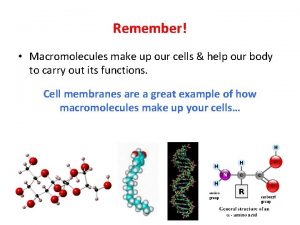
Macromolecules 1 Molecules of Life Macromolecules are large








- Slides: 45

Macromolecules 1

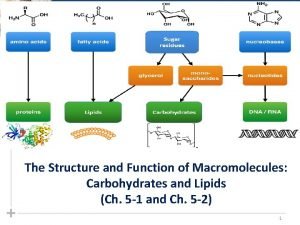
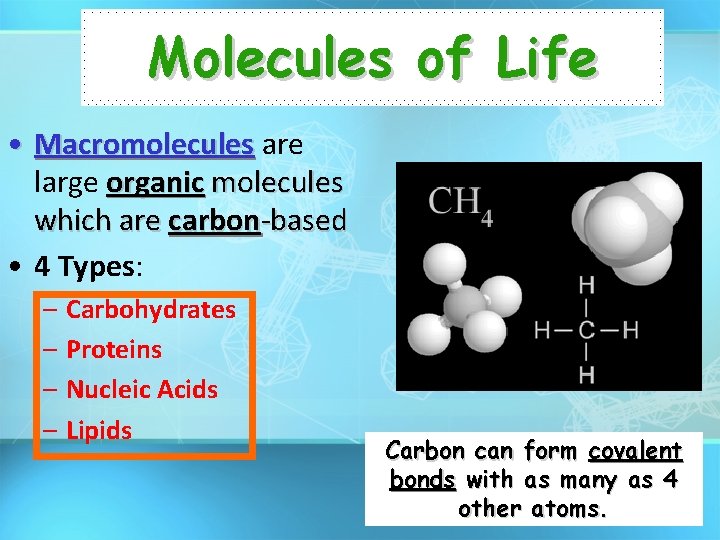
Molecules of Life • Macromolecules are large organic molecules which are carbon-based • 4 Types: – Carbohydrates – Proteins – Nucleic Acids – Lipids Carbon can form covalent bonds with as many as 4 other atoms.

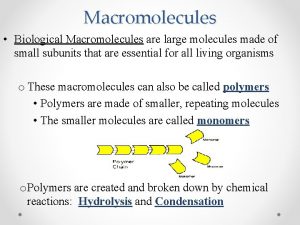
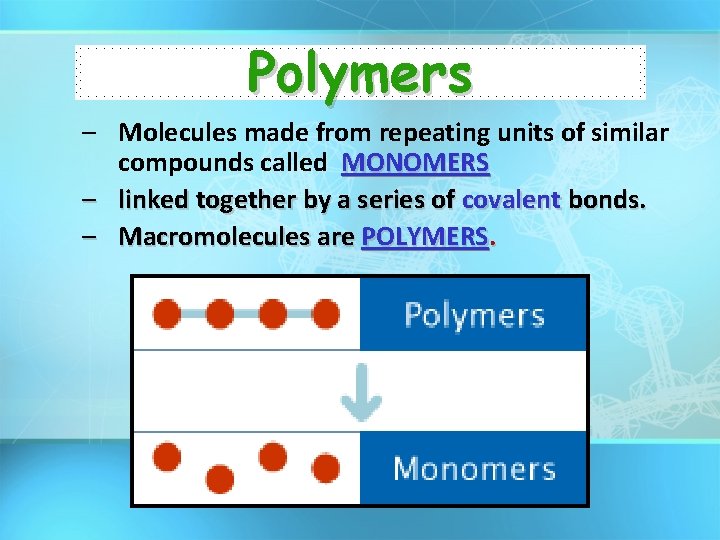
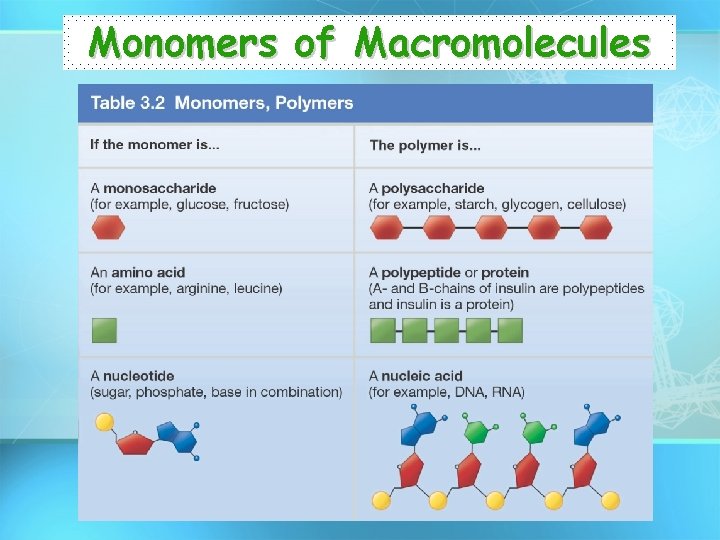
Polymers – Molecules made from repeating units of similar compounds called MONOMERS – linked together by a series of covalent bonds. – Macromolecules are POLYMERS.

Monomers of Macromolecules

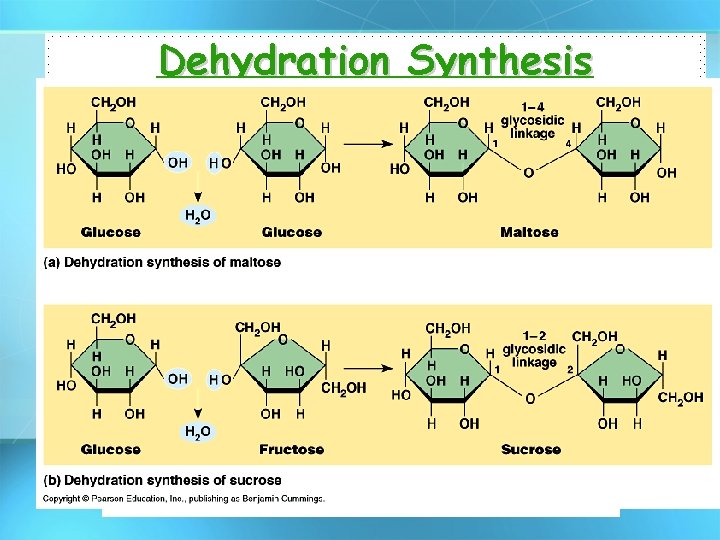
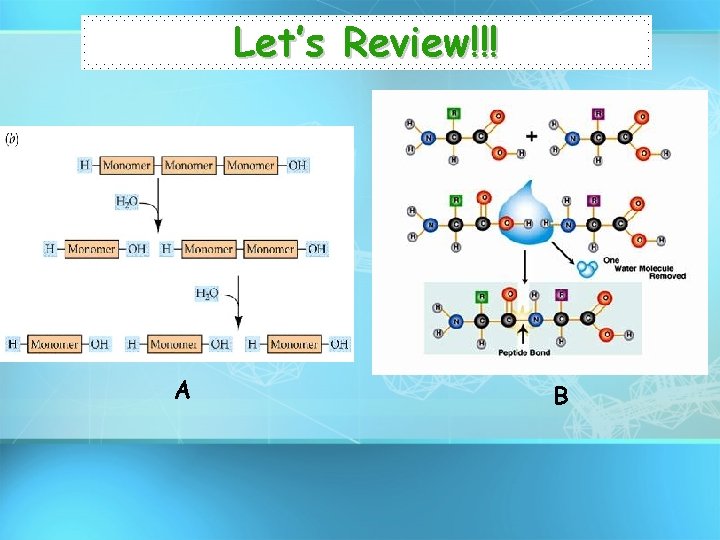
Dehydration Synthesis • Forms polymers by combining monomers by “removing water”.

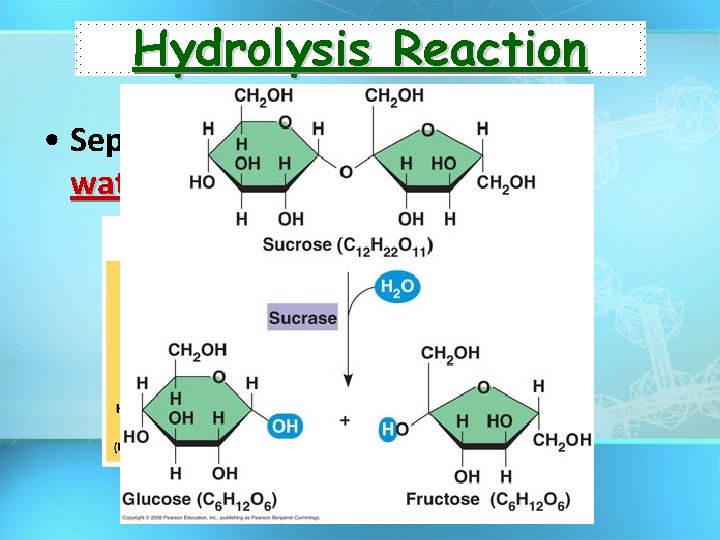
Hydrolysis Reaction • Separates monomers by “adding water”

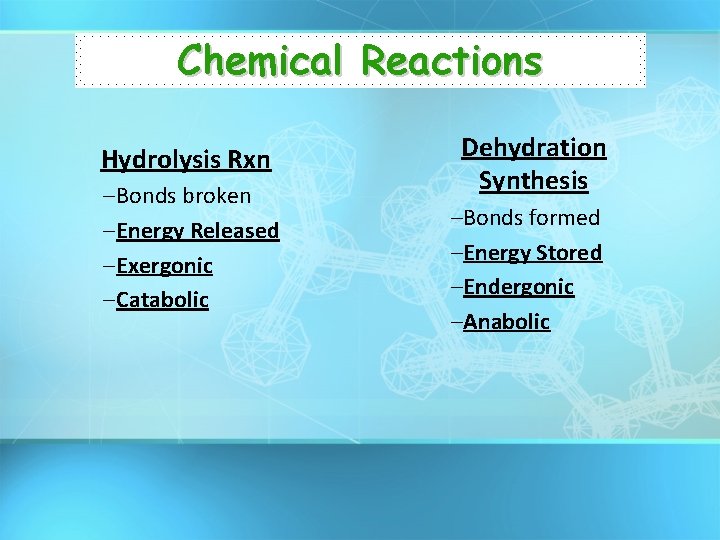
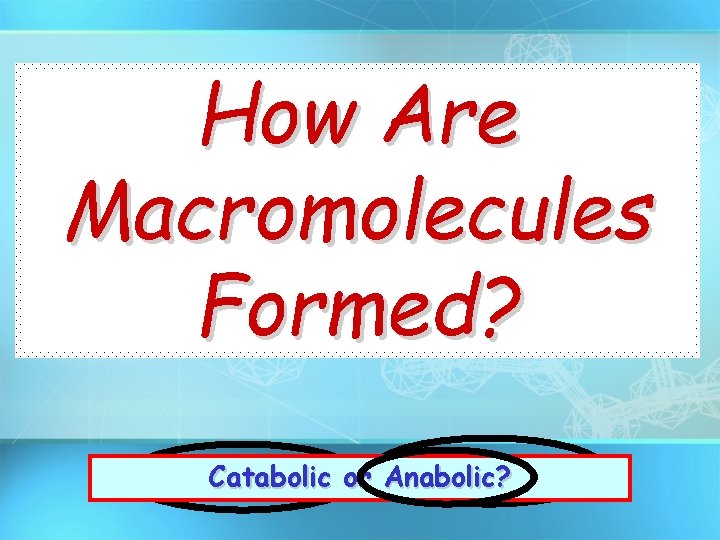
Chemical Reactions Hydrolysis Rxn – Bonds broken – Energy Released – Exergonic – Catabolic Dehydration Synthesis –Bonds formed –Energy Stored –Endergonic –Anabolic

Let’s Review!!! A B

How Are Macromolecules Formed? Release Endergonic Catabolic Energy or or Anabolic? Exergonic? Store Energy? or

How are Macromolecules separated or digested? Release Endergonic Catabolic Energy or or Anabolic? Exergonic? Store Energy? or

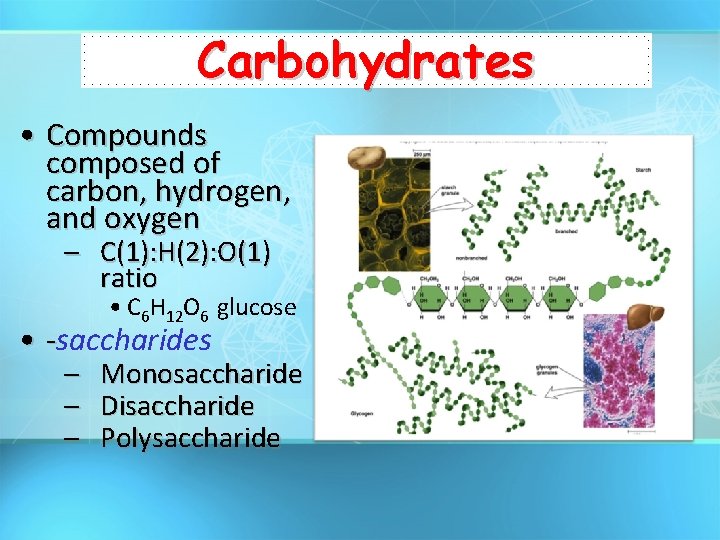
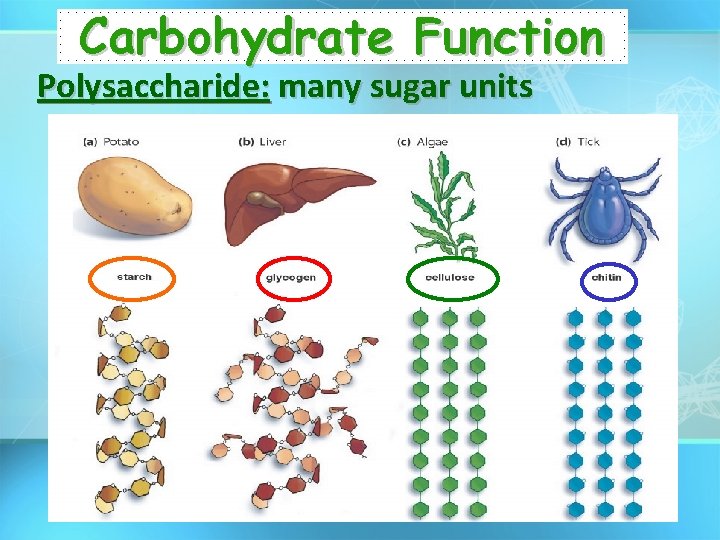
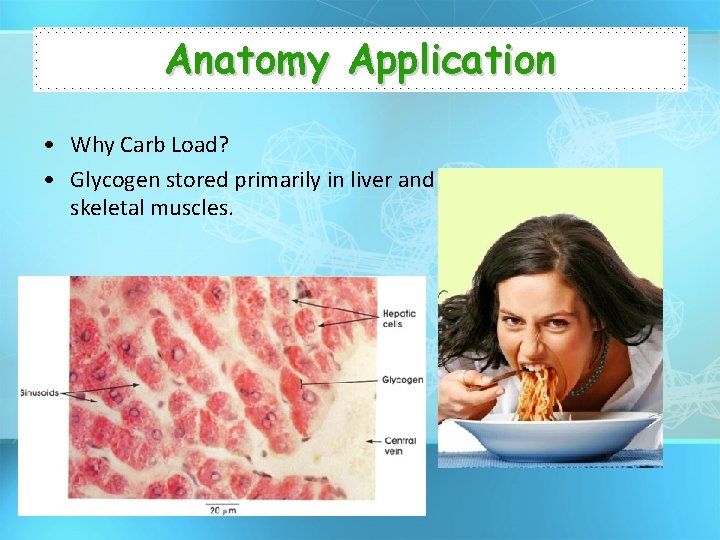
Carbohydrates • General Function: – Energy storage • Starch (Plants) • Glycogen (Liver) – Structural Support • Cellulose (Plant Cell Wall)

Carbohydrates • Compounds composed of carbon, hydrogen, and oxygen – C(1): H(2): O(1) ratio • C 6 H 12 O 6 glucose • -saccharides – – – Monosaccharide Disaccharide Polysaccharide

Carbohydrate Function Polysaccharide: many sugar units

Anatomy Application • Why Carb Load? • Glycogen stored primarily in liver and skeletal muscles.

Lipids • Functions: – ENERGY STORAGE – MAKING CELL MEMBRANES – STEROIDS

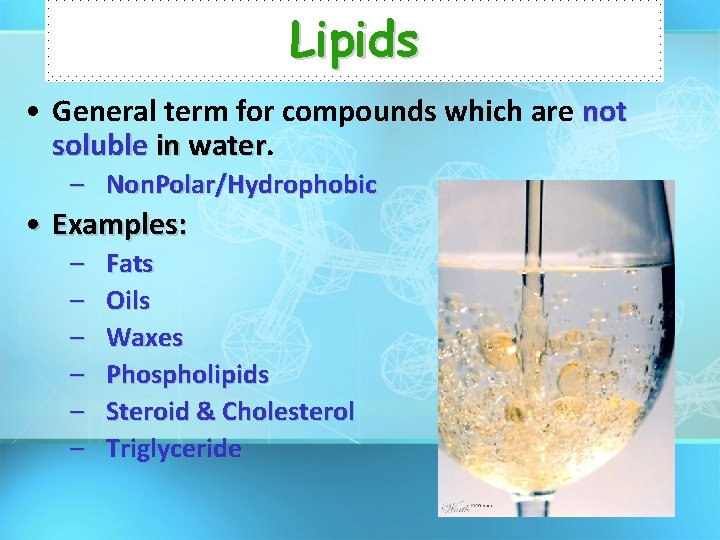
Lipids • General term for compounds which are not soluble in water – Non. Polar/Hydrophobic • Examples: – – – Fats Oils Waxes Phospholipids Steroid & Cholesterol Triglyceride

Fat storage for energy

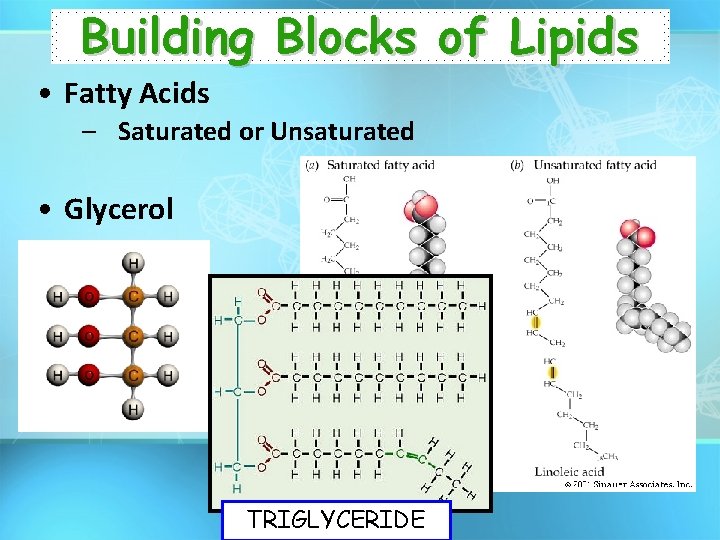
Building Blocks of Lipids • Fatty Acids – Saturated or Unsaturated • Glycerol TRIGLYCERIDE

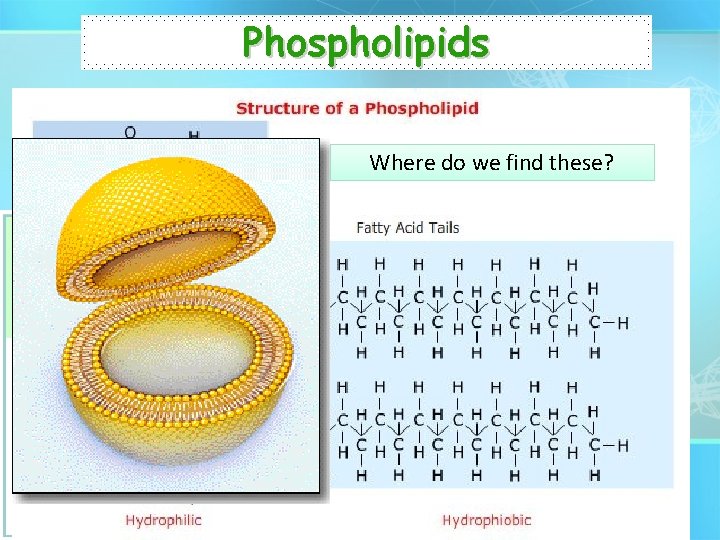
Phospholipids • Responsible for the structure and function of the cell membrane. Where do we find these? • http: //telstar. ote. cmu. edu/biology/downloads/membranes/in dex. html

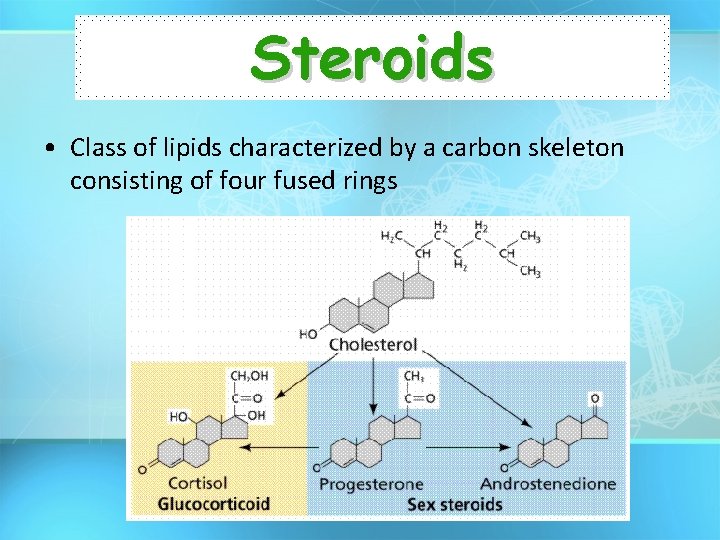
Steroids • Class of lipids characterized by a carbon skeleton consisting of four fused rings

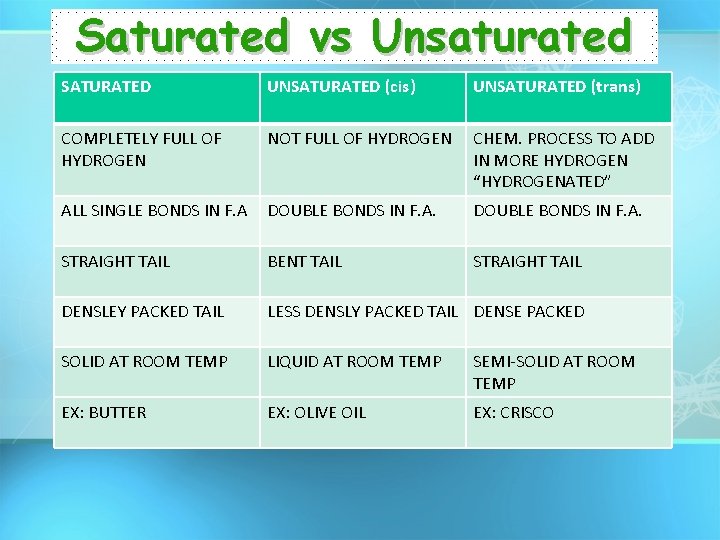
Saturated vs Unsaturated SATURATED UNSATURATED (cis) UNSATURATED (trans) COMPLETELY FULL OF HYDROGEN NOT FULL OF HYDROGEN CHEM. PROCESS TO ADD IN MORE HYDROGEN “HYDROGENATED” ALL SINGLE BONDS IN F. A DOUBLE BONDS IN F. A. STRAIGHT TAIL BENT TAIL STRAIGHT TAIL DENSLEY PACKED TAIL LESS DENSLY PACKED TAIL DENSE PACKED SOLID AT ROOM TEMP LIQUID AT ROOM TEMP SEMI-SOLID AT ROOM TEMP EX: BUTTER EX: OLIVE OIL EX: CRISCO


Hydrogenated things • What have you heard about hydrogenated and/or partially hydrogenated things? • Why so bad? • Can be bad: not easily broken down. Accumulates in your body tissue and arteries • Trans fat video

Proteins FUNCTION • • Enzymes Defense Transportation Support Motion Hormones storage

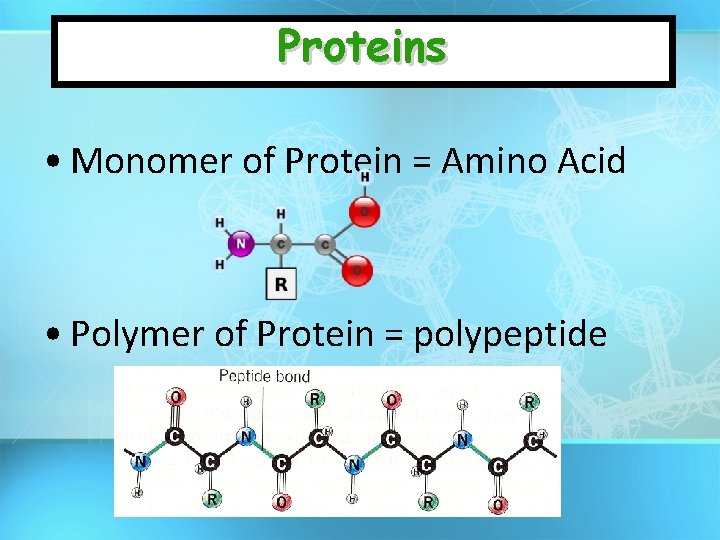
Proteins • Monomer of Protein = Amino Acid • Polymer of Protein = polypeptide

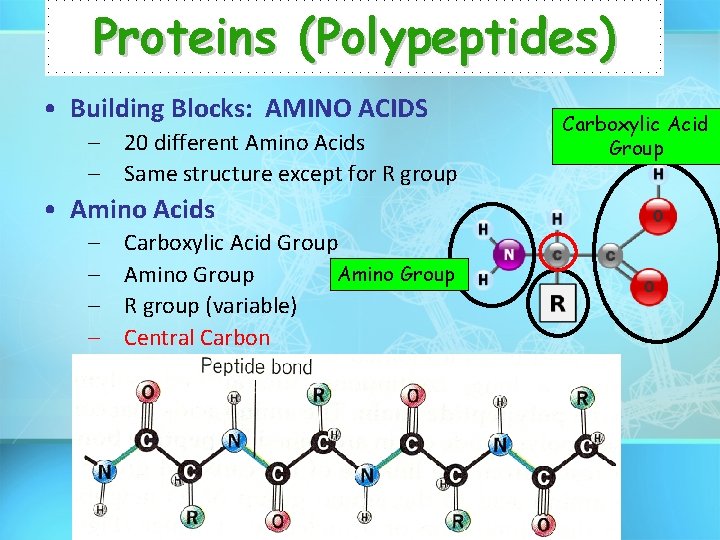
Proteins (Polypeptides) • Building Blocks: AMINO ACIDS – 20 different Amino Acids – Same structure except for R group • Amino Acids – – Carboxylic Acid Group Amino Group R group (variable) Central Carbon Carboxylic Acid Group


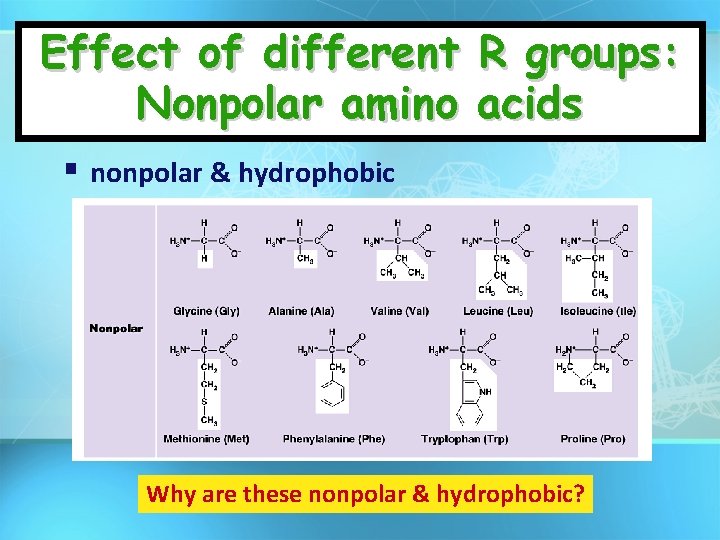
Effect of different R groups: Nonpolar amino acids nonpolar & hydrophobic Why are these nonpolar & hydrophobic?

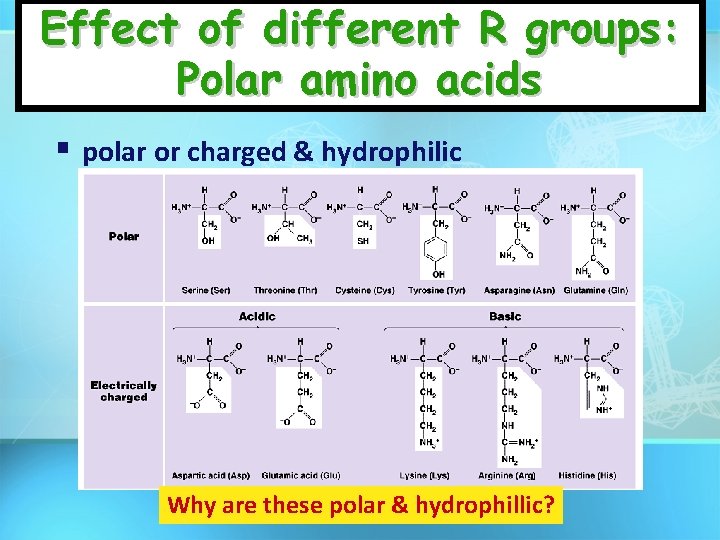
Effect of different R groups: Polar amino acids polar or charged & hydrophilic Why are these polar & hydrophillic?

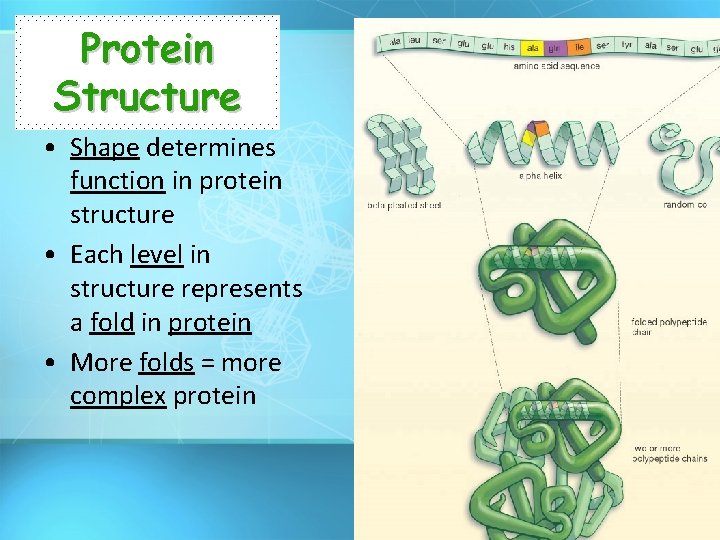
Protein Structure • Shape determines function in protein structure • Each level in structure represents a fold in protein • More folds = more complex protein

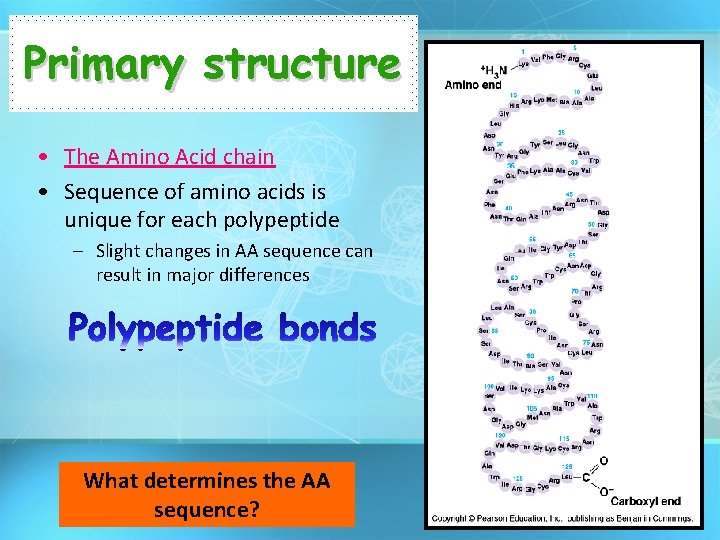
Primary structure • The Amino Acid chain • Sequence of amino acids is unique for each polypeptide – Slight changes in AA sequence can result in major differences What determines the AA sequence?

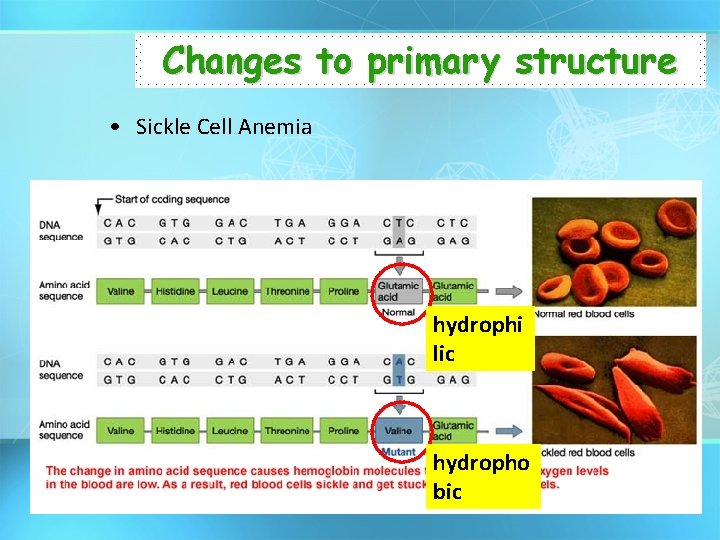
Changes to primary structure • Sickle Cell Anemia hydrophi lic hydropho bic

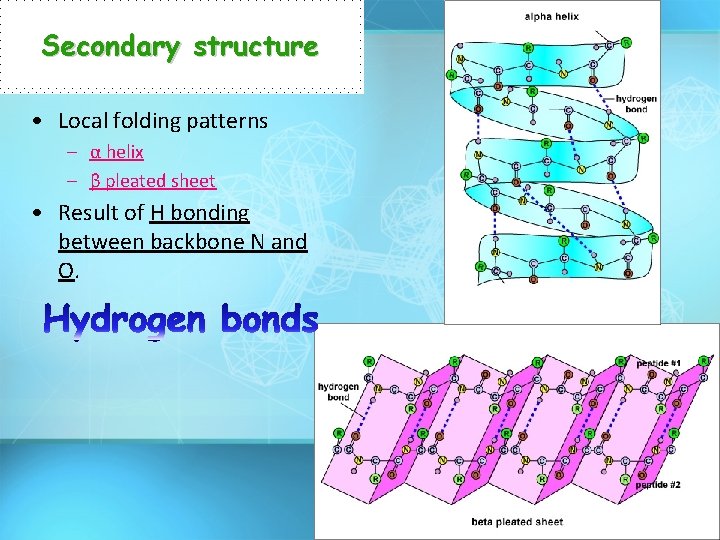
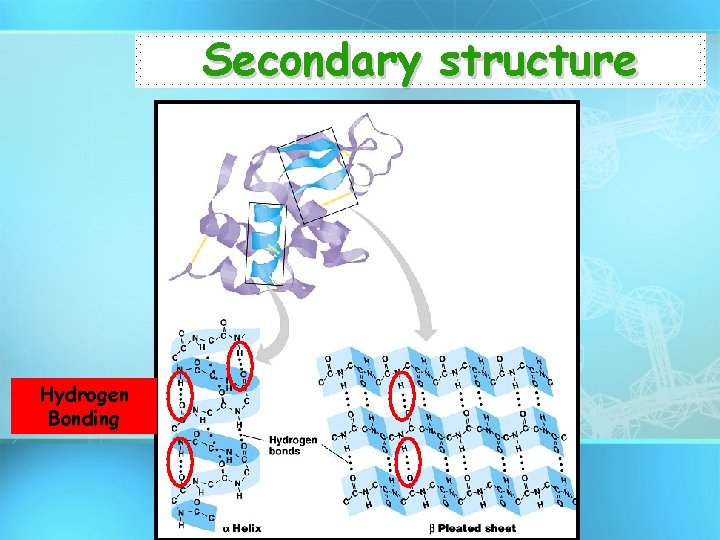
Secondary structure • Local folding patterns – α helix – β pleated sheet • Result of H bonding between backbone N and O.

Secondary structure Hydrogen Bonding

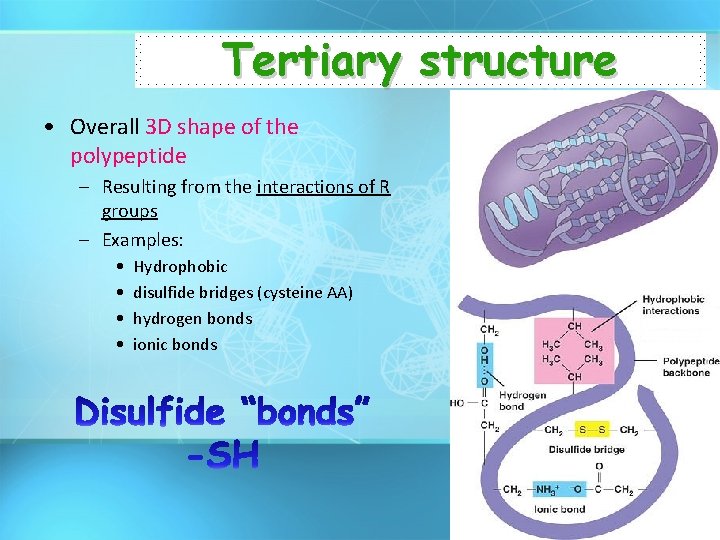
Tertiary structure • Overall 3 D shape of the polypeptide – Resulting from the interactions of R groups – Examples: • • Hydrophobic disulfide bridges (cysteine AA) hydrogen bonds ionic bonds

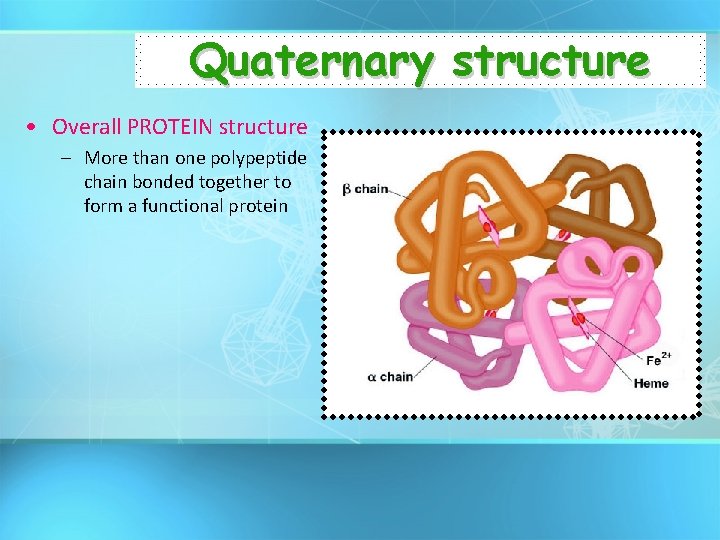
Quaternary structure • Overall PROTEIN structure – More than one polypeptide chain bonded together to form a functional protein

Proteins denature • when a protein unravels and loses its native conformation (shape) – Loss of shape = loss of function – Reversible or Irreversible Denaturation Normal protein Denatured protein Renaturation

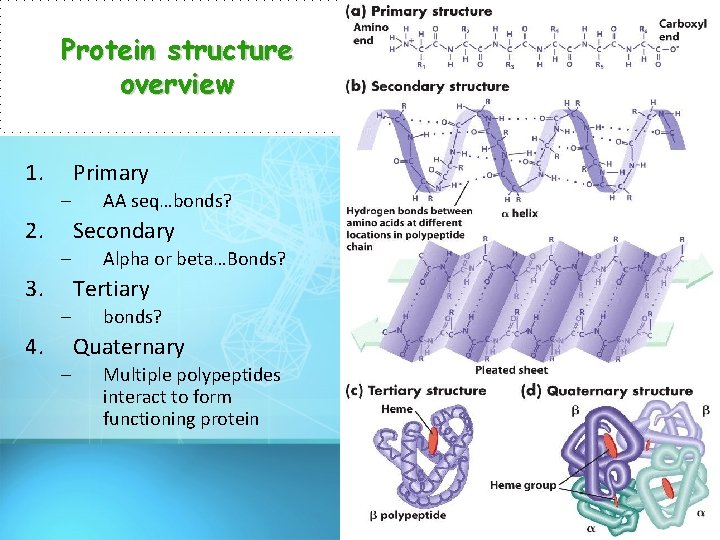
Protein structure overview 1. Primary – 2. AA seq…bonds? Secondary – 3. Alpha or beta…Bonds? Tertiary – 4. bonds? Quaternary – Multiple polypeptides interact to form functioning protein

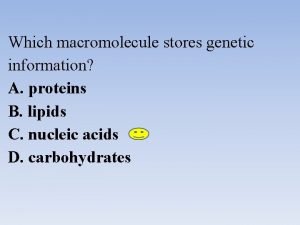
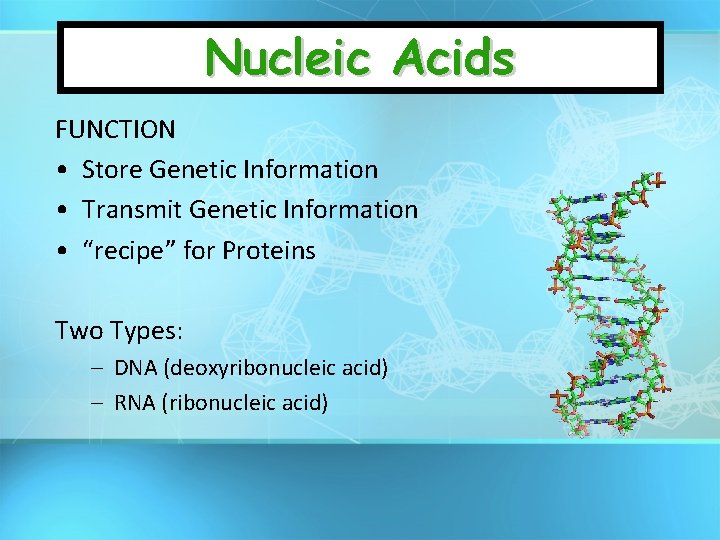
Nucleic Acids FUNCTION • Store Genetic Information • Transmit Genetic Information • “recipe” for Proteins Two Types: – DNA (deoxyribonucleic acid) – RNA (ribonucleic acid)

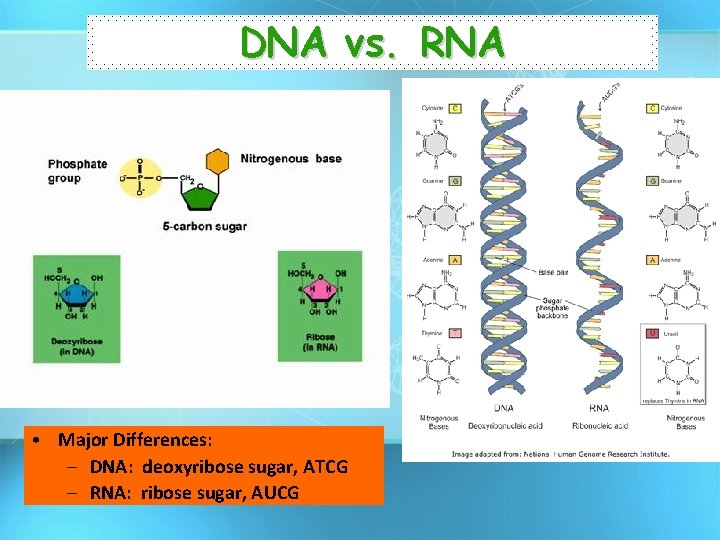
DNA vs. RNA • Major Differences: – DNA: deoxyribose sugar, ATCG – RNA: ribose sugar, AUCG

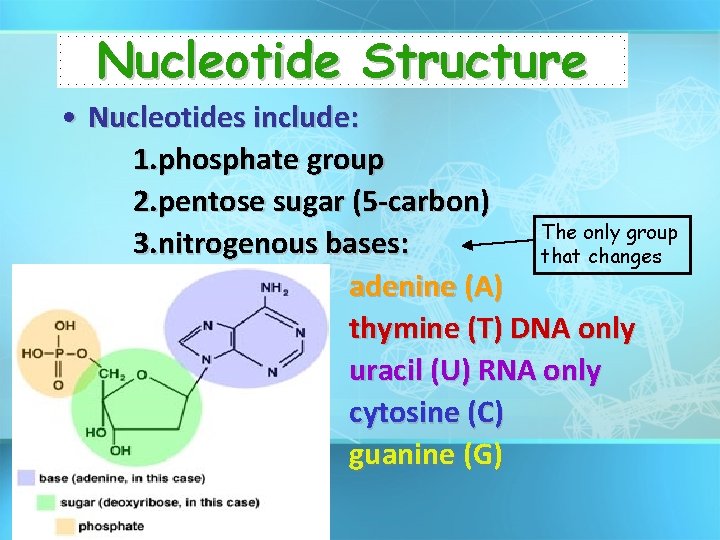
Nucleotide Structure • Nucleotides include: 1. phosphate group 2. pentose sugar (5 -carbon) The only group 3. nitrogenous bases: that changes adenine (A) thymine (T) DNA only uracil (U) RNA only cytosine (C) guanine (G)

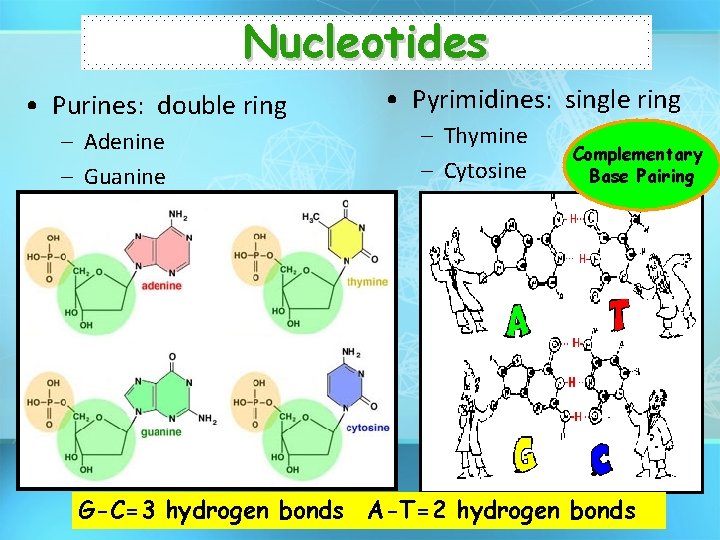
Nucleotides • Purines: double ring – Adenine – Guanine • Pyrimidines: single ring – Thymine – Cytosine Complementary Base Pairing G-C=3 hydrogen bonds A-T=2 hydrogen bonds

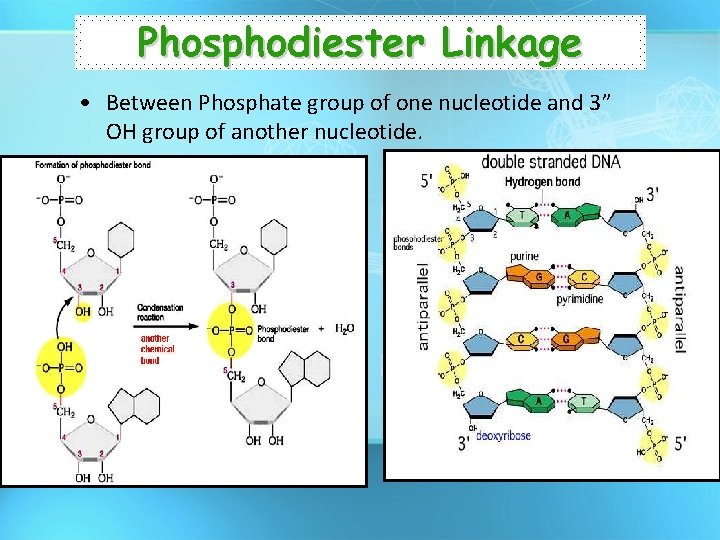
Phosphodiester Linkage • Between Phosphate group of one nucleotide and 3” OH group of another nucleotide.

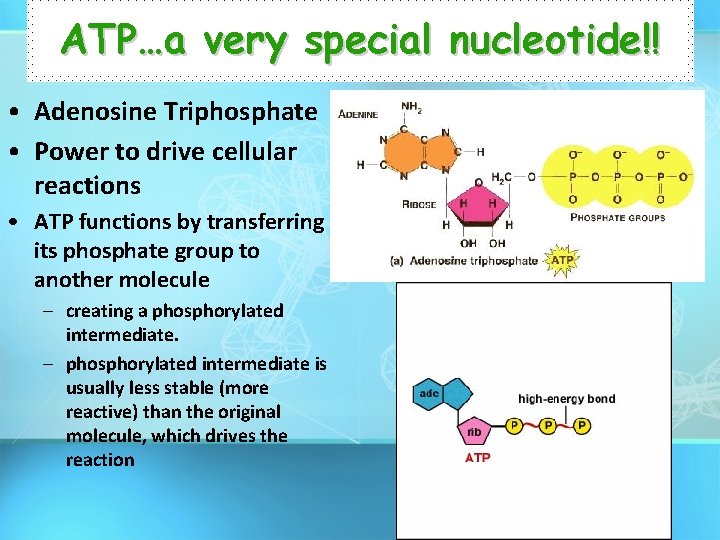
ATP…a very special nucleotide!! • Adenosine Triphosphate • Power to drive cellular reactions • ATP functions by transferring its phosphate group to another molecule – creating a phosphorylated intermediate. – phosphorylated intermediate is usually less stable (more reactive) than the original molecule, which drives the reaction

45
 Mikael ferm
Mikael ferm Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Biological molecules what are the building blocks of life
Biological molecules what are the building blocks of life Chapter 3 molecules of life
Chapter 3 molecules of life Protostar
Protostar Life cycle of a milkweed bug
Life cycle of a milkweed bug Macromolecules in steak
Macromolecules in steak Nitrogen cucle
Nitrogen cucle Macromolecules
Macromolecules What are macromolecules
What are macromolecules Picture of a macromolecule
Picture of a macromolecule Organic macromolecules chart
Organic macromolecules chart Fats subunits
Fats subunits Iki indicator
Iki indicator Micromolecules and macromolecules
Micromolecules and macromolecules What are macromolecules
What are macromolecules Macromolecules superheroes
Macromolecules superheroes Monomers building blocks
Monomers building blocks Macromolecule virtual lab
Macromolecule virtual lab Macromolecules poem
Macromolecules poem What macromolecules are in bread olive oil and pasta
What macromolecules are in bread olive oil and pasta Macromolecules summary
Macromolecules summary What are macromolecules
What are macromolecules Macromolecules you are what you eat
Macromolecules you are what you eat What is this image
What is this image Macromolecules worksheet
Macromolecules worksheet Macromolecules cheat sheet
Macromolecules cheat sheet Biological molecules you are what you eat
Biological molecules you are what you eat Are lipids long term energy storage
Are lipids long term energy storage Protein macromolecule
Protein macromolecule Macromolecules foldable
Macromolecules foldable Whats macromolecules
Whats macromolecules Macromolecules doodle notes
Macromolecules doodle notes Macromolecules in cellular respiration
Macromolecules in cellular respiration Macromolecules
Macromolecules Macromolecules
Macromolecules What macromolecules make up a macadamia nut
What macromolecules make up a macadamia nut Chapter 5 the structure and function of macromolecules
Chapter 5 the structure and function of macromolecules Pizza macromolecules
Pizza macromolecules Why are macromolecules important
Why are macromolecules important Structure of macromolecules
Structure of macromolecules Digestive organelle where macromolecules are hydrolyzed
Digestive organelle where macromolecules are hydrolyzed Poem for biomolecules
Poem for biomolecules What type of macromolecule stores genetic information
What type of macromolecule stores genetic information What is this
What is this Are vitamins and minerals macromolecules
Are vitamins and minerals macromolecules