiplication factor to get whole numbers in formula (if needed) 4) Make ratios of moles 3) Convert H, C, and O masses to moles © 2010 -13 J. E. Johnson 82

© 2010 -13 J. E. Johnson 83

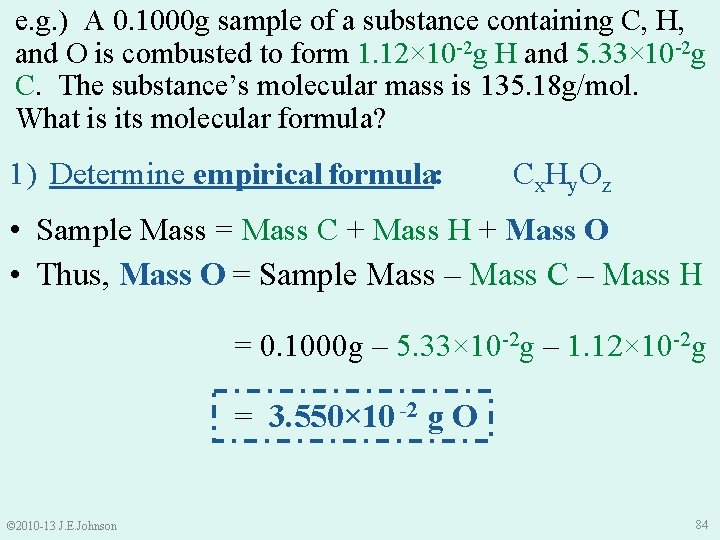
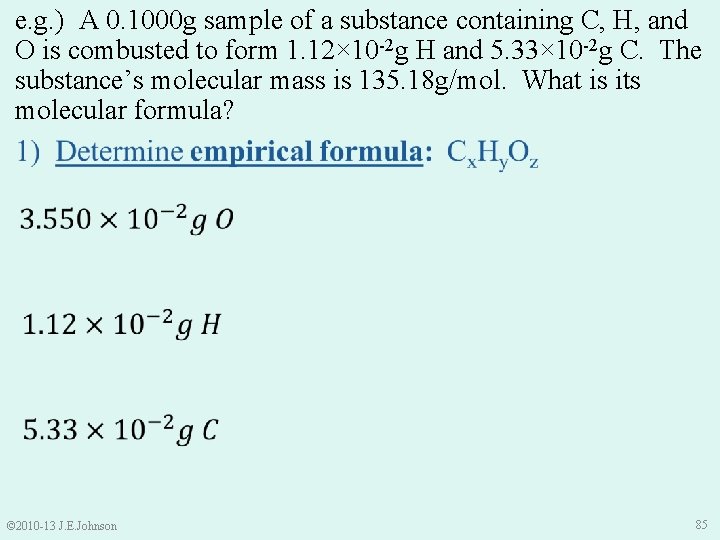
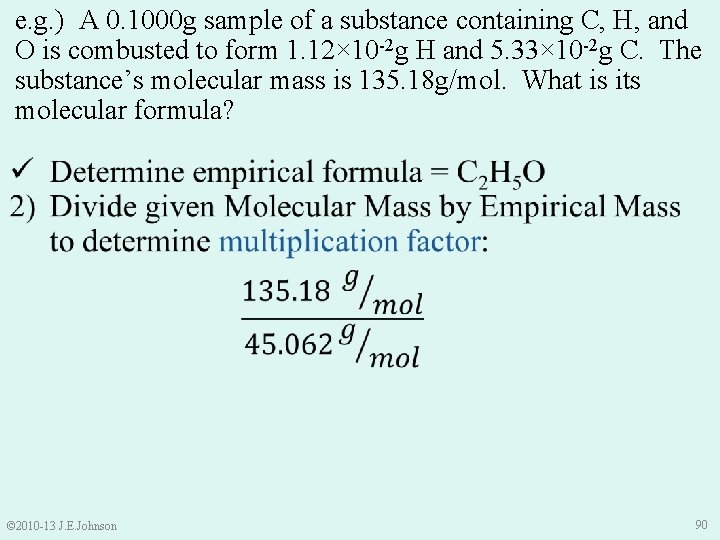
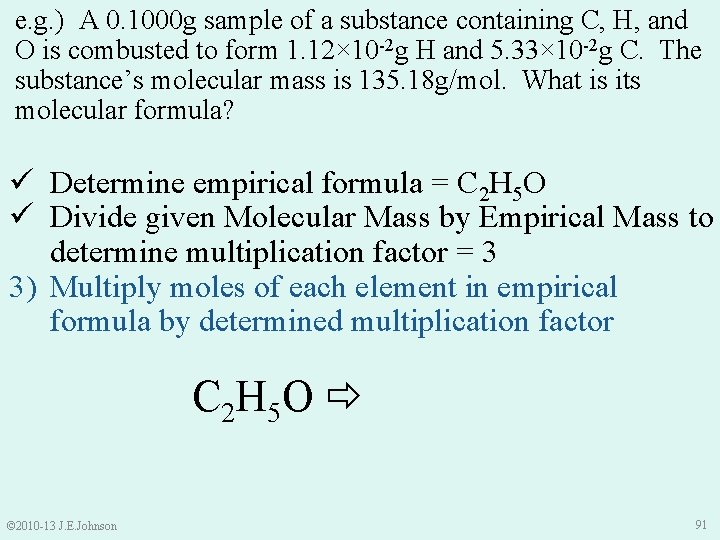
e. g. ) A 0. 1000 g sample of a substance containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s molecular mass is 135. 18 g/mol. What is its molecular formula? 1) Determine empirical formula: Cx. Hy. Oz • Sample Mass = Mass C + Mass H + Mass O • Thus, Mass O = Sample Mass – Mass C – Mass H = 0. 1000 g – 5. 33× 10 -2 g – 1. 12× 10 -2 g = 3. 550× 10 -2 g O © 2010 -13 J. E. Johnson 84

e. g. ) A 0. 1000 g sample of a substance containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s molecular mass is 135. 18 g/mol. What is its molecular formula? © 2010 -13 J. E. Johnson 85

e. g. ) A 0. 1000 g sample of a substance containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s molecular mass is 135. 18 g/mol. What is its molecular formula? © 2010 -13 J. E. Johnson 86

e. g. ) A 0. 1000 g sample of a substance containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s molecular mass is 135. 18 g/mol. What is its molecular formula? © 2010 -13 J. E. Johnson 87

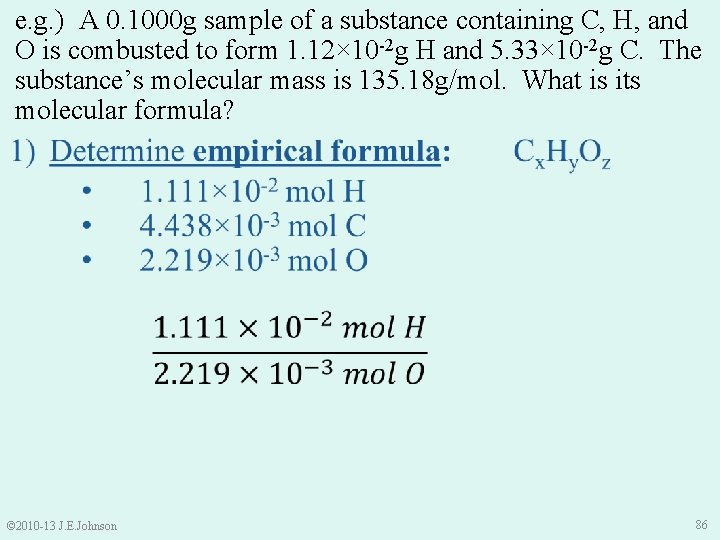
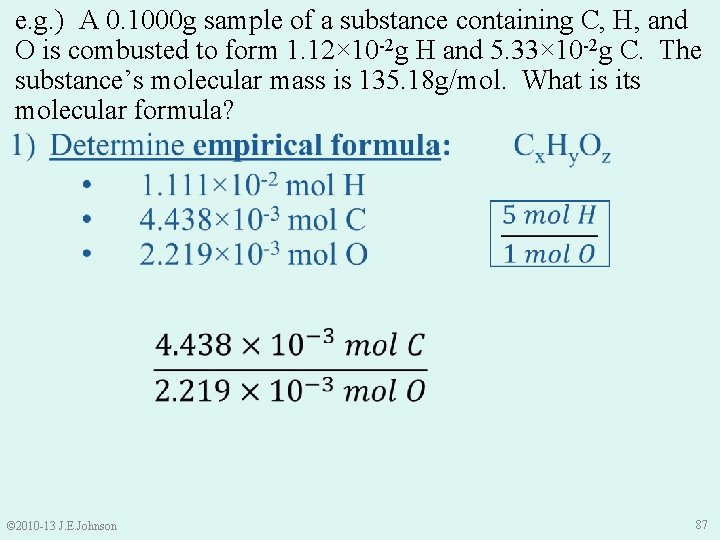
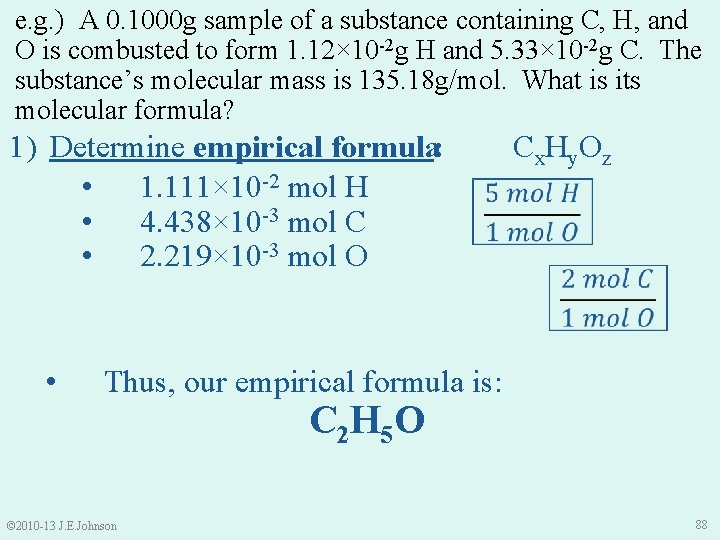
e. g. ) A 0. 1000 g sample of a substance containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s molecular mass is 135. 18 g/mol. What is its molecular formula? 1) Determine empirical formula: • 1. 111× 10 -2 mol H • 4. 438× 10 -3 mol C • 2. 219× 10 -3 mol O • Cx. Hy. Oz Thus, our empirical formula is: © 2010 -13 J. E. Johnson C 2 H 5 O 88

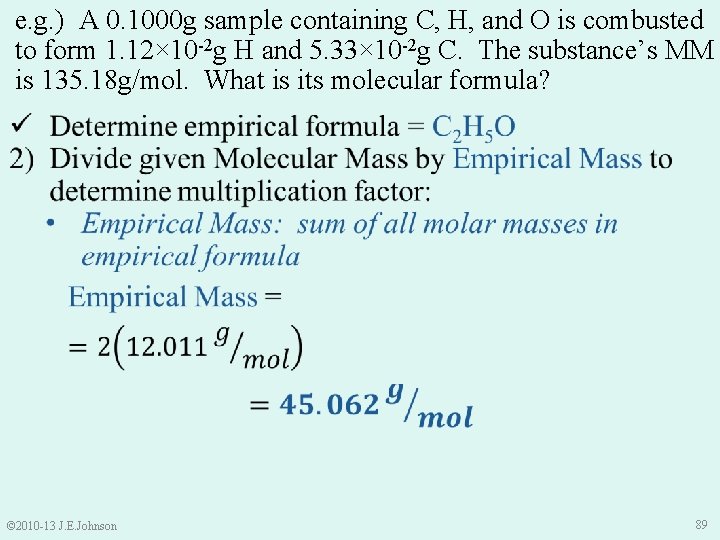
e. g. ) A 0. 1000 g sample containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s MM is 135. 18 g/mol. What is its molecular formula? © 2010 -13 J. E. Johnson 89

e. g. ) A 0. 1000 g sample of a substance containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s molecular mass is 135. 18 g/mol. What is its molecular formula? © 2010 -13 J. E. Johnson 90

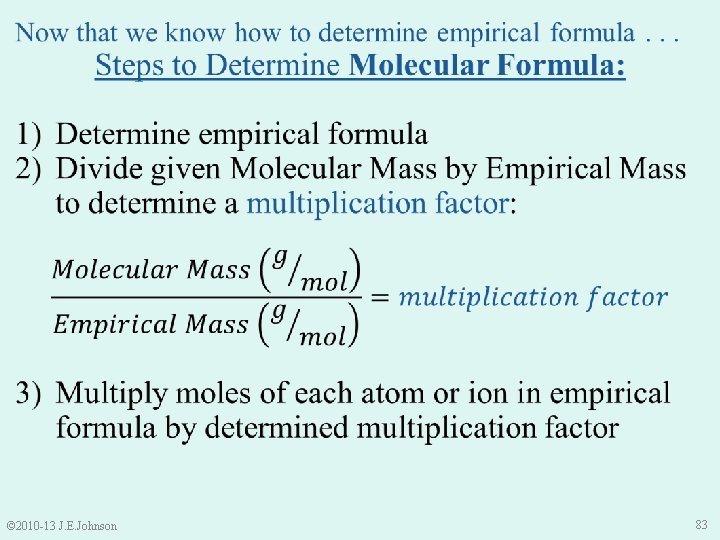
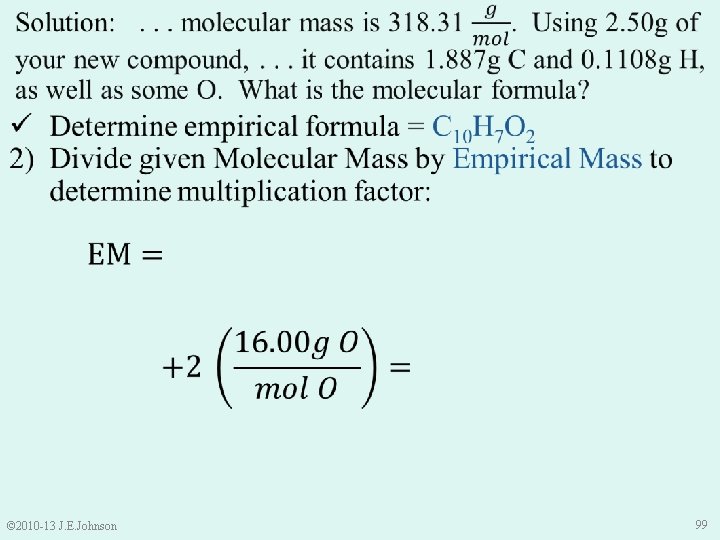
e. g. ) A 0. 1000 g sample of a substance containing C, H, and O is combusted to form 1. 12× 10 -2 g H and 5. 33× 10 -2 g C. The substance’s molecular mass is 135. 18 g/mol. What is its molecular formula? ü Determine empirical formula = C 2 H 5 O ü Divide given Molecular Mass by Empirical Mass to determine multiplication factor = 3 3) Multiply moles of each element in empirical formula by determined multiplication factor C 2 H 5 O C 6 H 15 O 3 © 2010 -13 J. E. Johnson 91

Let’s Practice: Limiting Reactants © 2010 -13 J. E. Johnson 92

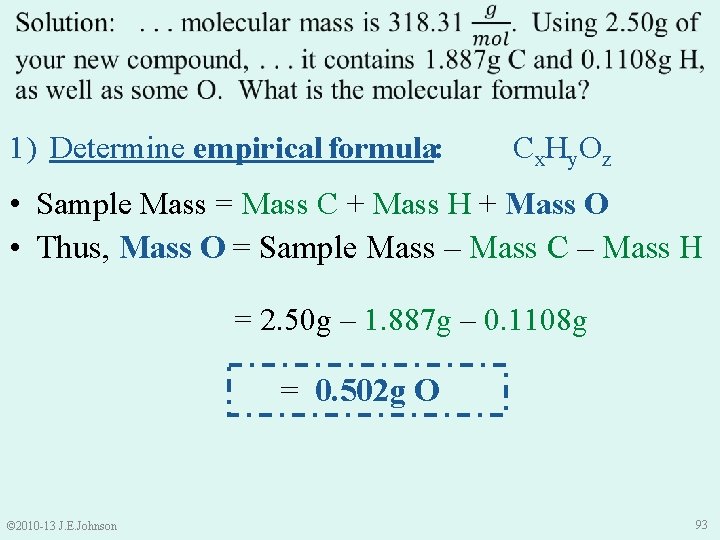
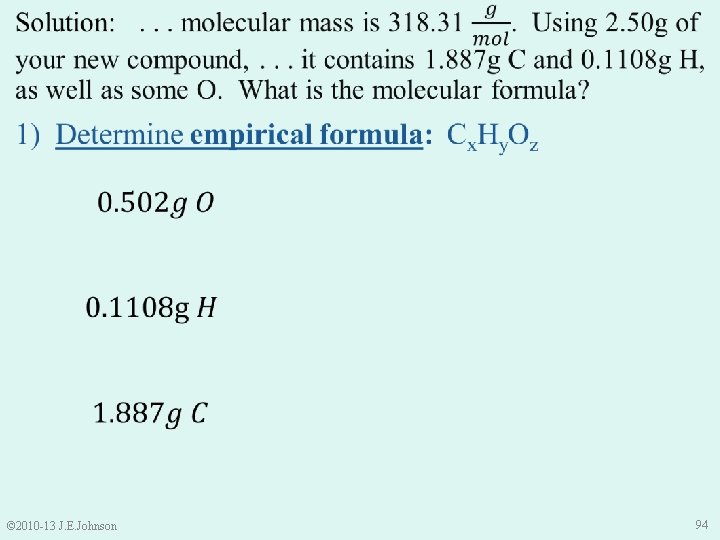
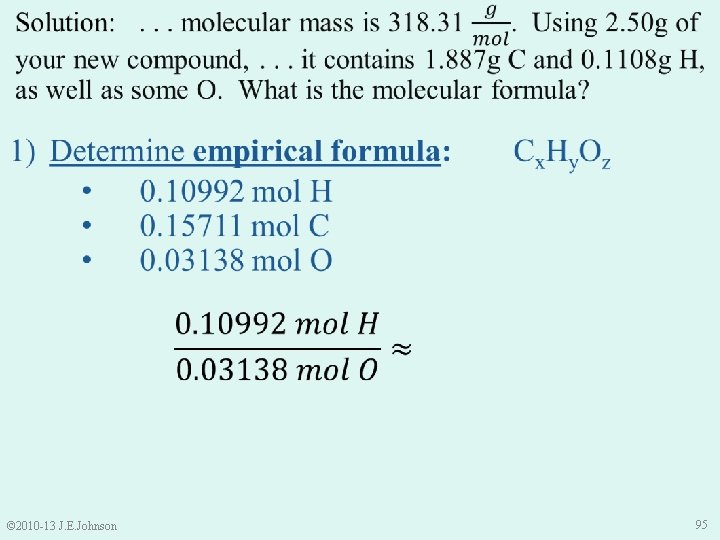
1) Determine empirical formula: Cx. Hy. Oz • Sample Mass = Mass C + Mass H + Mass O • Thus, Mass O = Sample Mass – Mass C – Mass H = 2. 50 g – 1. 887 g – 0. 1108 g = 0. 502 g O © 2010 -13 J. E. Johnson 93

© 2010 -13 J. E. Johnson 94

© 2010 -13 J. E. Johnson 95

© 2010 -13 J. E. Johnson 96

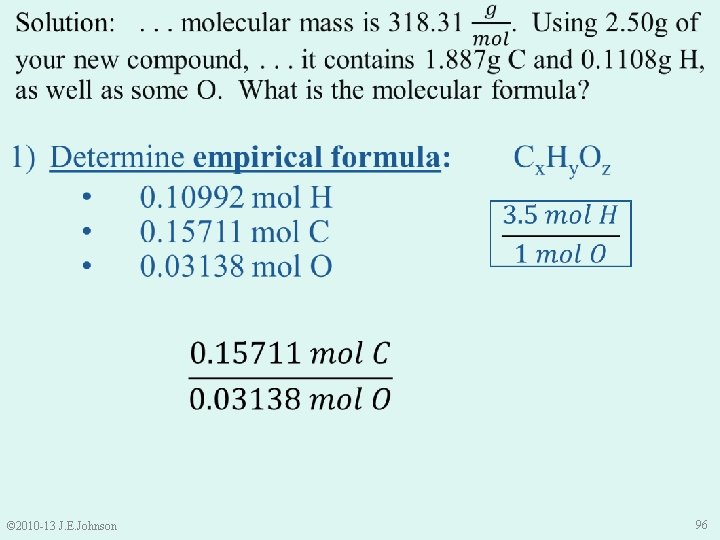
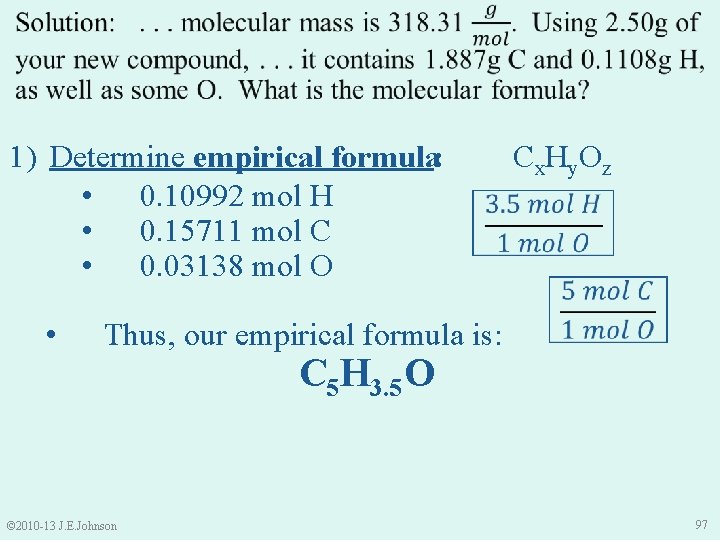
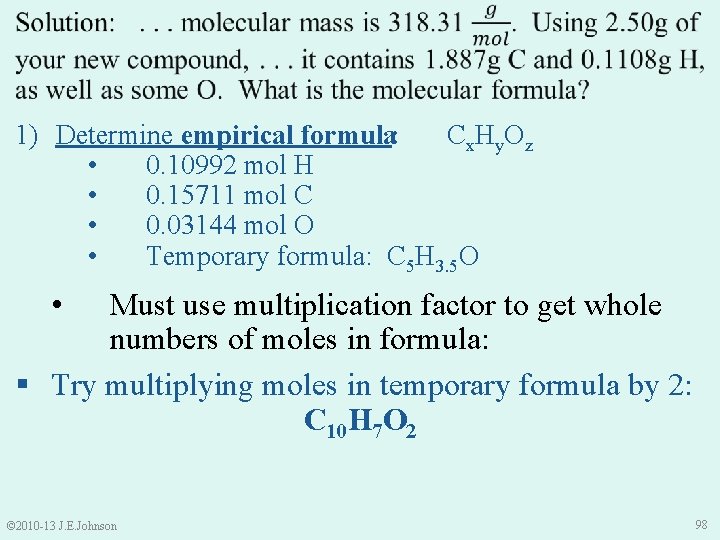
1) Determine empirical formula: • 0. 10992 mol H • 0. 15711 mol C • 0. 03138 mol O • Cx. Hy. Oz Thus, our empirical formula is: © 2010 -13 J. E. Johnson C 5 H 3. 5 O 97

1) Determine empirical formula: Cx. Hy. Oz • 0. 10992 mol H • 0. 15711 mol C • 0. 03144 mol O • Temporary formula: C 5 H 3. 5 O • Must use multiplication factor to get whole numbers of moles in formula: § Try multiplying moles in temporary formula by 2: C 10 H 7 O 2 © 2010 -13 J. E. Johnson 98

© 2010 -13 J. E. Johnson 99

© 2010 -13 J. E. Johnson 100

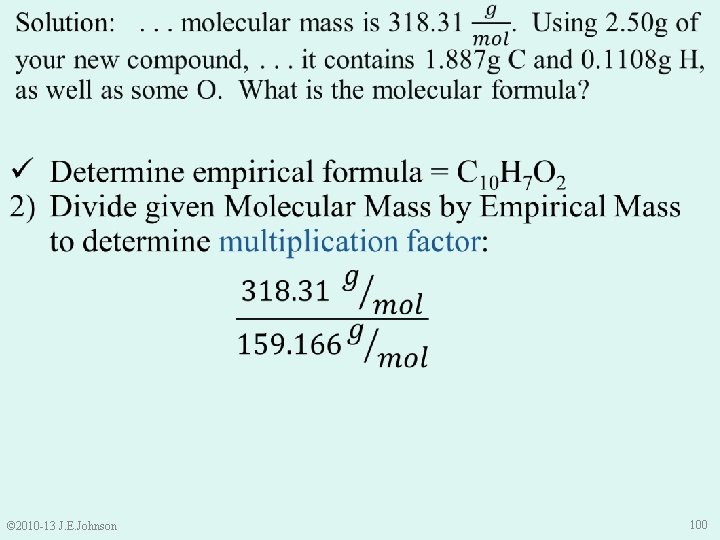
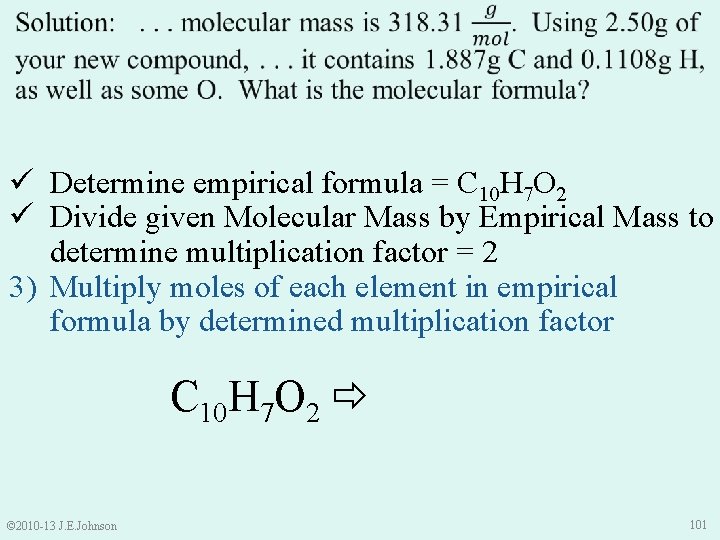
ü Determine empirical formula = C 10 H 7 O 2 ü Divide given Molecular Mass by Empirical Mass to determine multiplication factor = 2 3) Multiply moles of each element in empirical formula by determined multiplication factor C 10 H 7 O 2 C 20 H 14 O 4 © 2010 -13 J. E. Johnson 101

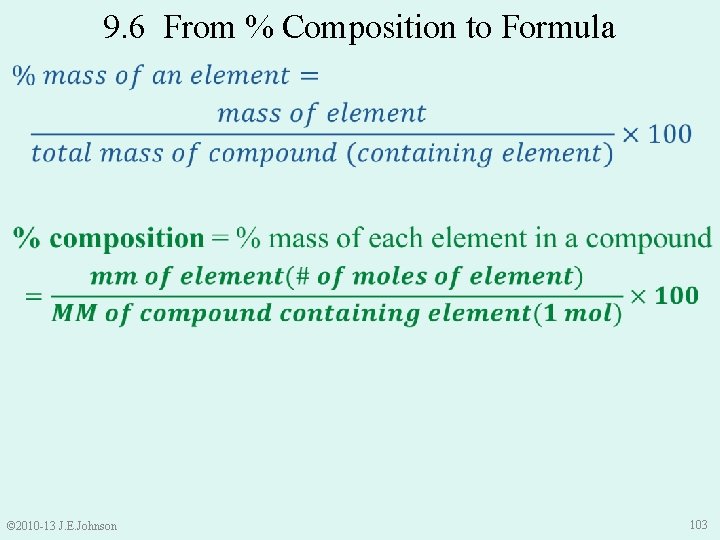
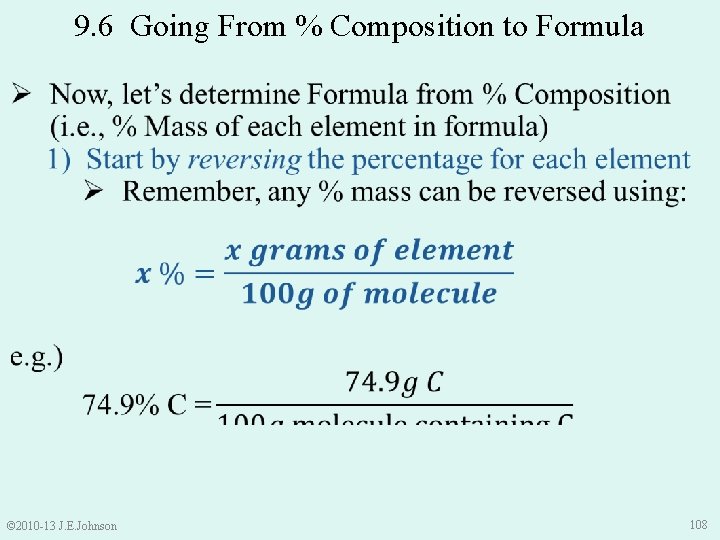
9. 6 From % Composition to Formula © 2010 -13 J. E. Johnson 102

9. 6 From % Composition to Formula © 2010 -13 J. E. Johnson 103

© 2010 -13 J. E. Johnson 104

© 2010 -13 J. E. Johnson 105

© 2010 -13 J. E. Johnson 106

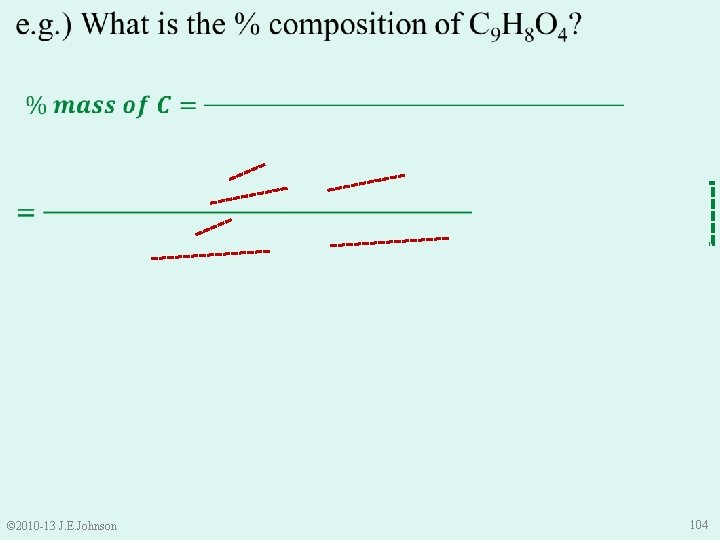
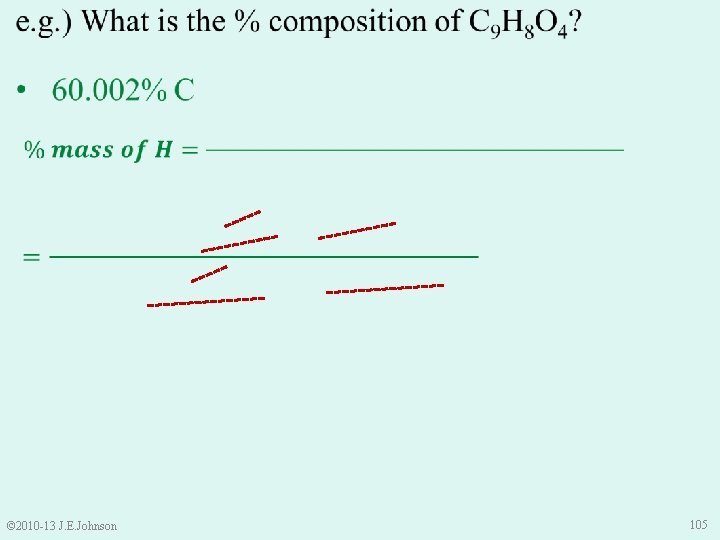
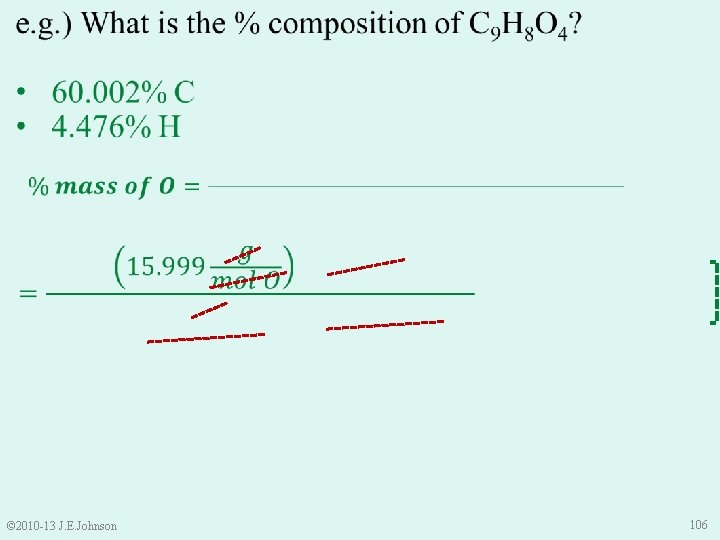
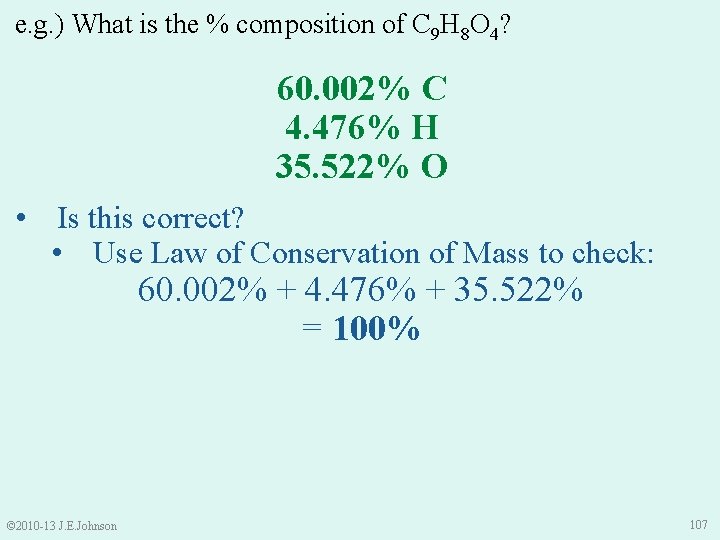
e. g. ) What is the % composition of C 9 H 8 O 4? 60. 002% C 4. 476% H 35. 522% O • Is this correct? • Use Law of Conservation of Mass to check: 60. 002% + 4. 476% + 35. 522% = 100% © 2010 -13 J. E. Johnson 107

9. 6 Going From % Composition to Formula © 2010 -13 J. E. Johnson 108

9. 6 Going From % Composition to Formula © 2010 -13 J. E. Johnson 109

© 2010 -13 J. E. Johnson 110

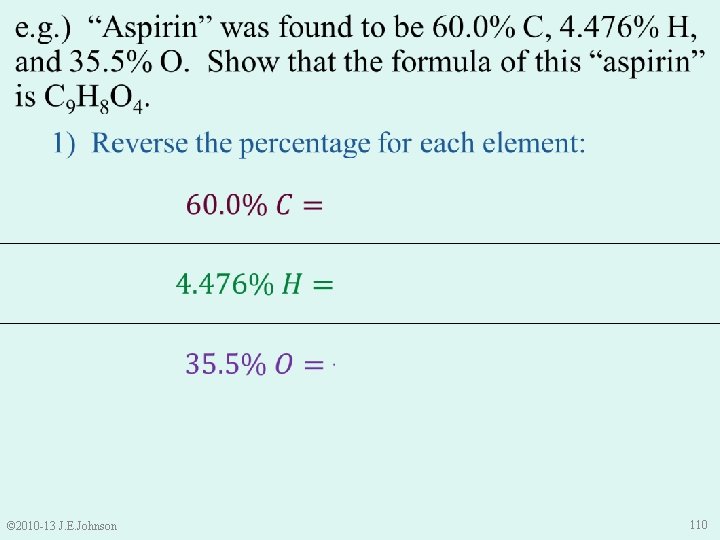
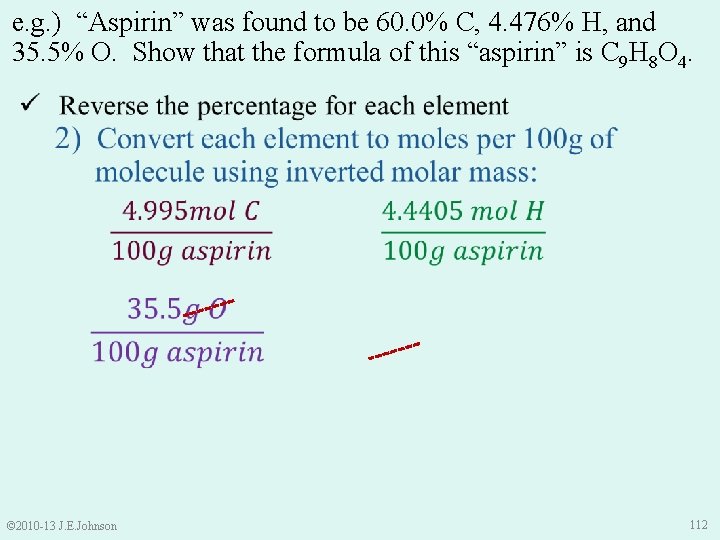
e. g. ) “Aspirin” was found to be 60. 0% C, 4. 476% H, and 35. 5% O. Show that the formula of this “aspirin” is C 9 H 8 O 4. © 2010 -13 J. E. Johnson 111

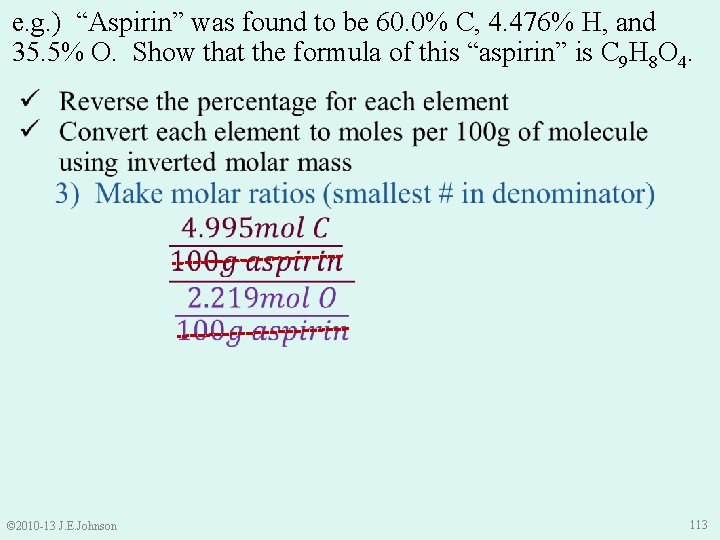
e. g. ) “Aspirin” was found to be 60. 0% C, 4. 476% H, and 35. 5% O. Show that the formula of this “aspirin” is C 9 H 8 O 4. © 2010 -13 J. E. Johnson 112

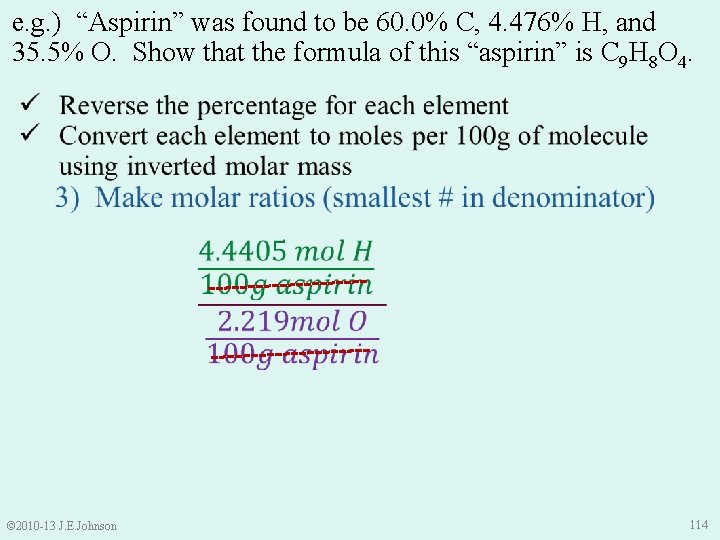
e. g. ) “Aspirin” was found to be 60. 0% C, 4. 476% H, and 35. 5% O. Show that the formula of this “aspirin” is C 9 H 8 O 4. © 2010 -13 J. E. Johnson 113

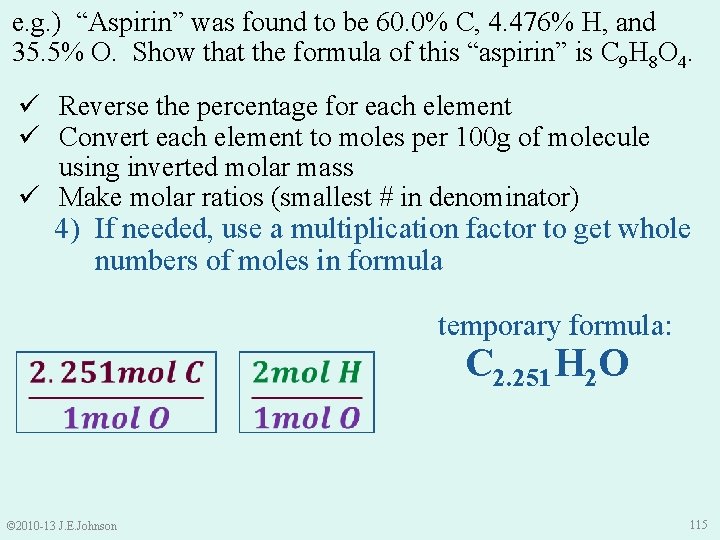
e. g. ) “Aspirin” was found to be 60. 0% C, 4. 476% H, and 35. 5% O. Show that the formula of this “aspirin” is C 9 H 8 O 4. © 2010 -13 J. E. Johnson 114

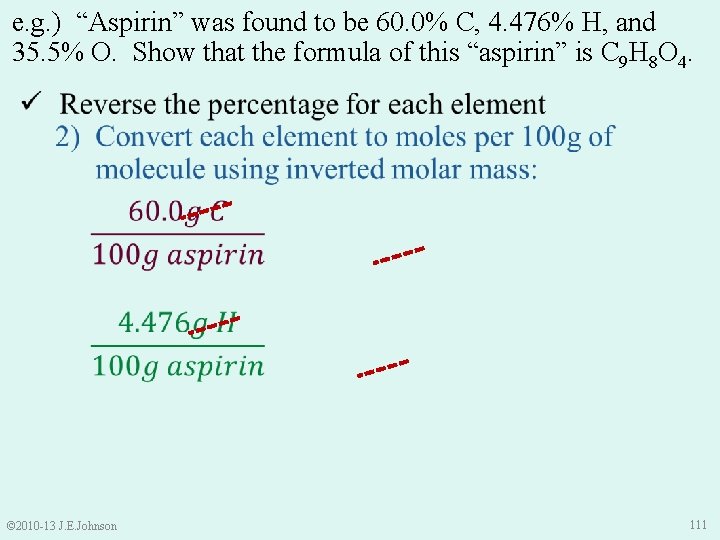
e. g. ) “Aspirin” was found to be 60. 0% C, 4. 476% H, and 35. 5% O. Show that the formula of this “aspirin” is C 9 H 8 O 4. ü Reverse the percentage for each element ü Convert each element to moles per 100 g of molecule using inverted molar mass ü Make molar ratios (smallest # in denominator) 4) If needed, use a multiplication factor to get whole numbers of moles in formula © 2010 -13 J. E. Johnson temporary formula: C 2. 251 H 2 O 115

e. g. ) “Aspirin” was found to be 60. 0% C, 4. 476% H, and 35. 5% O. Show that the formula of this “aspirin” is C 9 H 8 O 4. ü Reverse the percentage for each element ü Convert each element to moles per 100 g of molecule using inverted molar mass ü Make molar ratios (smallest # in denominator) 4) If needed, use a multiplication factor to get whole numbers of moles in formula v Multiply moles in temporary formula by 4: C 2. 251 H 2 O C 9 H 8 O 4 © 2010 -13 J. E. Johnson 116

** DON’T FORGET ** Take the Chapter 9 Quiz - it’s available on Web. CT after 7 pm tonight! http: //www. snn-rdr. ca/old/nov 99/moleday. html www. nisdtx. org/120820731141731840/ © 2010 -13 J. E. Johnson 117