Chemical Reactions definition Chemical reactions are processes in



- Slides: 12

Chemical Reactions

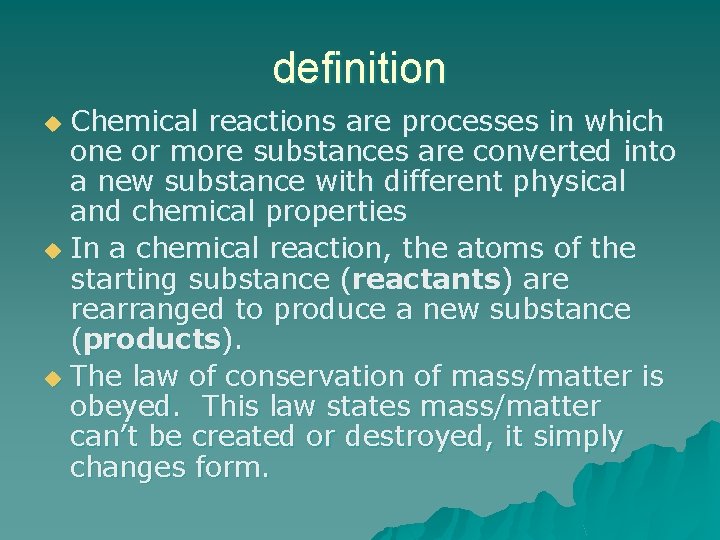
definition Chemical reactions are processes in which one or more substances are converted into a new substance with different physical and chemical properties u In a chemical reaction, the atoms of the starting substance (reactants) are rearranged to produce a new substance (products). u The law of conservation of mass/matter is obeyed. This law states mass/matter can’t be created or destroyed, it simply changes form. u

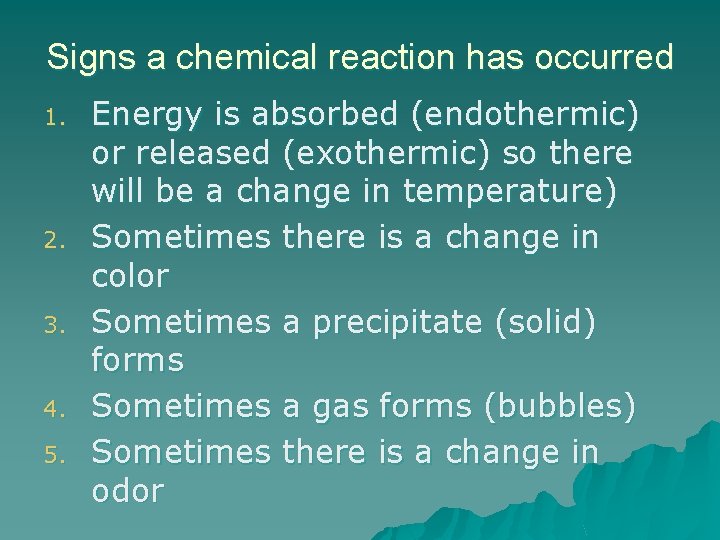
Signs a chemical reaction has occurred 1. 2. 3. 4. 5. Energy is absorbed (endothermic) or released (exothermic) so there will be a change in temperature) Sometimes there is a change in color Sometimes a precipitate (solid) forms Sometimes a gas forms (bubbles) Sometimes there is a change in odor

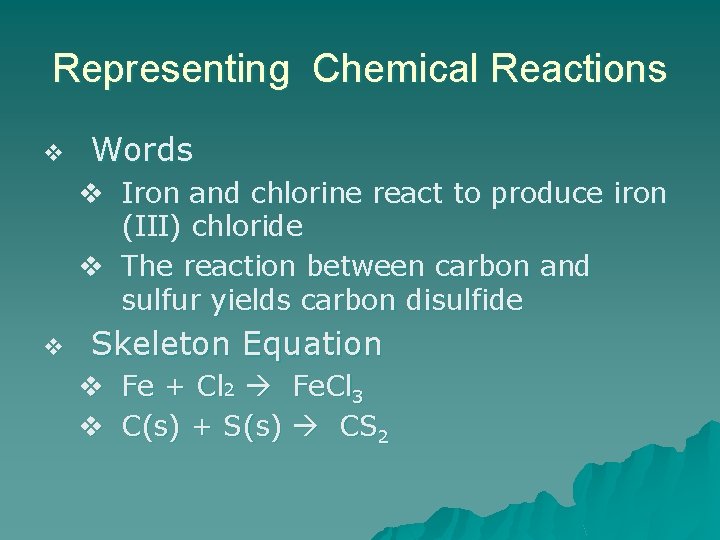
Representing Chemical Reactions v Words v Iron and chlorine react to produce iron (III) chloride v The reaction between carbon and sulfur yields carbon disulfide v Skeleton Equation v Fe + Cl 2 Fe. Cl 3 v C(s) + S(s) CS 2

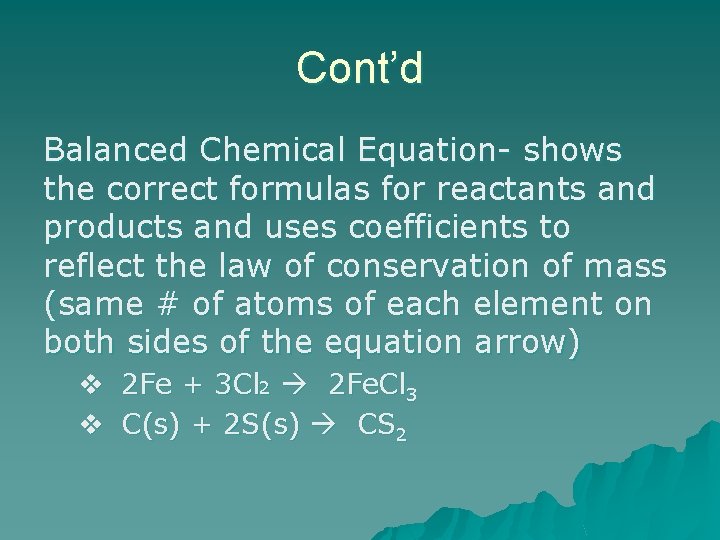
Cont’d Balanced Chemical Equation- shows the correct formulas for reactants and products and uses coefficients to reflect the law of conservation of mass (same # of atoms of each element on both sides of the equation arrow) v 2 Fe + 3 Cl 2 2 Fe. Cl 3 v C(s) + 2 S(s) CS 2

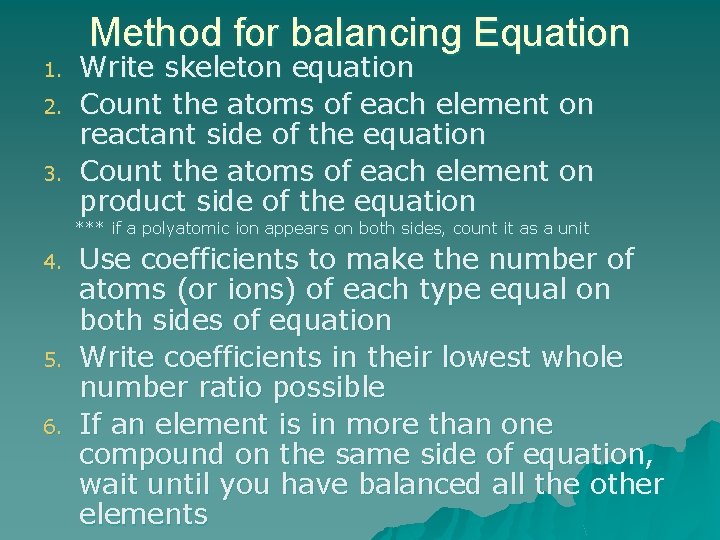
Method for balancing Equation 1. 2. 3. Write skeleton equation Count the atoms of each element on reactant side of the equation Count the atoms of each element on product side of the equation *** if a polyatomic ion appears on both sides, count it as a unit 4. 5. 6. Use coefficients to make the number of atoms (or ions) of each type equal on both sides of equation Write coefficients in their lowest whole number ratio possible If an element is in more than one compound on the same side of equation, wait until you have balanced all the other elements

Classifying Chemical reactions u There are five main types of chemical reactions: – Synthesis – Decomposition – Single replacement (or displacement) – Double replacement (or displacement) – Combustion

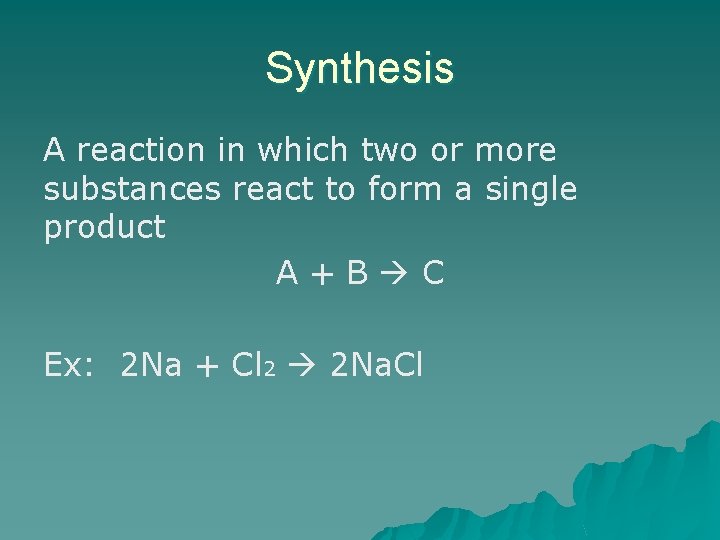
Synthesis A reaction in which two or more substances react to form a single product A+B C Ex: 2 Na + Cl 2 2 Na. Cl

Decomposition A reaction in which a single substance breaks down into two or more elements or new compounds C A+B Ex: 2 KCl. O 3 2 KCl + 3 O 2

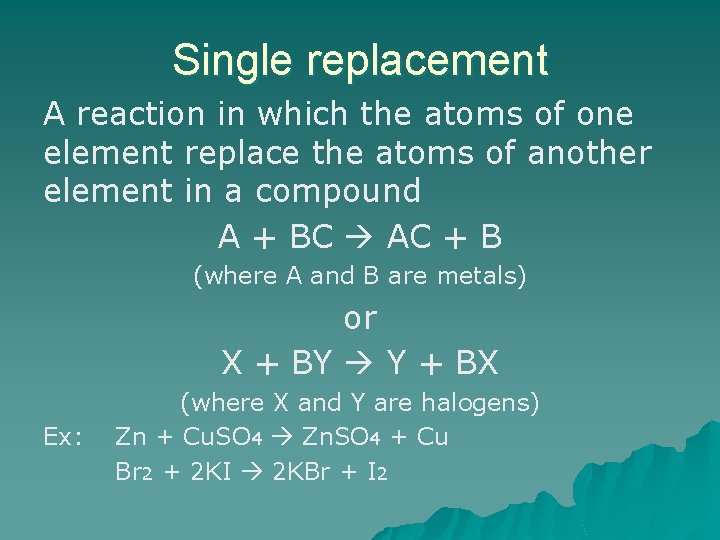
Single replacement A reaction in which the atoms of one element replace the atoms of another element in a compound A + BC AC + B (where A and B are metals) or X + BY Y + BX Ex: (where X and Y are halogens) Zn + Cu. SO 4 Zn. SO 4 + Cu Br 2 + 2 KI 2 KBr + I 2

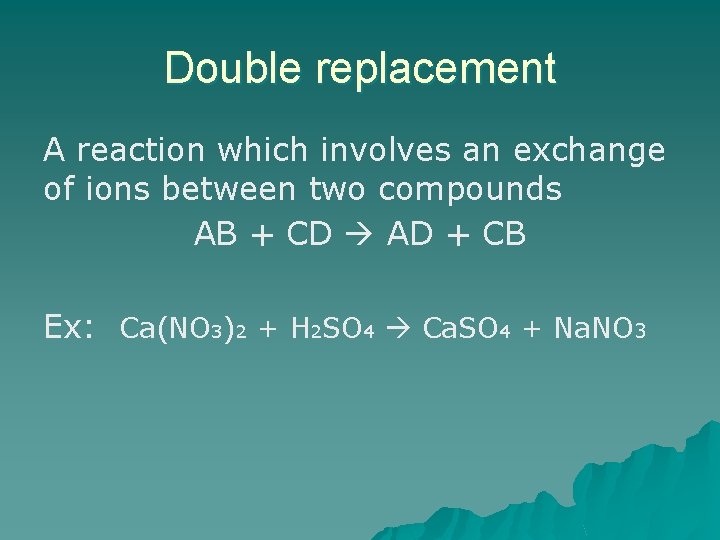
Double replacement A reaction which involves an exchange of ions between two compounds AB + CD AD + CB Ex: Ca(NO 3)2 + H 2 SO 4 Ca. SO 4 + Na. NO 3

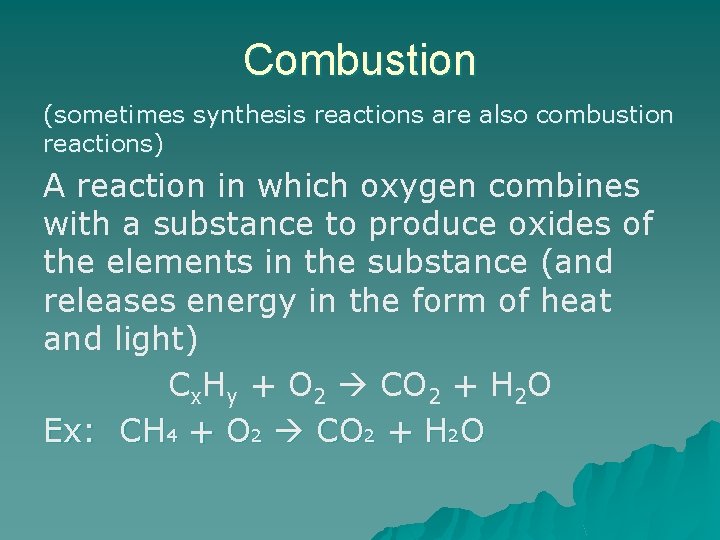
Combustion (sometimes synthesis reactions are also combustion reactions) A reaction in which oxygen combines with a substance to produce oxides of the elements in the substance (and releases energy in the form of heat and light) Cx. Hy + O 2 CO 2 + H 2 O Ex: CH 4 + O 2 CO 2 + H 2 O
 Insidan region jh
Insidan region jh Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Concurrent processes are processes that
Concurrent processes are processes that Balancing redox reactions
Balancing redox reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers A market form of meat preserved by salting smoking or aging
A market form of meat preserved by salting smoking or aging Chemical processes
Chemical processes Stoichiometry island diagram
Stoichiometry island diagram Examples of chemical change
Examples of chemical change























