MAJOR CHEMICAL PROCESSES The chemical processes in the


- Slides: 40

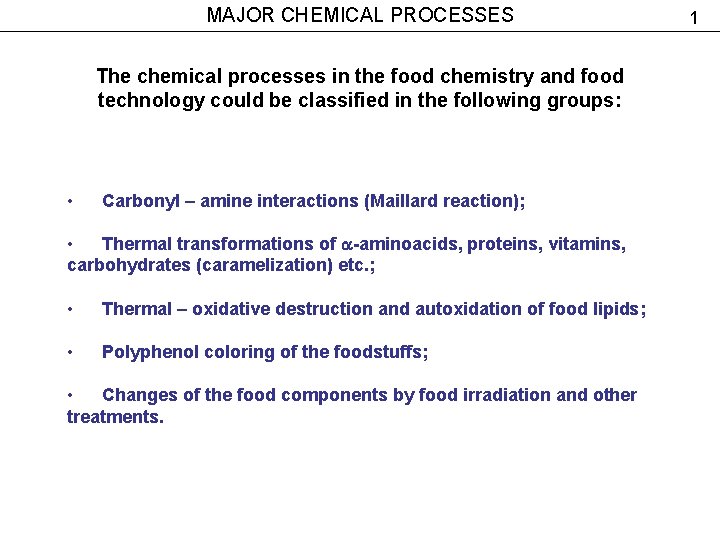
MAJOR CHEMICAL PROCESSES The chemical processes in the food chemistry and food technology could be classified in the following groups: • Carbonyl – amine interactions (Maillard reaction); • Thermal transformations of -aminoacids, proteins, vitamins, carbohydrates (caramelization) etc. ; • Thermal – oxidative destruction and autoxidation of food lipids; • Polyphenol coloring of the foodstuffs; • Changes of the food components by food irradiation and other treatments. 1

MAILLARD REACTION OBJECTIVES 1. Importance of Maillard reaction for food chemistry; 2. Major steps and some reactions; 3. Study the kinetics and parameters influencing the Maillard reaction; 4. Control and inhibition of Maillard reaction. 2

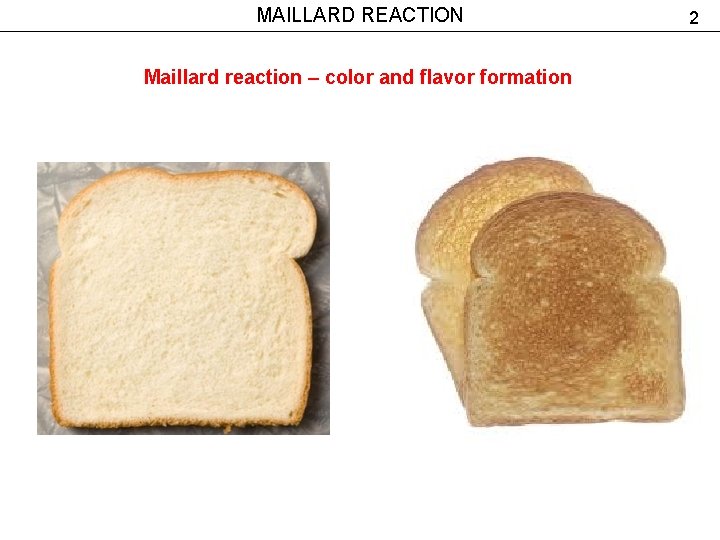
MAILLARD REACTION Maillard reaction – color and flavor formation 2

MAILLARD REACTION Maillard reaction - history 1912 г. Louis Camille Maillard a French physician and chemist - 3 scientific papers: C. R. Acad. Sci. , France, 54, 66 - 68, 1912; C. R. Soc. Biol. , 72, 559 - 561, 1912; Ann. Chem. , 9 (5), 258 - 317, 1916. Major conclusions: 1. All reducing sugars (aldoses, ketoses; penthoses and hexoses) could react with amino acids; 2. Reaction takes place in broad temperature range – 150 -200 о. С; 3. Reaction has autocatalytic character and the color is changed with accelerating speed and finally reach dark brown color; 4. During the reaction the amino acid undergo decarboxylation (lost of CO 2) – a sign that Strecker degradation takes place; 5. The participating in the reaction carbohydrates and amino acids can not be hydrolyzed (i. e. restored) by the known methods; 6. The melanoidins and the humic acids (building the humus) have similar nature; 3

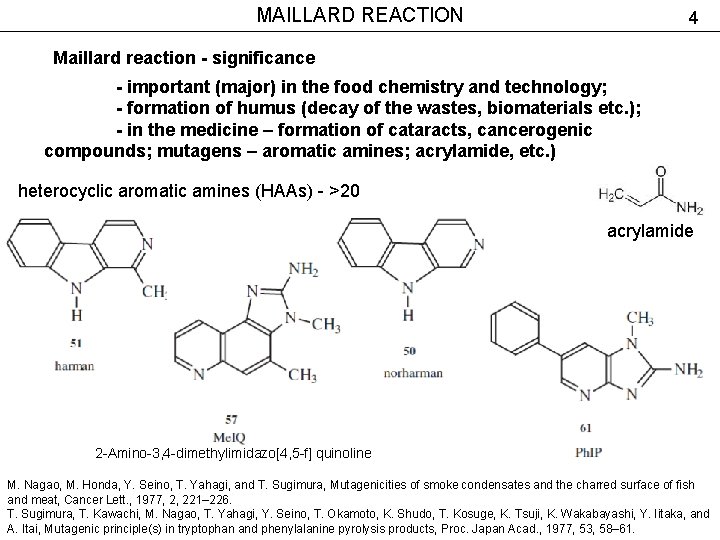
MAILLARD REACTION 4 Maillard reaction - significance - important (major) in the food chemistry and technology; - formation of humus (decay of the wastes, biomaterials etc. ); - in the medicine – formation of cataracts, cancerogenic compounds; mutagens – aromatic amines; acrylamide, etc. ) heterocyclic aromatic amines (HAAs) - >20 acrylamide 2 -Amino-3, 4 -dimethylimidazo[4, 5 -f] quinoline M. Nagao, M. Honda, Y. Seino, T. Yahagi, and T. Sugimura, Mutagenicities of smoke condensates and the charred surface of fish and meat, Cancer Lett. , 1977, 2, 221– 226. T. Sugimura, T. Kawachi, M. Nagao, T. Yahagi, Y. Seino, T. Okamoto, K. Shudo, T. Kosuge, K. Tsuji, K. Wakabayashi, Y. Iitaka, and A. Itai, Mutagenic principle(s) in tryptophan and phenylalanine pyrolysis products, Proc. Japan Acad. , 1977, 53, 58– 61.

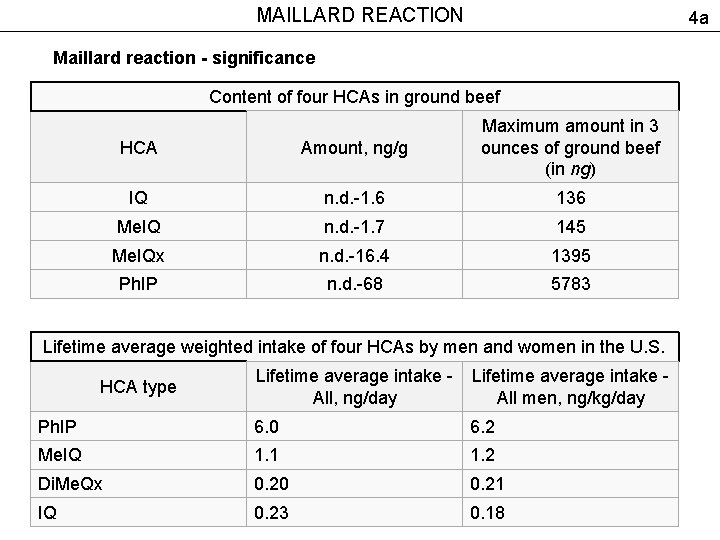
MAILLARD REACTION 4 a Maillard reaction - significance Content of four HCAs in ground beef HCA Amount, ng/g Maximum amount in 3 ounces of ground beef (in ng) IQ n. d. -1. 6 136 Me. IQ n. d. -1. 7 145 Me. IQx n. d. -16. 4 1395 Ph. IP n. d. -68 5783 Lifetime average weighted intake of four HCAs by men and women in the U. S. HCA type Lifetime average intake - All, ng/day All men, ng/kg/day Ph. IP 6. 0 6. 2 Me. IQ 1. 1 1. 2 Di. Me. Qx 0. 20 0. 21 IQ 0. 23 0. 18

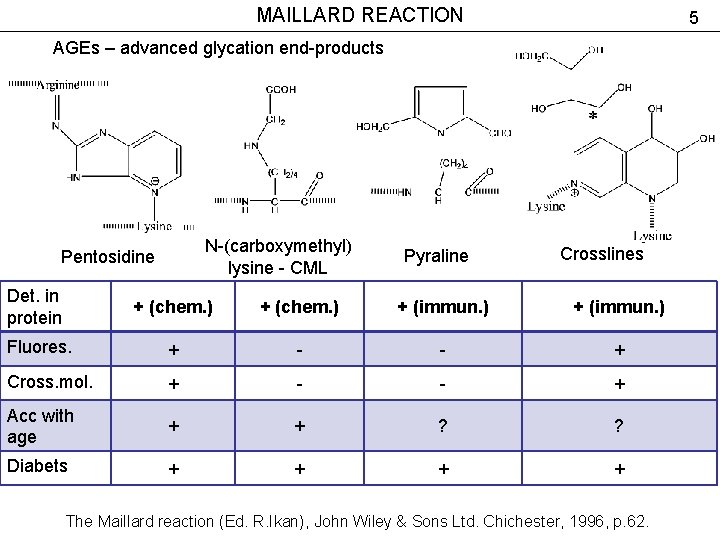
MAILLARD REACTION 5 AGEs – advanced glycation end-products N-(carboxymethyl) lysine - CML Pentosidine Pyraline Crosslines Det. in protein + (chem. ) + (immun. ) Fluores. + - - + Cross. mol. + - - + Acc with age + + ? ? Diabets + + The Maillard reaction (Ed. R. Ikan), John Wiley & Sons Ltd. Chichester, 1996, p. 62.

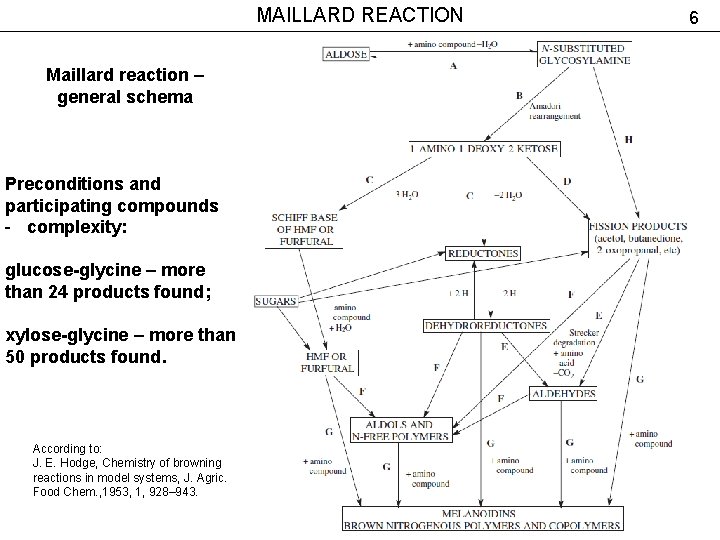
MAILLARD REACTION Maillard reaction – general schema Preconditions and participating compounds - complexity: glucose-glycine – more than 24 products found; xylose-glycine – more than 50 products found. According to: J. E. Hodge, Chemistry of browning reactions in model systems, J. Agric. Food Chem. , 1953, 1, 928– 943. 6

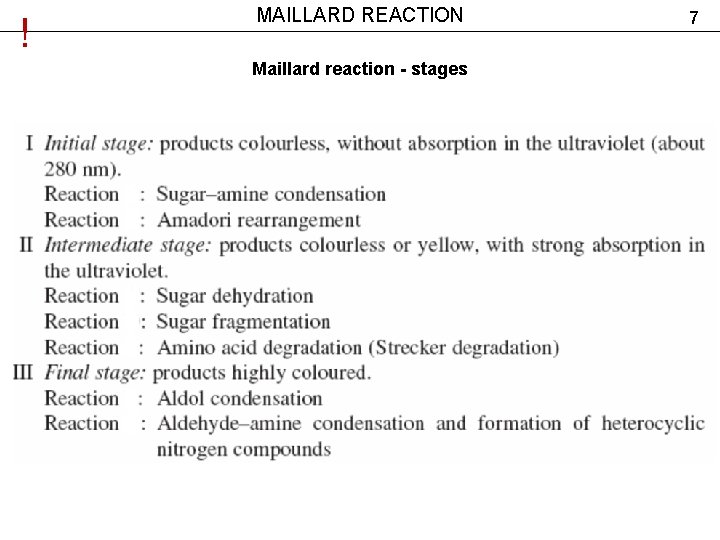
! MAILLARD REACTION Maillard reaction - stages 7

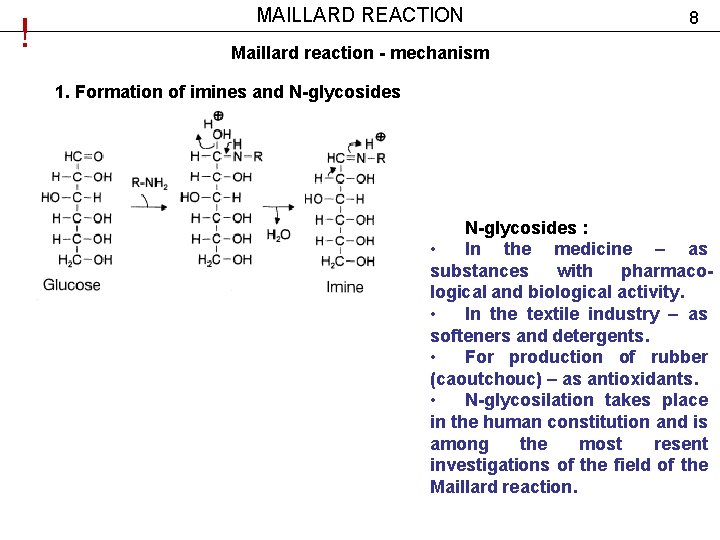
! MAILLARD REACTION 8 Maillard reaction - mechanism 1. Formation of imines and N-glycosides : • In the medicine – as substances with pharmacological and biological activity. • In the textile industry – as softeners and detergents. • For production of rubber (caoutchouc) – as antioxidants. • N-glycosilation takes place in the human constitution and is among the most resent investigations of the field of the Maillard reaction.

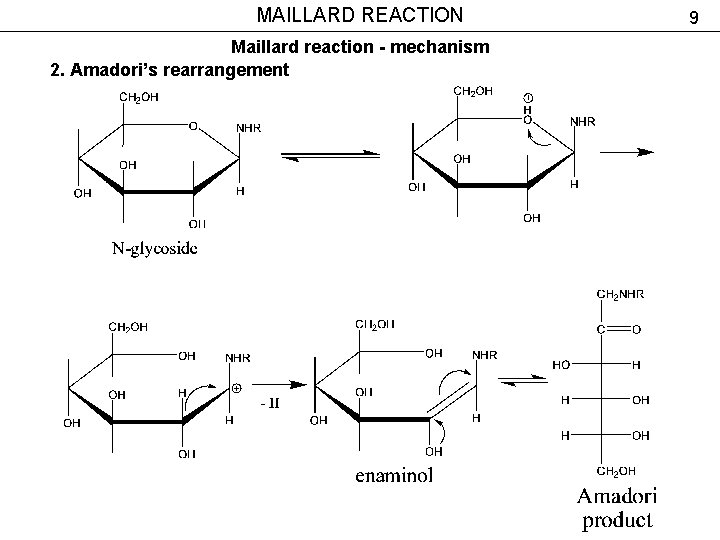
MAILLARD REACTION Maillard reaction - mechanism 2. Amadori’s rearrangement 9

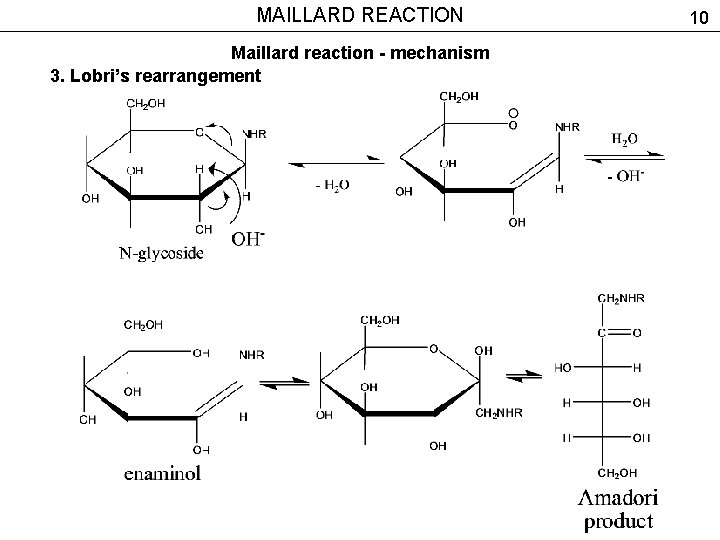
MAILLARD REACTION Maillard reaction - mechanism 3. Lobri’s rearrangement 10

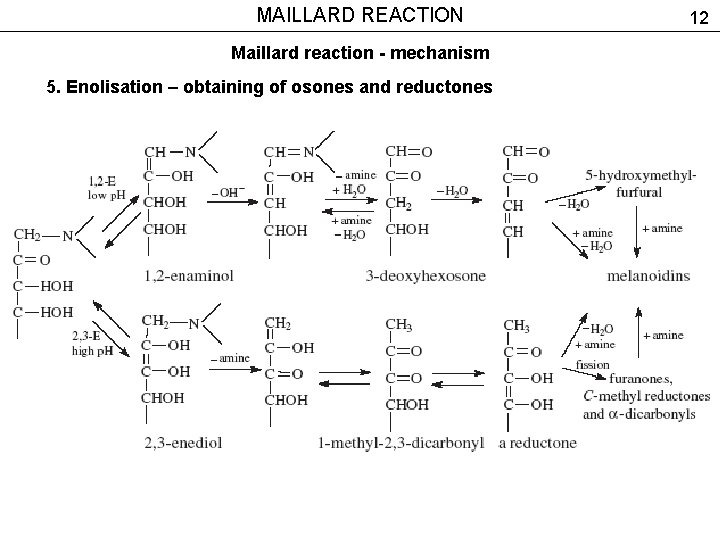
MAILLARD REACTION Maillard reaction - mechanism 5. Enolisation – obtaining of osones and reductones 12

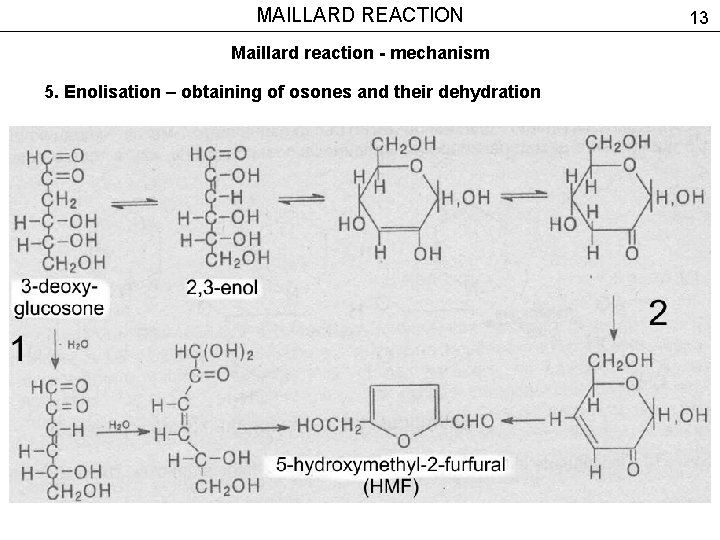
MAILLARD REACTION Maillard reaction - mechanism 5. Enolisation – obtaining of osones and their dehydration 13

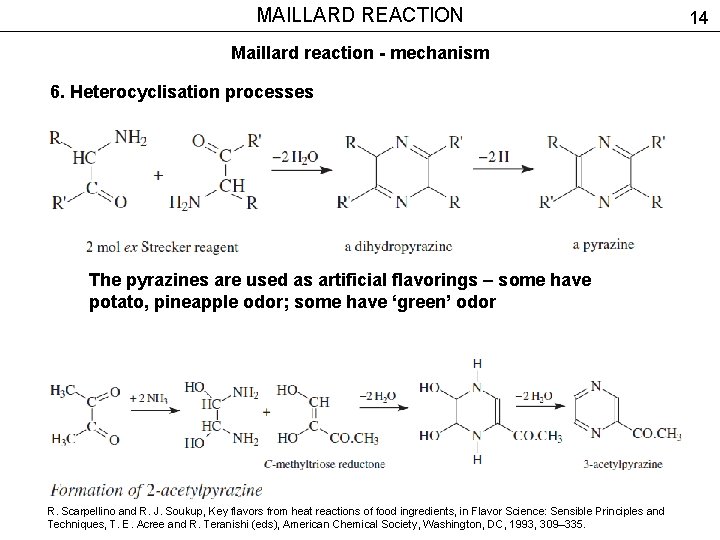
MAILLARD REACTION Maillard reaction - mechanism 6. Heterocyclisation processes The pyrazines are used as artificial flavorings – some have potato, pineapple odor; some have ‘green’ odor R. Scarpellino and R. J. Soukup, Key flavors from heat reactions of food ingredients, in Flavor Science: Sensible Principles and Techniques, T. E. Acree and R. Teranishi (eds), American Chemical Society, Washington, DC, 1993, 309– 335. 14

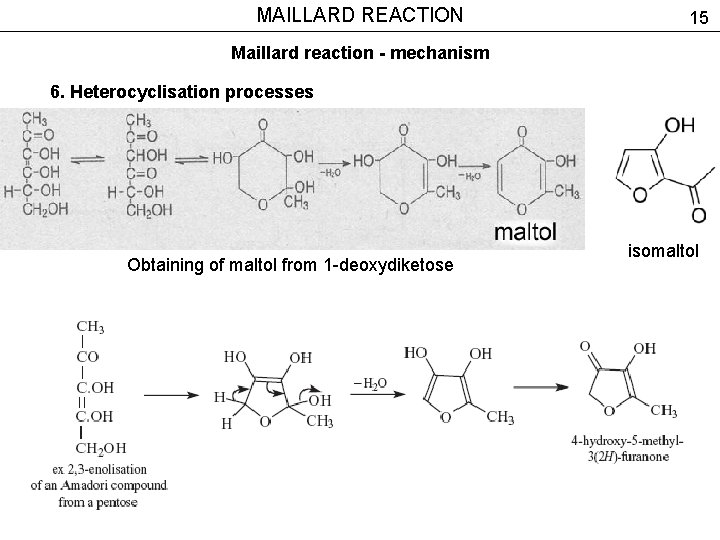
MAILLARD REACTION 15 Maillard reaction - mechanism 6. Heterocyclisation processes Obtaining of maltol from 1 -deoxydiketose isomaltol

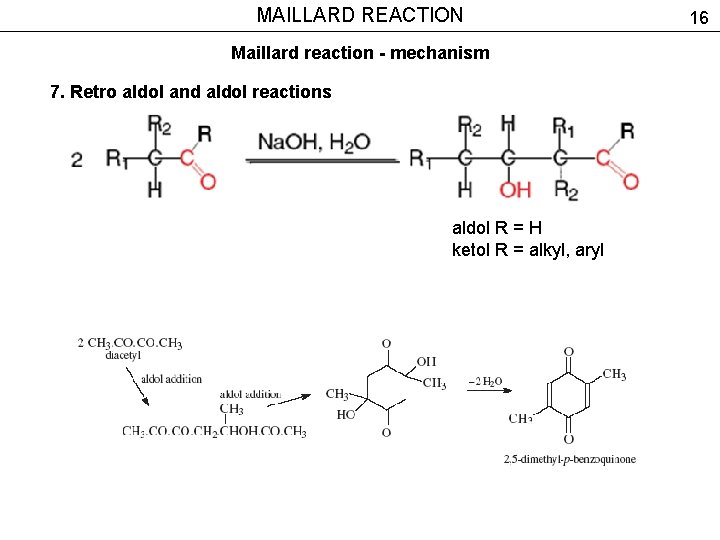
MAILLARD REACTION Maillard reaction - mechanism 7. Retro aldol and aldol reactions aldol R = H ketol R = alkyl, aryl 16

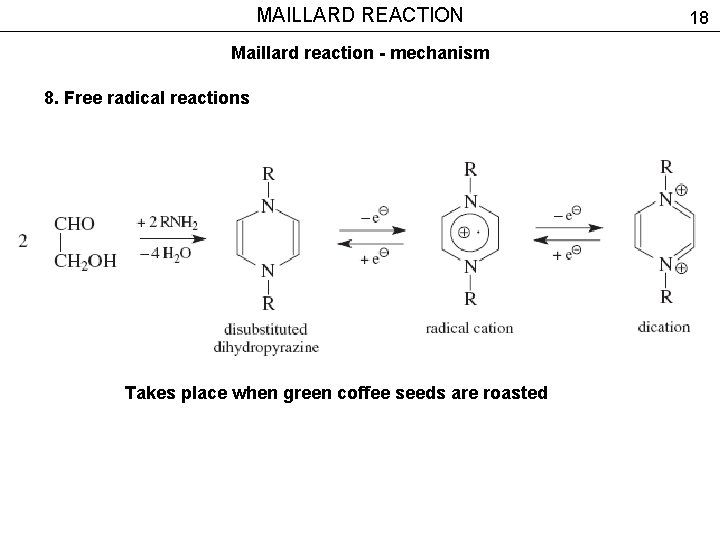
MAILLARD REACTION Maillard reaction - mechanism 8. Free radical reactions Takes place when green coffee seeds are roasted 18

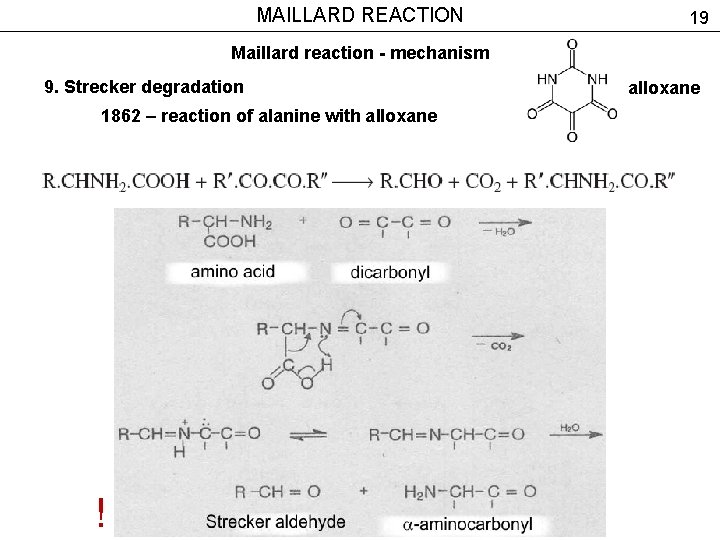
MAILLARD REACTION 19 Maillard reaction - mechanism 9. Strecker degradation 1862 – reaction of alanine with alloxane ! alloxane

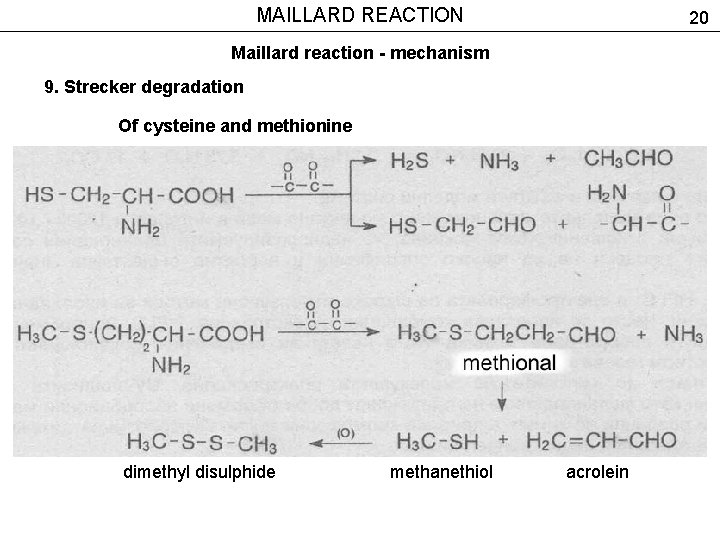
MAILLARD REACTION 20 Maillard reaction - mechanism 9. Strecker degradation Of cysteine and methionine dimethyl disulphide methanethiol acrolein

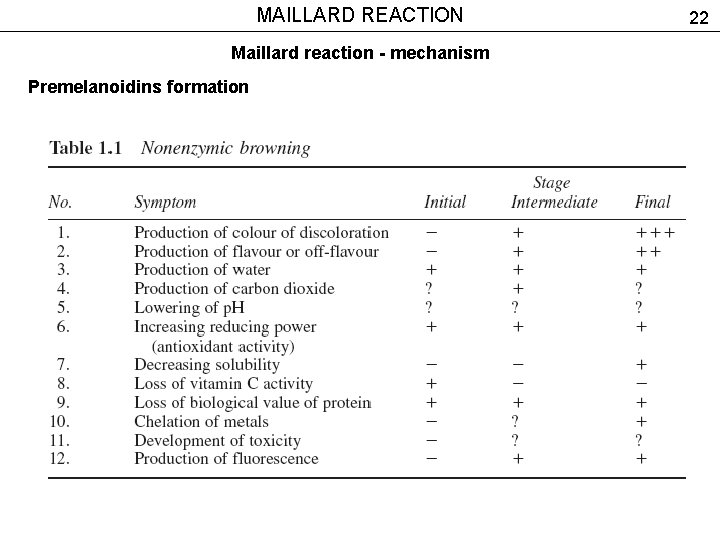
MAILLARD REACTION Maillard reaction - mechanism Premelanoidins formation 22

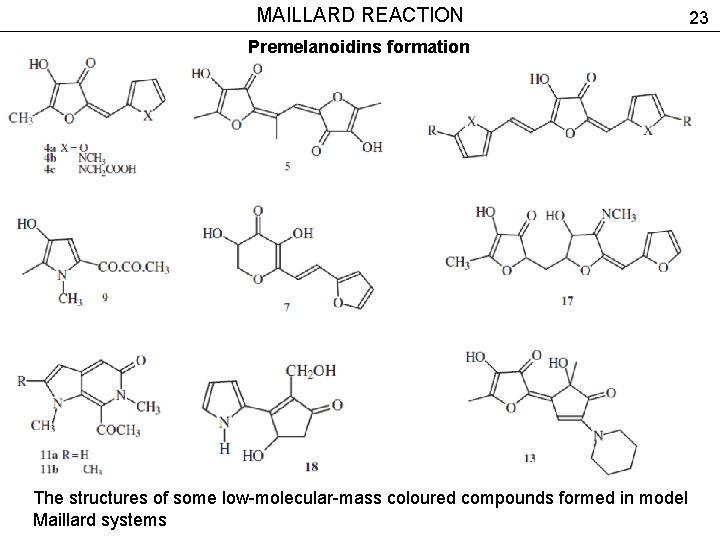
MAILLARD REACTION 23 Premelanoidins formation The structures of some low-molecular-mass coloured compounds formed in model Maillard systems

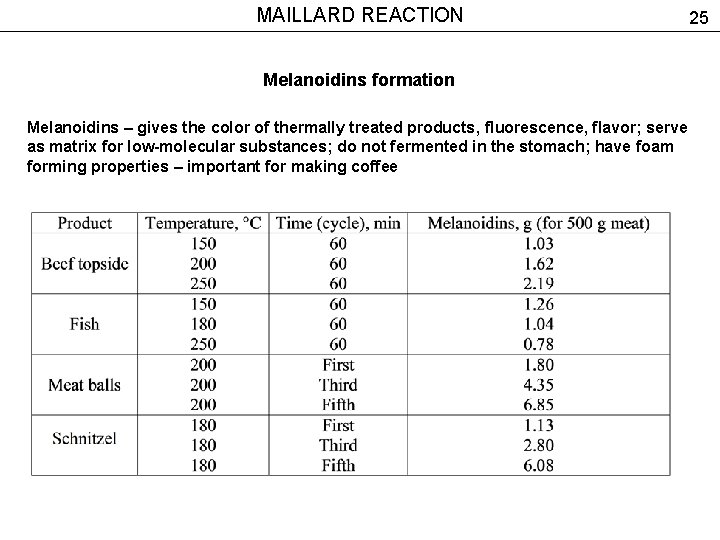
MAILLARD REACTION Melanoidins formation Melanoidins – gives the color of thermally treated products, fluorescence, flavor; serve as matrix for low-molecular substances; do not fermented in the stomach; have foam forming properties – important for making coffee 25

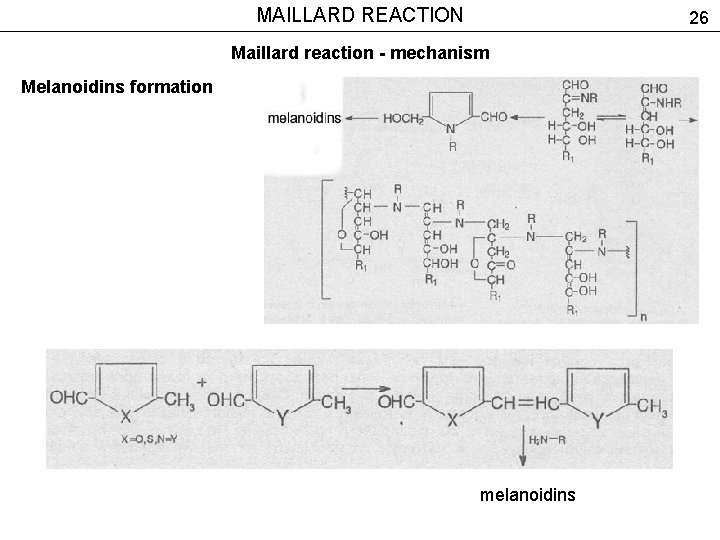
MAILLARD REACTION 26 Maillard reaction - mechanism Melanoidins formation melanoidins

MAILLARD REACTION Maillard reaction – analysis and isolation of melanoidins Model systems and standard melanoidins • Glucose (Sigma; min 99. 5%, Cat. No. G 8270) • Glycine (Sigma; min 99%, Cat. No. G 7126) 0. 05 mol from each in 100 ml H 2 O; 2 hrs / 125 ºС. Dialysis of the mixture – 24 h, 4°С; lyophilization 28

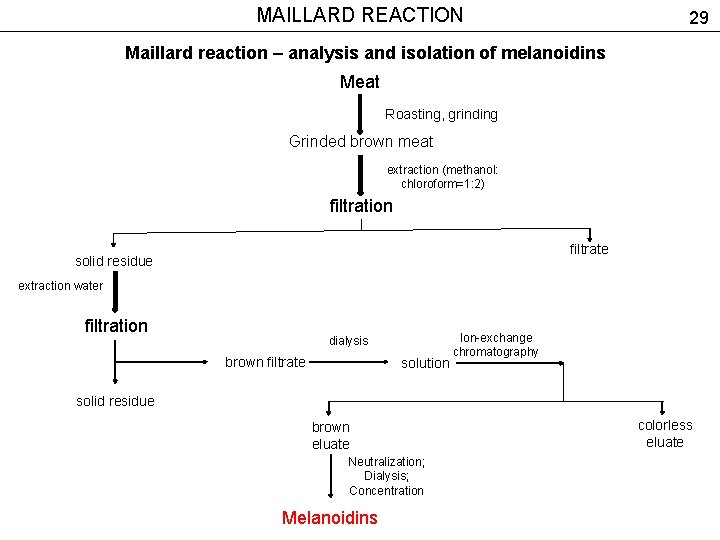
MAILLARD REACTION 29 Maillard reaction – analysis and isolation of melanoidins Meat Roasting, grinding Grinded brown meat extraction (methanol: chloroform=1: 2) filtration filtrate solid residue extraction water filtration dialysis brown filtrate solution Ion-exchange chromatography solid residue brown eluate Neutralization; Dialysis; Concentration Melanoidins colorless eluate

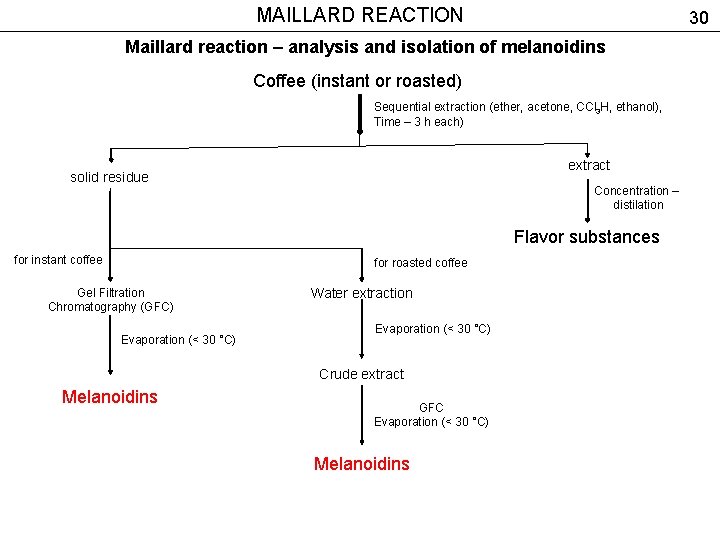
MAILLARD REACTION 30 Maillard reaction – analysis and isolation of melanoidins Coffee (instant or roasted) Sequential extraction (ether, acetone, CCl 3 H, ethanol), Time – 3 h each) extract solid residue Concentration – distilation Flavor substances for instant coffee for roasted coffee Gel Filtration Chromatography (GFC) Evaporation (< 30 °C) Water extraction Evaporation (< 30 °C) Crude extract Melanoidins GFC Evaporation (< 30 °C) Melanoidins

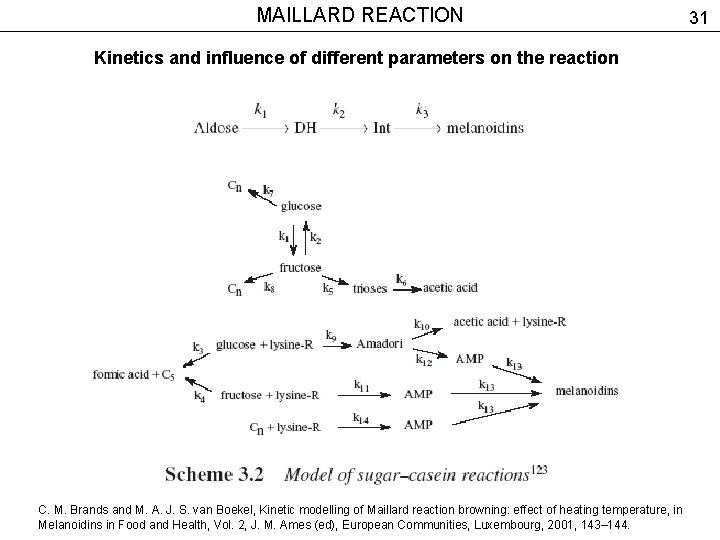
MAILLARD REACTION Kinetics and influence of different parameters on the reaction C. M. Brands and M. A. J. S. van Boekel, Kinetic modelling of Maillard reaction browning: effect of heating temperature, in Melanoidins in Food and Health, Vol. 2, J. M. Ames (ed), European Communities, Luxembourg, 2001, 143– 144. 31

MAILLARD REACTION Kinetics and influence of different parameters on the reaction 1. Type of the carbohydrate pentoses > hexoses ribose – the most reactive sugar (pentoses) ! galactose – the most reactive sugar (hexoses) ribose : xylose: galactose = 100 : 6 : 1 lactose – the most reactive disaccharides 2. Type of the amino compound ! Lysine – the most reactive amino acid 32

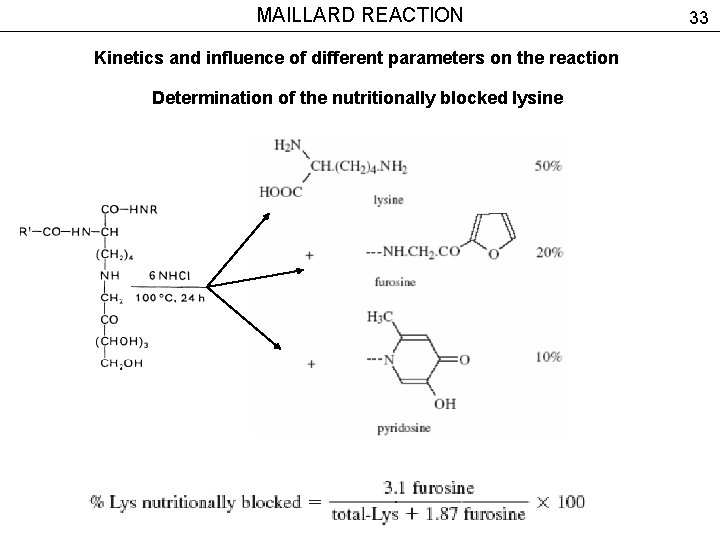
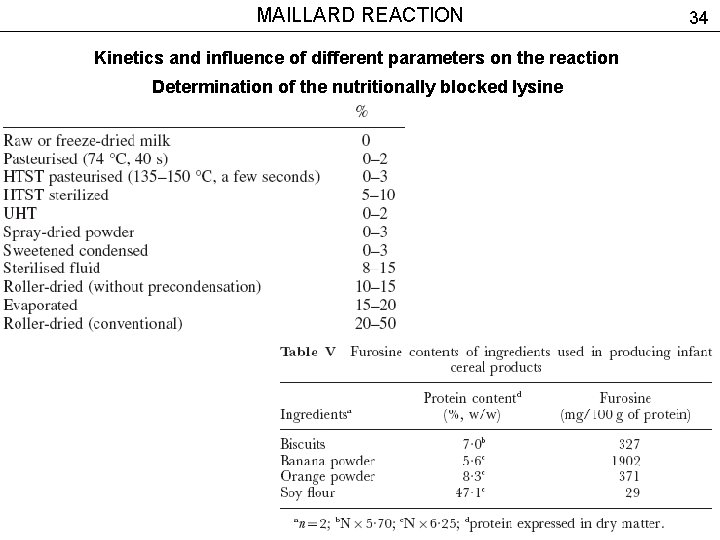
MAILLARD REACTION Kinetics and influence of different parameters on the reaction Determination of the nutritionally blocked lysine 33

MAILLARD REACTION Kinetics and influence of different parameters on the reaction Determination of the nutritionally blocked lysine 34

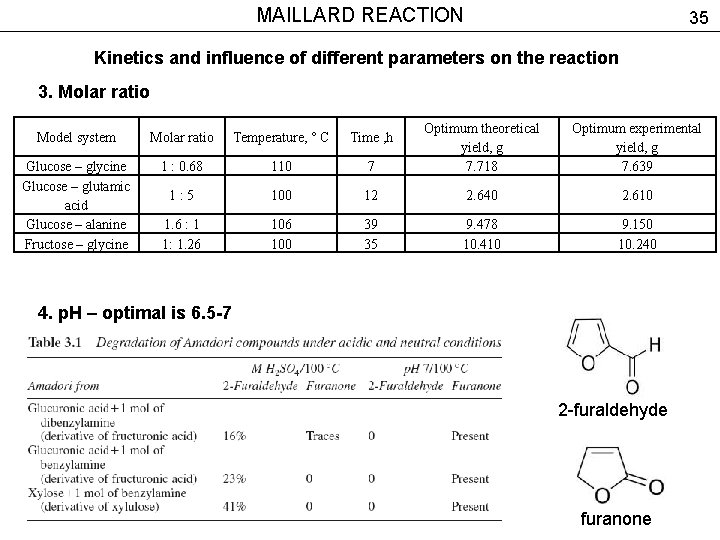
MAILLARD REACTION 35 Kinetics and influence of different parameters on the reaction 3. Molar ratio 7 Optimum theoretical yield, g 7. 718 Optimum experimental yield, g 7. 639 100 12 2. 640 2. 610 106 100 39 35 9. 478 10. 410 9. 150 10. 240 Model system Molar ratio Temperature, ° C Time , h Glucose – glycine Glucose – glutamic acid Glucose – alanine Fructose – glycine 1 : 0. 68 110 1: 5 1. 6 : 1 1: 1. 26 4. p. H – optimal is 6. 5 -7 2 -furaldehyde furanone

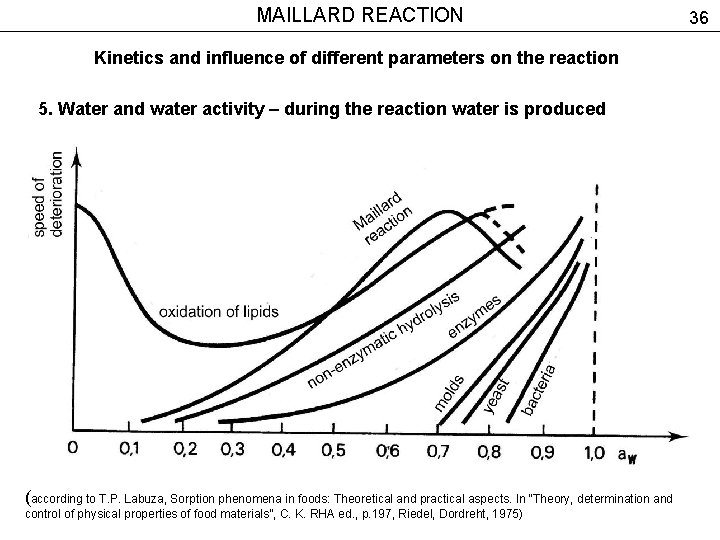
MAILLARD REACTION Kinetics and influence of different parameters on the reaction 5. Water and water activity – during the reaction water is produced (according to T. P. Labuza, Sorption phenomena in foods: Theoretical and practical aspects. In “Theory, determination and control of physical properties of food materials”, C. K. RHA ed. , p. 197, Riedel, Dordreht, 1975) 36

MAILLARD REACTION Kinetics and influence of different parameters on the reaction 6. Temperature - 180 -220ºС 7. Metal cations – Cu, Fe – catalyzers (speeding); Mn, Sn – inhitors; 8. Other compounds – lipids, chitosan, phospholipids 37

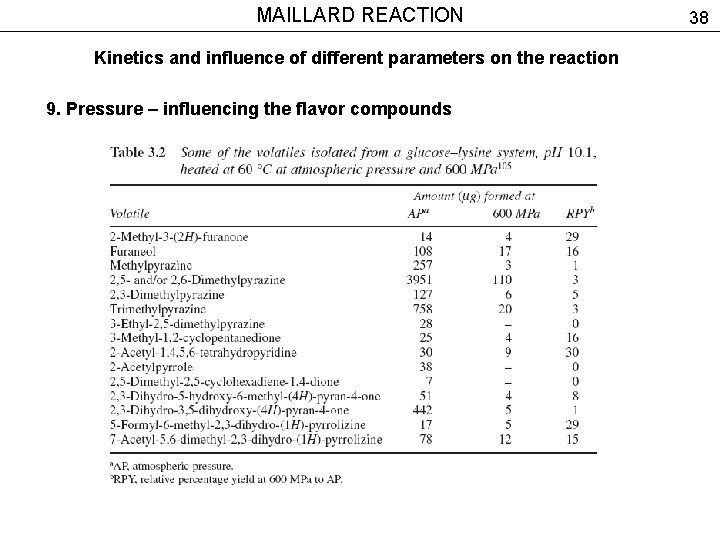
MAILLARD REACTION Kinetics and influence of different parameters on the reaction 9. Pressure – influencing the flavor compounds 38

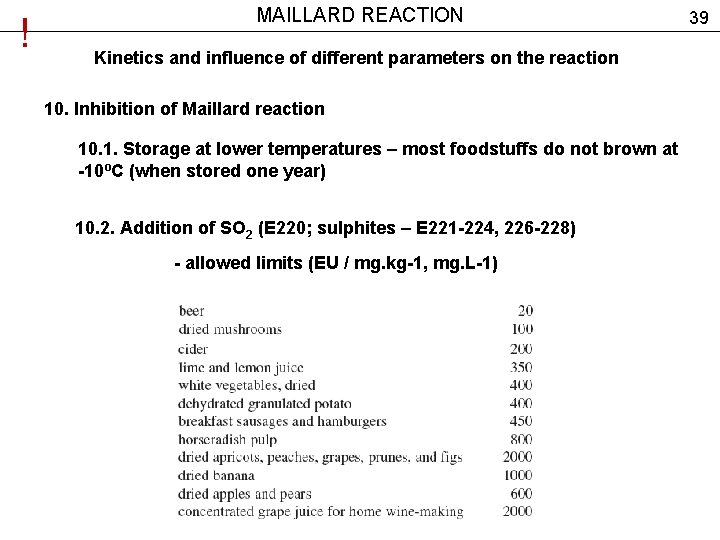
! MAILLARD REACTION Kinetics and influence of different parameters on the reaction 10. Inhibition of Maillard reaction 10. 1. Storage at lower temperatures – most foodstuffs do not brown at -10ºС (when stored one year) 10. 2. Addition of SO 2 (E 220; sulphites – Е 221 -224, 226 -228) - allowed limits (EU / mg. kg-1, mg. L-1) 39

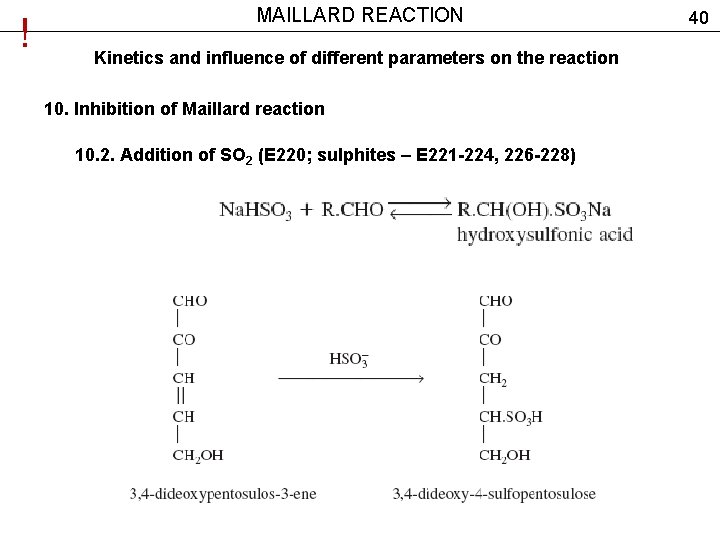
! MAILLARD REACTION Kinetics and influence of different parameters on the reaction 10. Inhibition of Maillard reaction 10. 2. Addition of SO 2 (E 220; sulphites – Е 221 -224, 226 -228) 40

! MAILLARD REACTION 41 Kinetics and influence of different parameters on the reaction 10. Inhibition of Maillard reaction 3. Lowering the р. Н 4. Dehydration – example: fruit juices (with combination with addition of SO 2 5. Removal of some of the foods’ components 5. 1. Carbohydrates dried Chinese egg white – removal of glucose by fermentation (! Microbial contamination) potato chips – dipping in the solution with glucose oxidase removal of lactose from milk

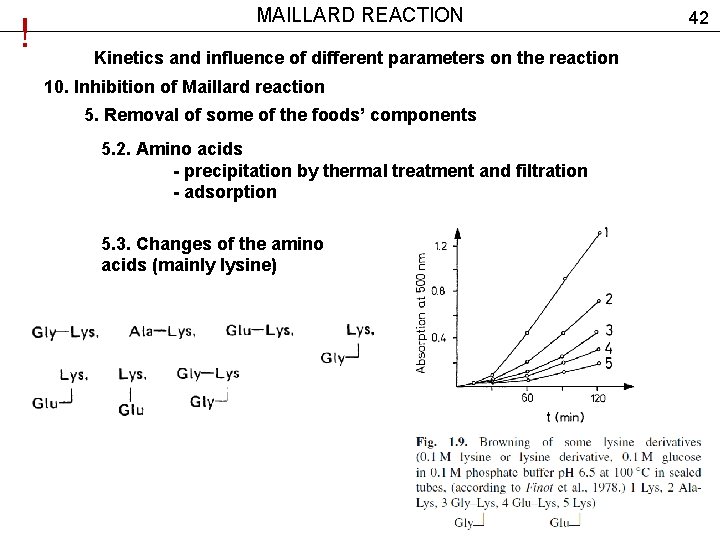
! MAILLARD REACTION Kinetics and influence of different parameters on the reaction 10. Inhibition of Maillard reaction 5. Removal of some of the foods’ components 5. 2. Amino acids - precipitation by thermal treatment and filtration - adsorption 5. 3. Changes of the amino acids (mainly lysine) 42

MAILLARD REACTION NO! 43
 Concurrent processes are processes that
Concurrent processes are processes that Three types of printmaking
Three types of printmaking Make a research about market forms of poultry
Make a research about market forms of poultry Chemical processes
Chemical processes The major reason for chemical bonding is
The major reason for chemical bonding is Strengthscape
Strengthscape Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Bổ thể
Bổ thể Phản ứng thế ankan
Phản ứng thế ankan Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Số nguyên tố là gì
Số nguyên tố là gì Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Fecboak
Fecboak đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Hệ hô hấp
Hệ hô hấp ưu thế lai là gì
ưu thế lai là gì Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Tư thế ngồi viết
Tư thế ngồi viết Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Tư thế ngồi viết
Tư thế ngồi viết Chó sói
Chó sói Thẻ vin
Thẻ vin V cc
V cc Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Slidetodoc
Slidetodoc 101012 bằng
101012 bằng Lời thề hippocrates
Lời thề hippocrates