Organic Chemistry For Dental Students Dr Nesma Mamdouh








- Slides: 28

Organic Chemistry For Dental Students Dr. Nesma Mamdouh Bayoumy Lecture (1) Spring, 2015 1

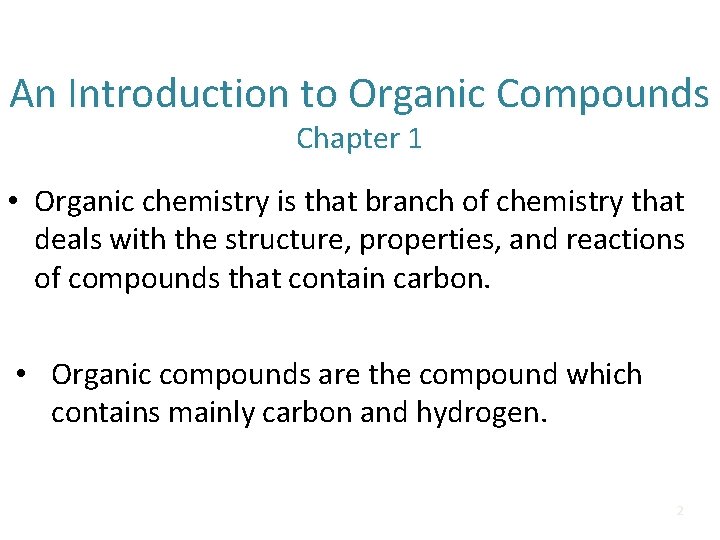
An Introduction to Organic Compounds Chapter 1 • Organic chemistry is that branch of chemistry that deals with the structure, properties, and reactions of compounds that contain carbon. • Organic compounds are the compound which contains mainly carbon and hydrogen. 2

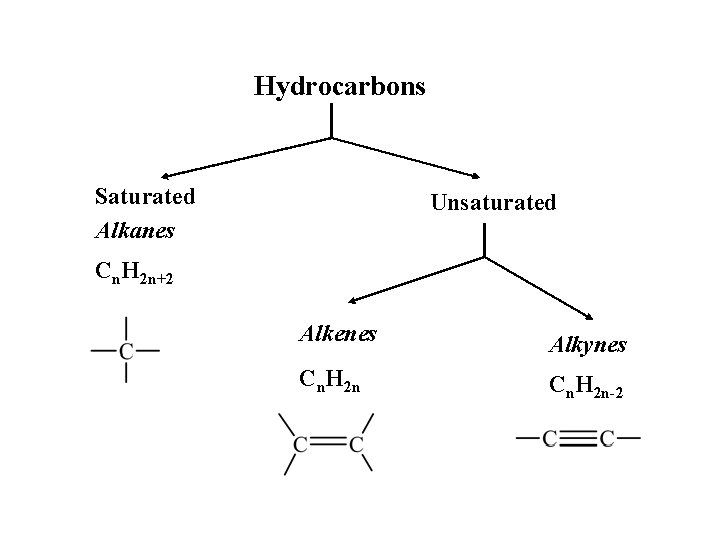
Hydrocarbons Saturated Alkanes Unsaturated Cn. H 2 n+2 Alkenes Alkynes Cn. H 2 n-2

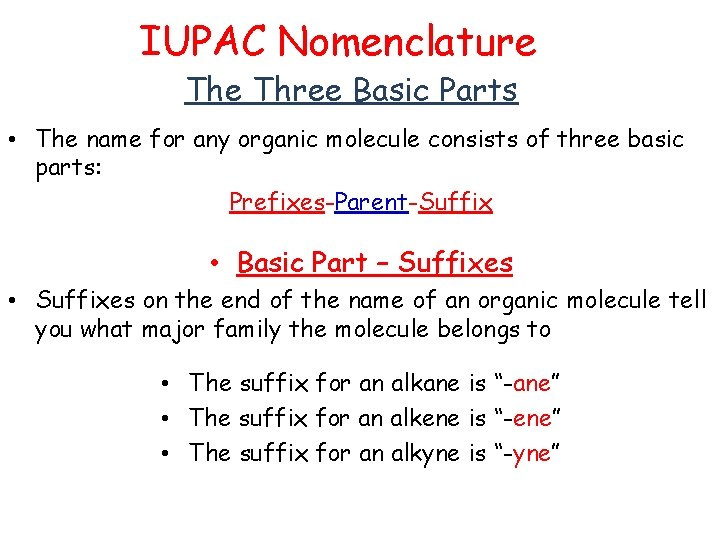
IUPAC Nomenclature Three Basic Parts • The name for any organic molecule consists of three basic parts: Prefixes-Parent-Suffix • Basic Part – Suffixes • Suffixes on the end of the name of an organic molecule tell you what major family the molecule belongs to • The suffix for an alkane is “-ane” • The suffix for an alkene is “-ene” • The suffix for an alkyne is “-yne”

• Basic Part – the Parent • The “parent” part of the name tells you how many carbons are in the longest chain “main chain” of the molecule. • Counting to Ten in Organic • • • 01 = meth 02 = eth 03 = prop 04 = but 05 = pent 06 = hex 07 = hept 08 = oct 09 = non 10 = dec

• Parent and suffix… • The parent is named based on the number of carbons • 1 carbon = “meth” • So a one-carbon alkane is called methane CH 4 • 2 carbons = “eth” • So a two carbon alkane is called ethane. CH 3 Parent and suffix… • 3 carbons = “prop” • So a three carbon alkane is called propane. CH 3 CH 2 CH 3 • 4 carbons = “but” • So a four carbon alkane is called butane. CH 3 CH 2 CH 3

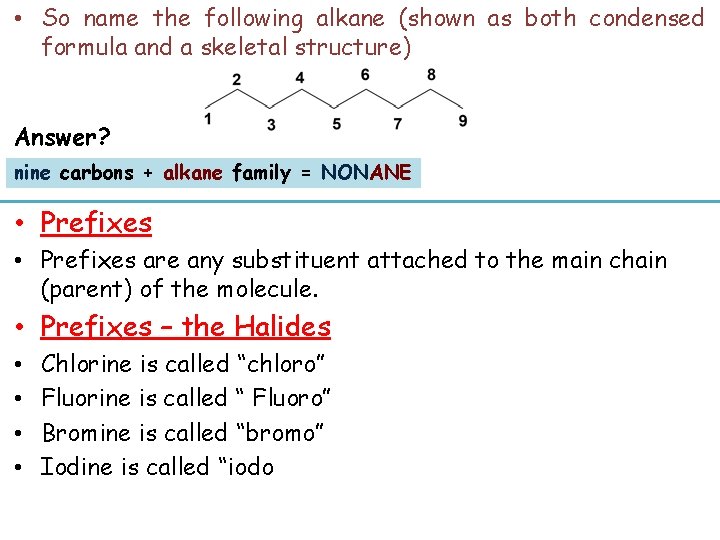
• So name the following alkane (shown as both condensed formula and a skeletal structure) Answer? nine carbons + alkane family = NONANE • Prefixes are any substituent attached to the main chain (parent) of the molecule. • Prefixes – the Halides • • Chlorine is called “chloro” Fluorine is called “ Fluoro” Bromine is called “bromo” Iodine is called “iodo

Putting together a name… • The rules for IUPAC nomenclature include: • Step 1: Find the main chain (Longest chain) • Step 2: Number the main chain • Step 3: Identify all prefixes and their position numbers • Step 4: Write the full name: Prefixes-Parent-Suffix. • Step 5: Add punctuation. o Put commas between numbers (2 5 5 becomes 2, 5, 5) o Put a hyphen between a number and a letter (2 5 5 trimethylheptane becomes 2, 5, 5 -trimethylheptane) o Successive words are merged into one word (trimethyl heptane becomes trimethylheptane)

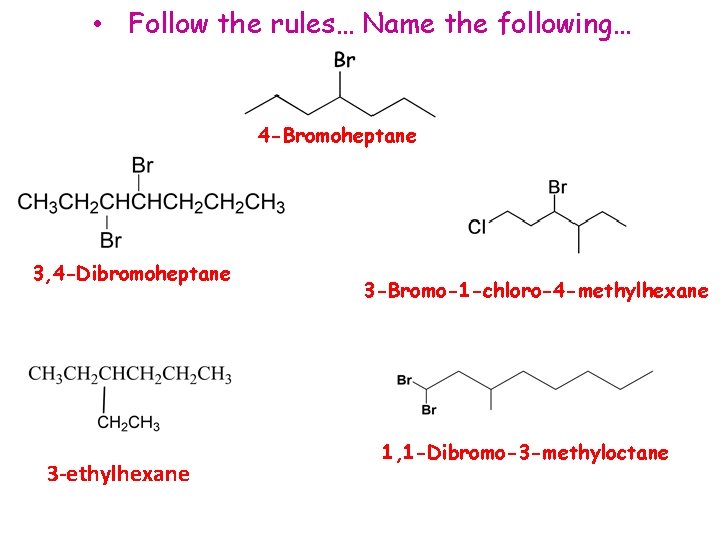
• Follow the rules… Name the following… 4 -Bromoheptane 3, 4 -Dibromoheptane 3 -ethylhexane 3 -Bromo-1 -chloro-4 -methylhexane 1, 1 -Dibromo-3 -methyloctane

• Alkyl Groups • Alkyl groups are named similarly to alkanes. • If you remove a hydrogen atom from one of these you get an alkyl group. • A fragment of methane, CH 4, would be CH 3 - called “methyl” • A fragment of ethane, CH 3, would be CH 3 CH 2 -, called “ethyl”. Example: 3 -ethylhexane

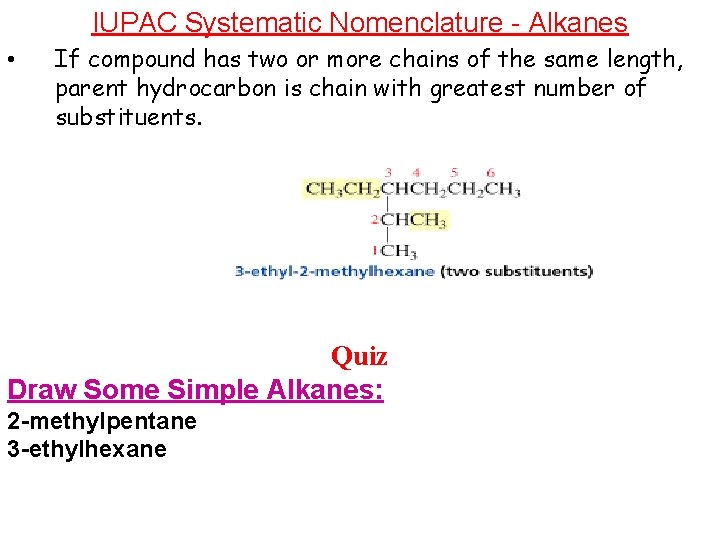
IUPAC Systematic Nomenclature - Alkanes • If compound has two or more chains of the same length, parent hydrocarbon is chain with greatest number of substituents. Quiz Draw Some Simple Alkanes: 2 -methylpentane 3 -ethylhexane

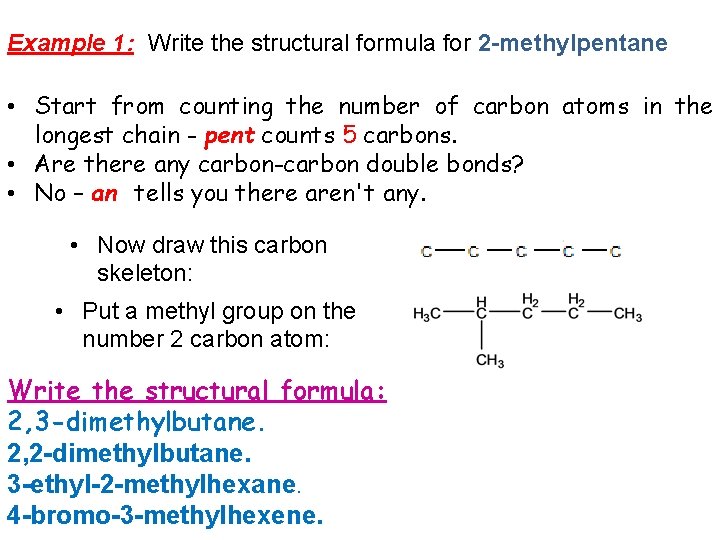
Example 1: Write the structural formula for 2 -methylpentane • Start from counting the number of carbon atoms in the longest chain - pent counts 5 carbons. • Are there any carbon-carbon double bonds? • No – an tells you there aren't any. • Now draw this carbon skeleton: • Put a methyl group on the number 2 carbon atom: Write the structural formula: 2, 3 -dimethylbutane. 2, 2 -dimethylbutane. 3 -ethyl-2 -methylhexane. 4 -bromo-3 -methylhexene.

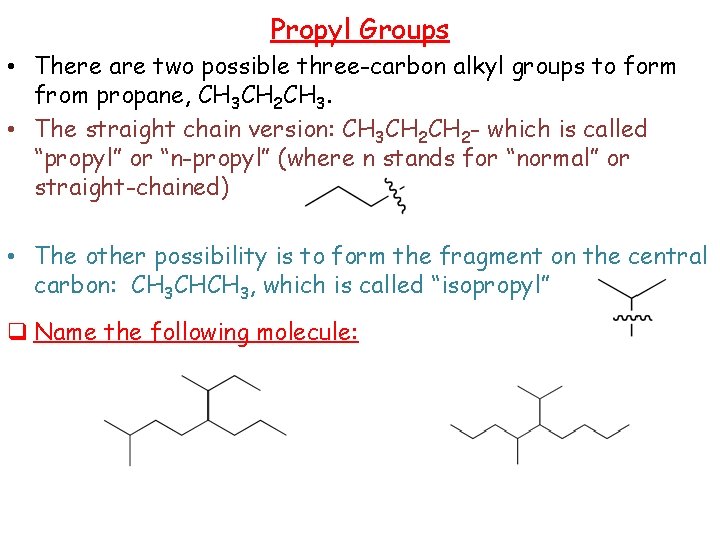
Propyl Groups • There are two possible three-carbon alkyl groups to form from propane, CH 3 CH 2 CH 3. • The straight chain version: CH 3 CH 2 - which is called “propyl” or “n-propyl” (where n stands for “normal” or straight-chained) • The other possibility is to form the fragment on the central carbon: CH 3 CHCH 3, which is called “isopropyl” q Name the following molecule:

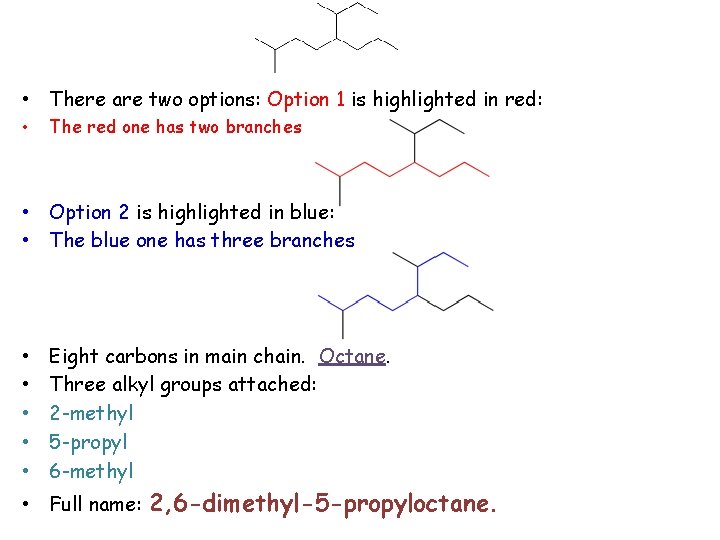
• There are two options: Option 1 is highlighted in red: • The red one has two branches • Option 2 is highlighted in blue: • The blue one has three branches • • • Eight carbons in main chain. Octane. Three alkyl groups attached: 2 -methyl 5 -propyl 6 -methyl • Full name: 2, 6 -dimethyl-5 -propyloctane.

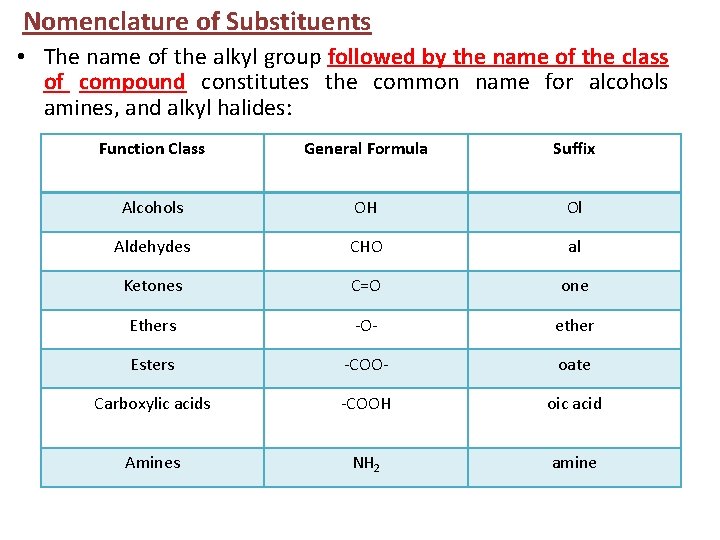
Nomenclature of Substituents • The name of the alkyl group followed by the name of the class of compound constitutes the common name for alcohols amines, and alkyl halides: Function Class General Formula Suffix Alcohols OH Ol Aldehydes CHO al Ketones C=O one Ethers -O- ether Esters -COO- oate Carboxylic acids -COOH oic acid Amines NH 2 amine

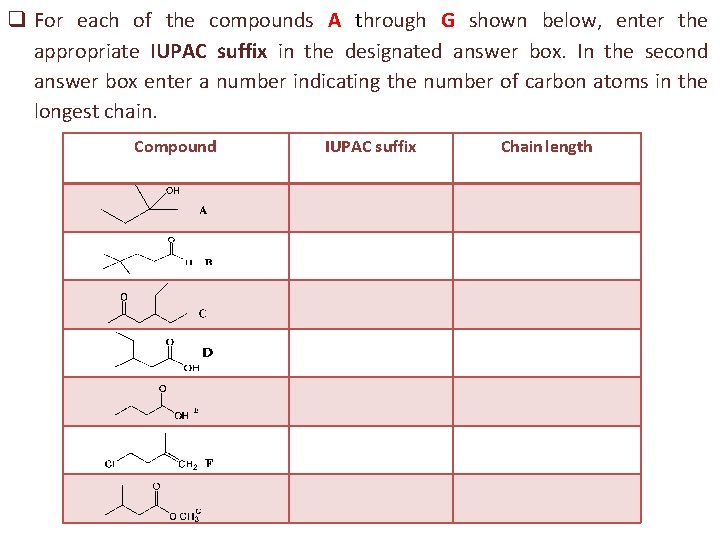
q For each of the compounds A through G shown below, enter the appropriate IUPAC suffix in the designated answer box. In the second answer box enter a number indicating the number of carbon atoms in the longest chain. Compound IUPAC suffix Chain length

Organic Chemistry For Dental Students Dr. Nesma Mamdouh Bayoumy Lecture (2) Spring, 2015 17

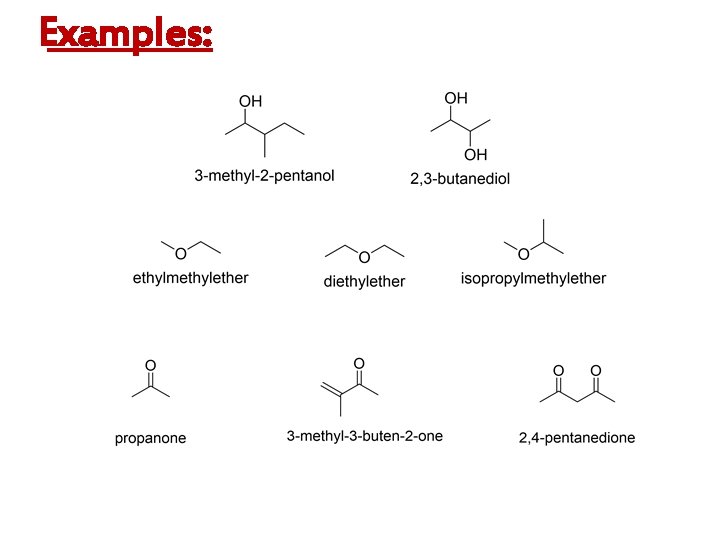
Examples:

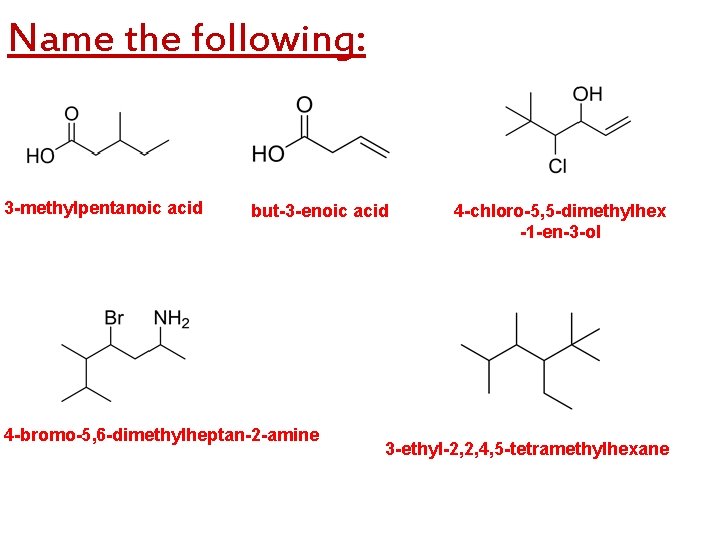
Name the following: 3 -methylpentanoic acid but-3 -enoic acid 4 -bromo-5, 6 -dimethylheptan-2 -amine 4 -chloro-5, 5 -dimethylhex -1 -en-3 -ol 3 -ethyl-2, 2, 4, 5 -tetramethylhexane

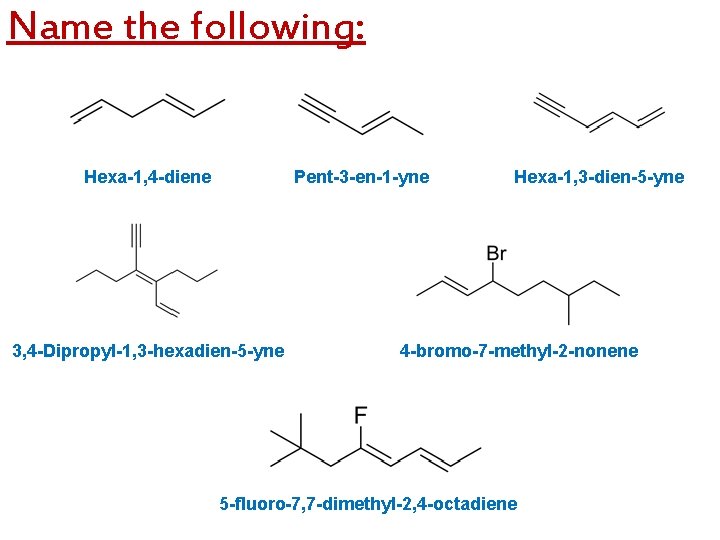
Name the following: Hexa-1, 4 -diene Pent-3 -en-1 -yne 3, 4 -Dipropyl-1, 3 -hexadien-5 -yne Hexa-1, 3 -dien-5 -yne 4 -bromo-7 -methyl-2 -nonene 5 -fluoro-7, 7 -dimethyl-2, 4 -octadiene

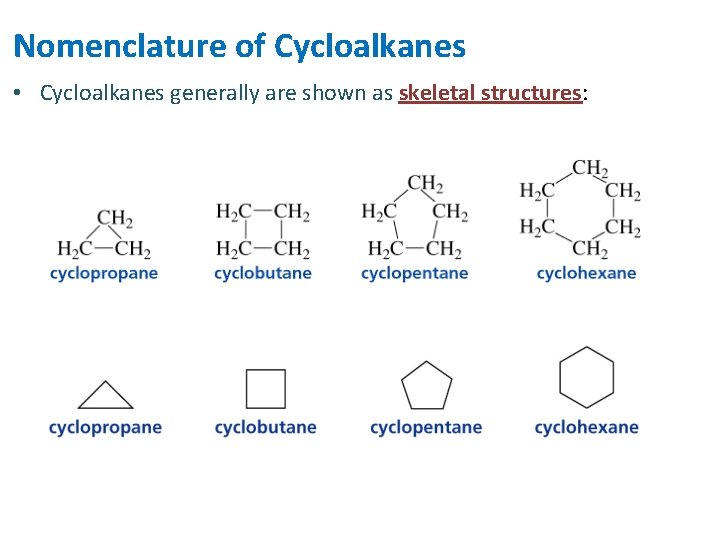
Nomenclature of Cycloalkanes • Cycloalkanes generally are shown as skeletal structures:

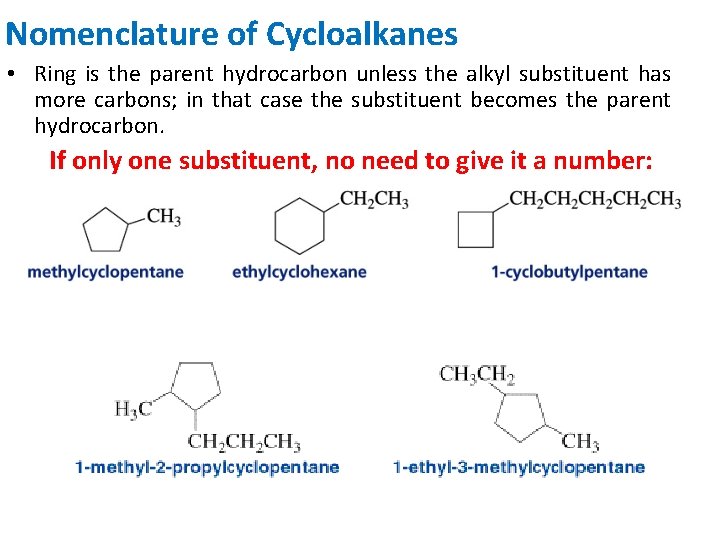
Nomenclature of Cycloalkanes • Ring is the parent hydrocarbon unless the alkyl substituent has more carbons; in that case the substituent becomes the parent hydrocarbon. If only one substituent, no need to give it a number:

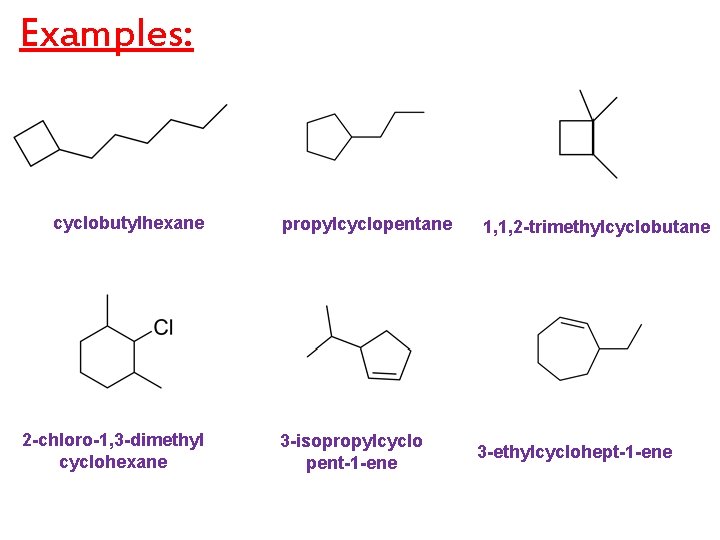
Examples: cyclobutylhexane 2 -chloro-1, 3 -dimethyl cyclohexane propylcyclopentane 3 -isopropylcyclo pent-1 -ene 1, 1, 2 -trimethylcyclobutane 3 -ethylcyclohept-1 -ene

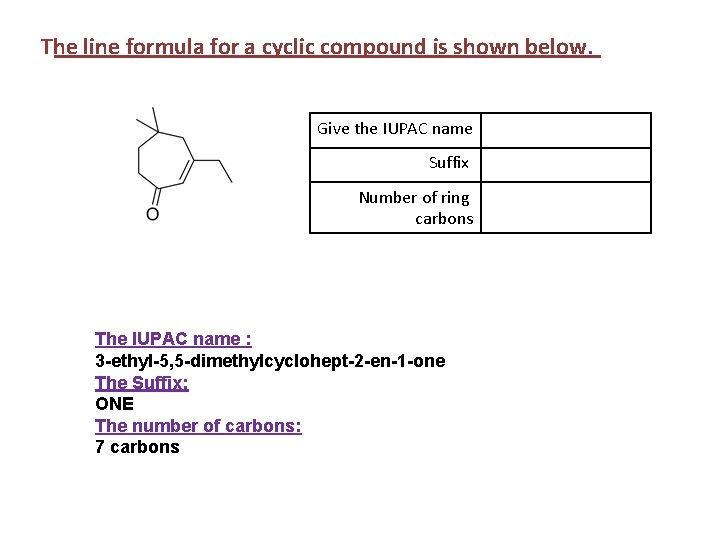
The line formula for a cyclic compound is shown below. Give the IUPAC name Suffix Number of ring carbons The IUPAC name : 3 -ethyl-5, 5 -dimethylcyclohept-2 -en-1 -one The Suffix: ONE The number of carbons: 7 carbons

• A line formula for a compound is shown below. • How many carbon atoms are in the root chain? • Give the IUPAC name for this compound? Answer: Number of carbons 5 The IUPAC name: 4 -chloro-2 -ethyl-3 -methylpent-4 -enoic acid

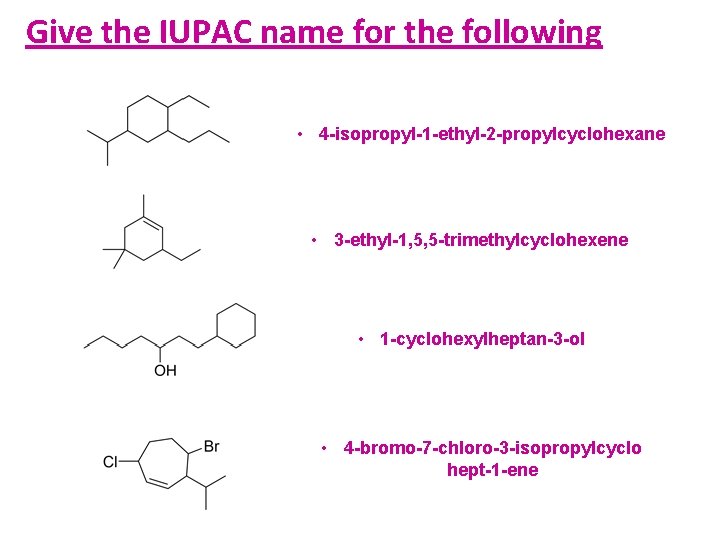
Give the IUPAC name for the following • 4 -isopropyl-1 -ethyl-2 -propylcyclohexane • 3 -ethyl-1, 5, 5 -trimethylcyclohexene • 1 -cyclohexylheptan-3 -ol • 4 -bromo-7 -chloro-3 -isopropylcyclo hept-1 -ene


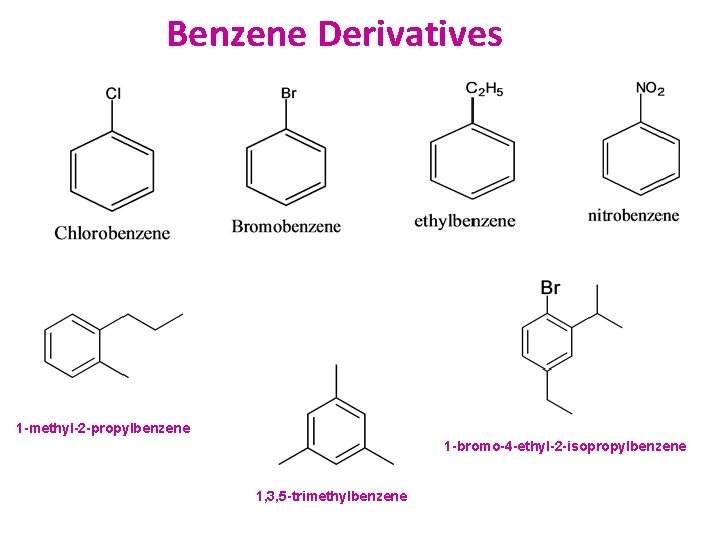
Benzene Derivatives 1 -methyl-2 -propylbenzene 1 -bromo-4 -ethyl-2 -isopropylbenzene 1, 3, 5 -trimethylbenzene
 Mamdouh mahfouz
Mamdouh mahfouz Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Lewis dot structure ch4
Lewis dot structure ch4 Organic chemistry lab report example
Organic chemistry lab report example Klein
Klein Conjugation organic chemistry
Conjugation organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry
Organic chemistry Cn functional group
Cn functional group Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Organic chemistry
Organic chemistry Mass spec of chlorine
Mass spec of chlorine Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Cyclopentane condensed structural formula
Cyclopentane condensed structural formula Organic chemistry cheat sheet
Organic chemistry cheat sheet What is organic chemistry
What is organic chemistry Organic chemistry
Organic chemistry Organic synthesis via enolates
Organic synthesis via enolates Nonene
Nonene Met et prop but
Met et prop but Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry
Organic chemistry Nomenclature of ethers
Nomenclature of ethers What is organic chemistry like
What is organic chemistry like Organic chemistry
Organic chemistry Canola oil
Canola oil The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms What is chemistry
What is chemistry