Organic Molecules AKA Macromolecules Organic Molecules Organic Molecule




- Slides: 28

Organic Molecules AKA Macromolecules

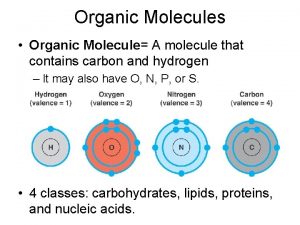
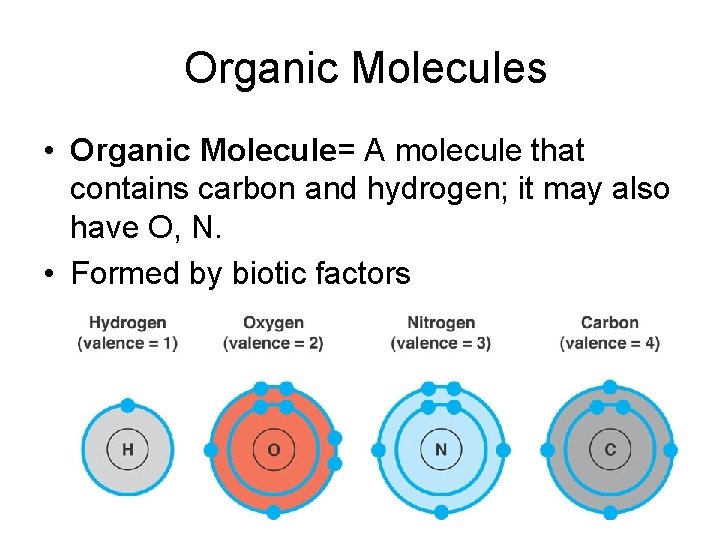
Organic Molecules • Organic Molecule= A molecule that contains carbon and hydrogen; it may also have O, N. • Formed by biotic factors

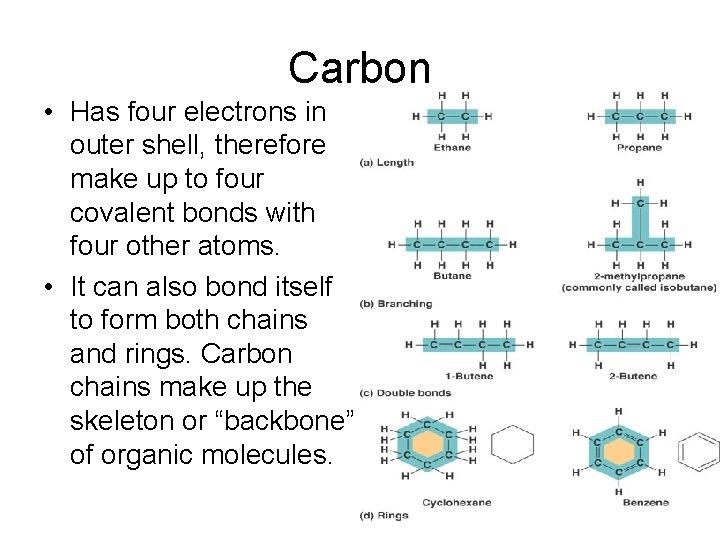
Carbon • Has four electrons in outer shell, therefore make up to four covalent bonds with four other atoms. • It can also bond itself to form both chains and rings. Carbon chains make up the skeleton or “backbone” of organic molecules.

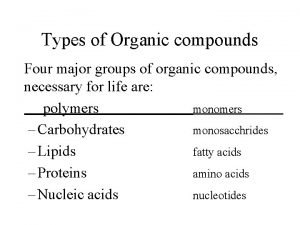
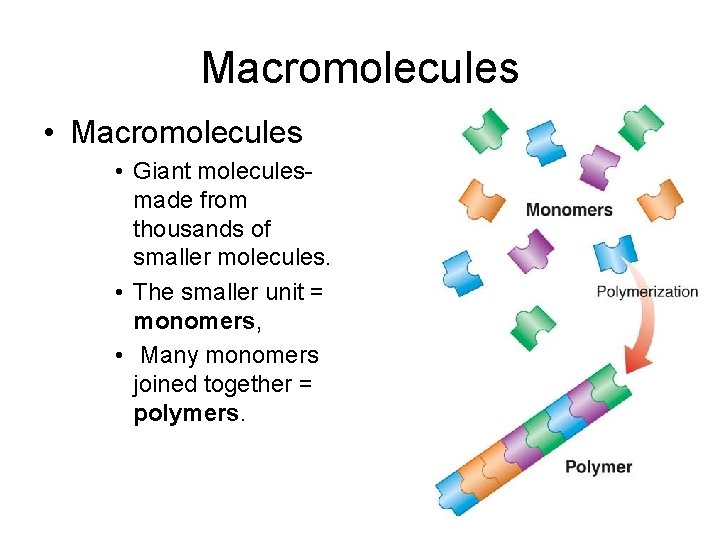
Macromolecules • Giant moleculesmade from thousands of smaller molecules. • The smaller unit = monomers, • Many monomers joined together = polymers.

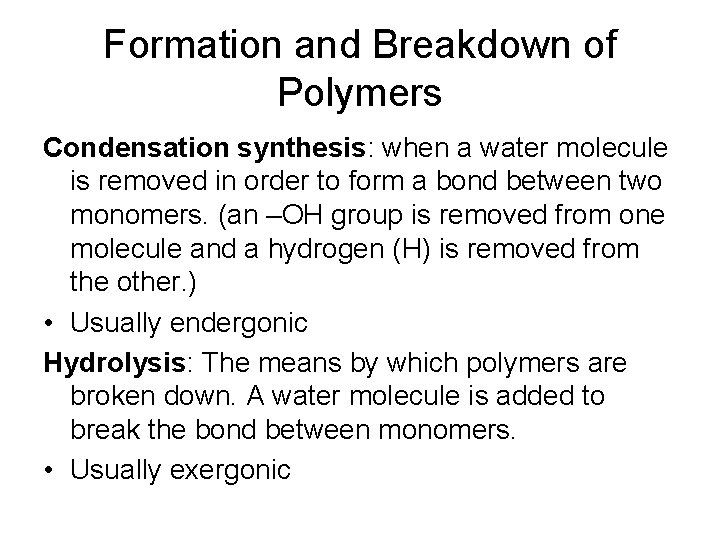
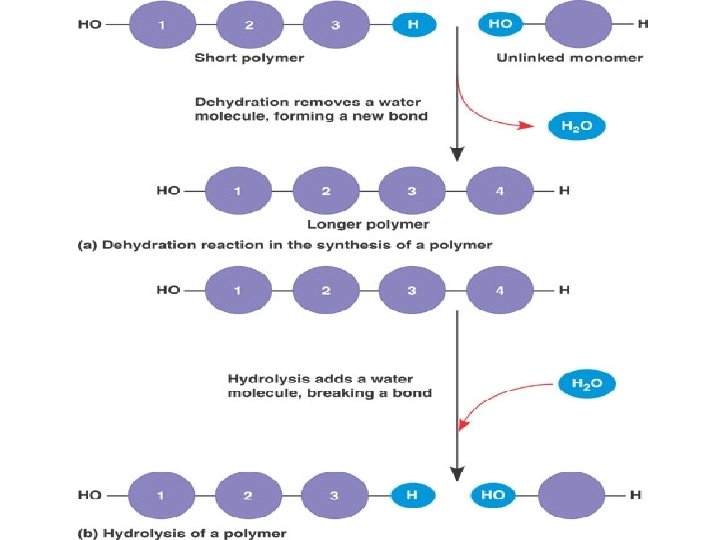
Formation and Breakdown of Polymers Condensation synthesis: when a water molecule is removed in order to form a bond between two monomers. (an –OH group is removed from one molecule and a hydrogen (H) is removed from the other. ) • Usually endergonic Hydrolysis: The means by which polymers are broken down. A water molecule is added to break the bond between monomers. • Usually exergonic


The 4 Types of Macromolecules

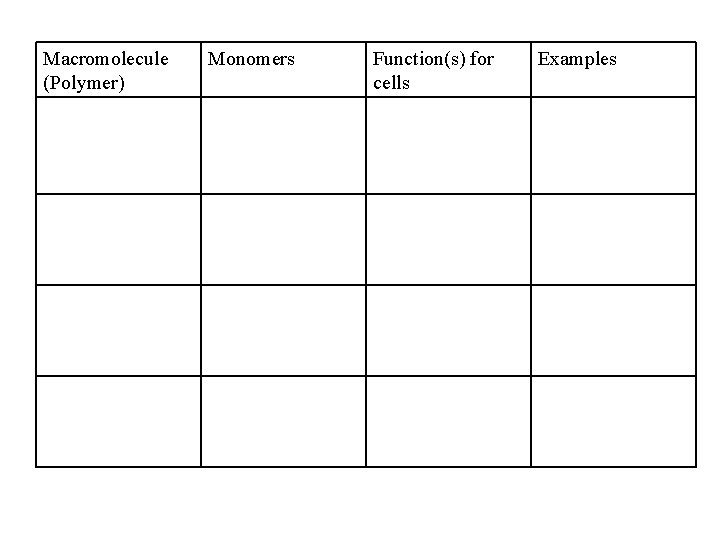
Macromolecule (Polymer) Monomers Function(s) for cells Examples

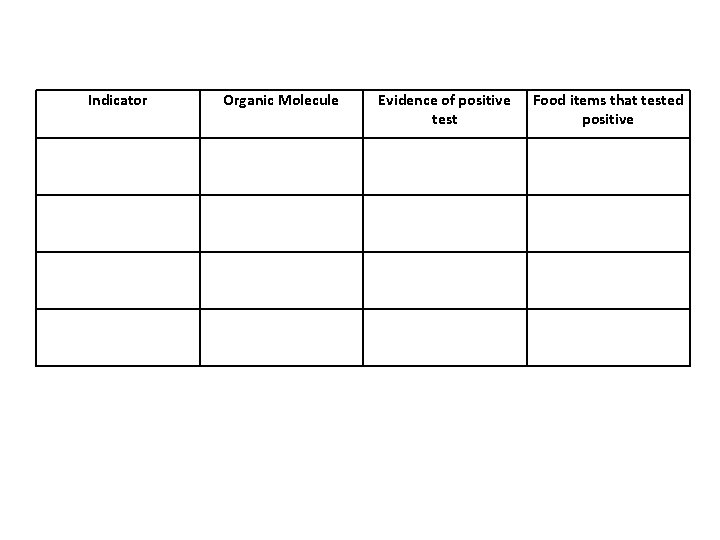
Indicator Organic Molecule Evidence of positive test Food items that tested positive

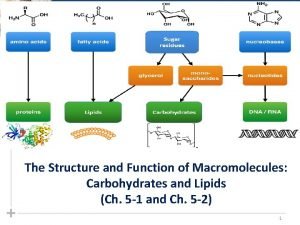
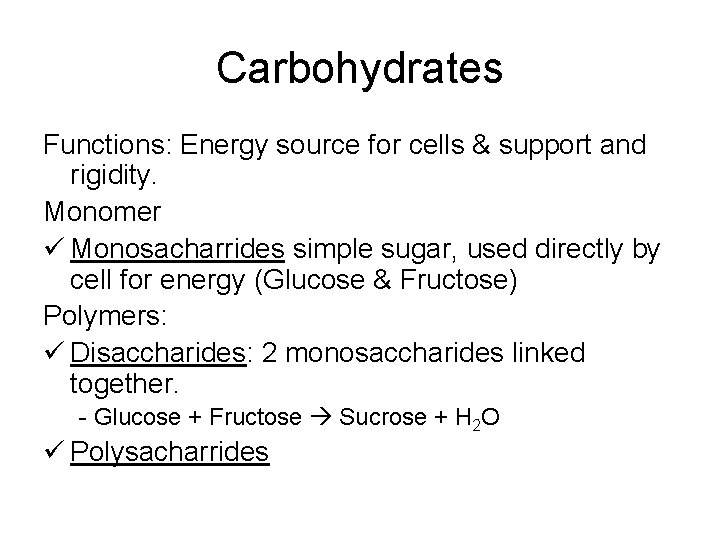
Carbohydrates Functions: Energy source for cells & support and rigidity. Monomer ü Monosacharrides simple sugar, used directly by cell for energy (Glucose & Fructose) Polymers: ü Disaccharides: 2 monosaccharides linked together. - Glucose + Fructose Sucrose + H 2 O ü Polysacharrides

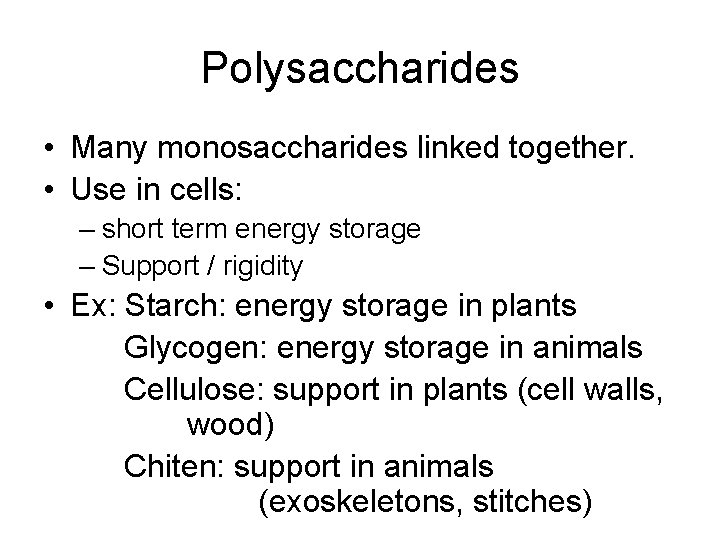
Polysaccharides • Many monosaccharides linked together. • Use in cells: – short term energy storage – Support / rigidity • Ex: Starch: energy storage in plants Glycogen: energy storage in animals Cellulose: support in plants (cell walls, wood) Chiten: support in animals (exoskeletons, stitches)

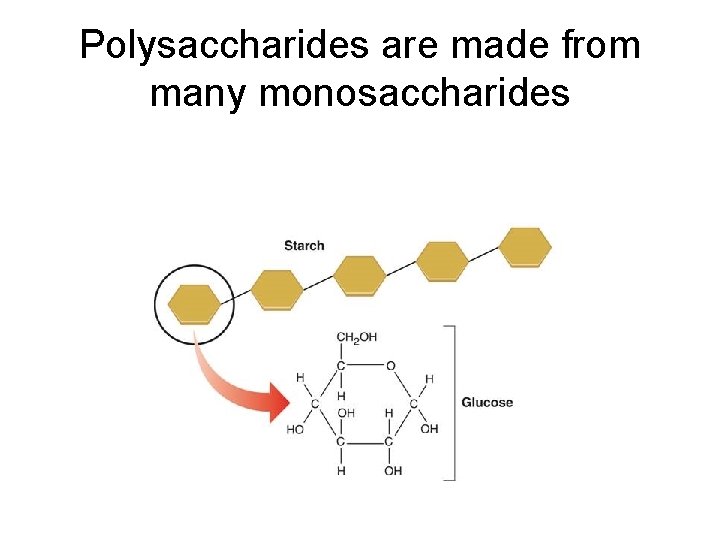
Polysaccharides are made from many monosaccharides

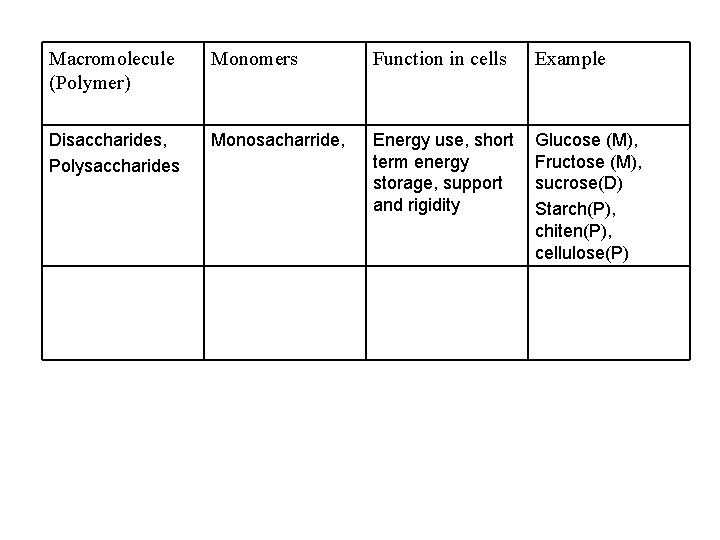
Macromolecule (Polymer) Monomers Function in cells Example Disaccharides, Polysaccharides Monosacharride, Energy use, short term energy storage, support and rigidity Glucose (M), Fructose (M), sucrose(D) Starch(P), chiten(P), cellulose(P)

Indicator demo Fill out the graphic organizer after observing the carbohydrate demonstration 1) Why didn’t sugar water test positive for sugars?

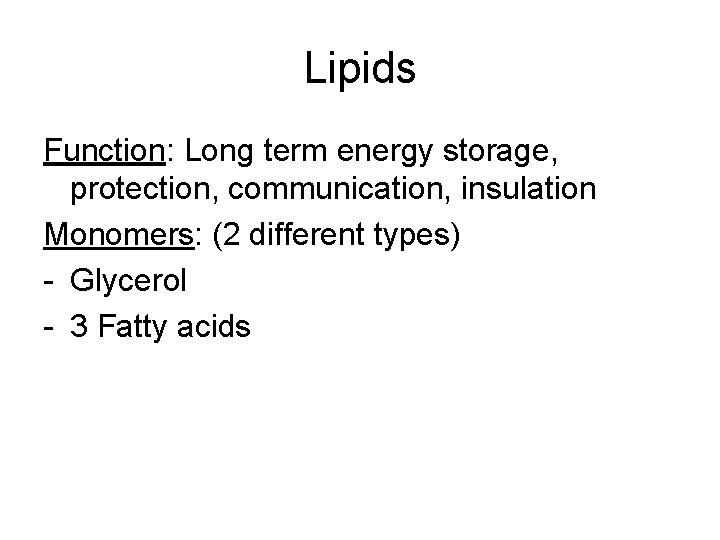
Lipids Function: Long term energy storage, protection, communication, insulation Monomers: (2 different types) - Glycerol - 3 Fatty acids

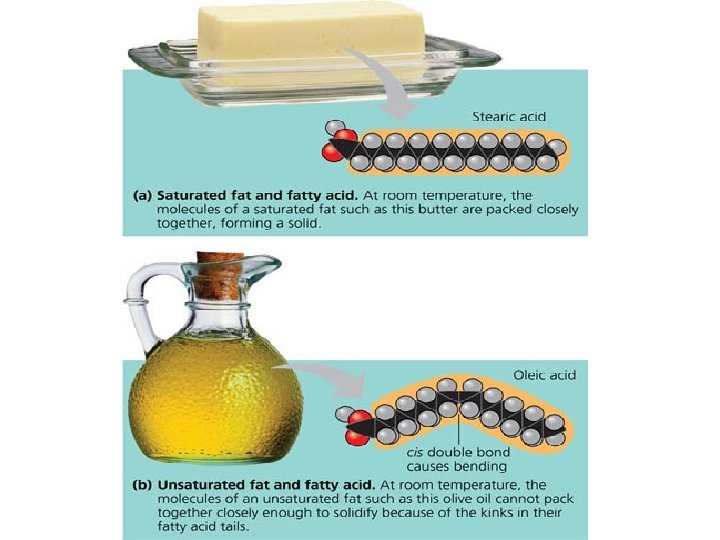
2 Types of Fatty Acids Saturated: If each carbon atom in a lipid’s fatty acid has single covalent bonds. - Results in straight chains(Solid at room temp. ) - Animal fats Unsaturated: If there is at least one carbon double covalent bond in a fatty acid. - Results in wavy chains(Liquid at room temp. ) - Plant fat


Other Lipids • Waxes: Used by cells for protection (from what? ) • Steroids: Cell to cell communication (what to say? )

Indicator demo Fill out the graphic organizer after observing the results to the Lipid demonstration 2) Using the concepts of polarity (and your water demo), explain why the lipids made a translucent spot on the brown paper towel, while the water did not.

Proteins Functions: • Transport (hemoglobin in blood O 2 ) • Defense (antibodies) • Regulation (enzymes = speed up chemical reactions) • Physical Variety (eye color, hair texture, skin pigment) • Muscle contraction

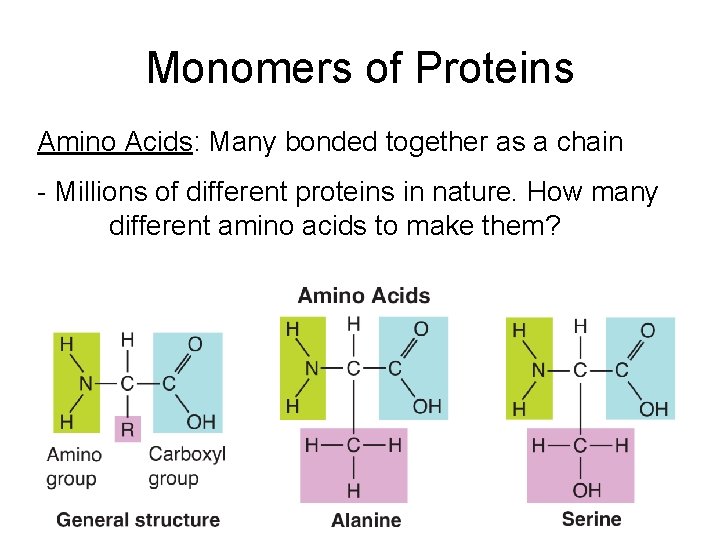
Monomers of Proteins Amino Acids: Many bonded together as a chain - Millions of different proteins in nature. How many different amino acids to make them?

Amino Acids Contd • 20 different Amino Acids use to make all of life’s proteins. • How can there be so many different proteins when there are only 20 different amino acids? • Amino acids are connected by peptide bonds (protein aka polypeptide)

Indicator demo Fill out the graphic organizer after observing the results to the protein demonstration

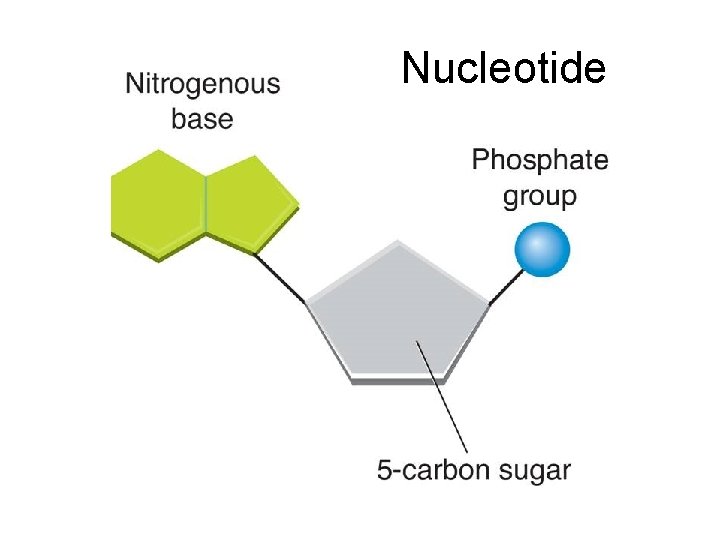
Nucleic Acids Function: Complex macromolecule that stores information in the form of a code for building all of a cells different proteins. Monomers: • Nucleotides consist of three parts: • a 5 -carbon sugar • a phosphate group • a nitrogenous base

Nucleotide

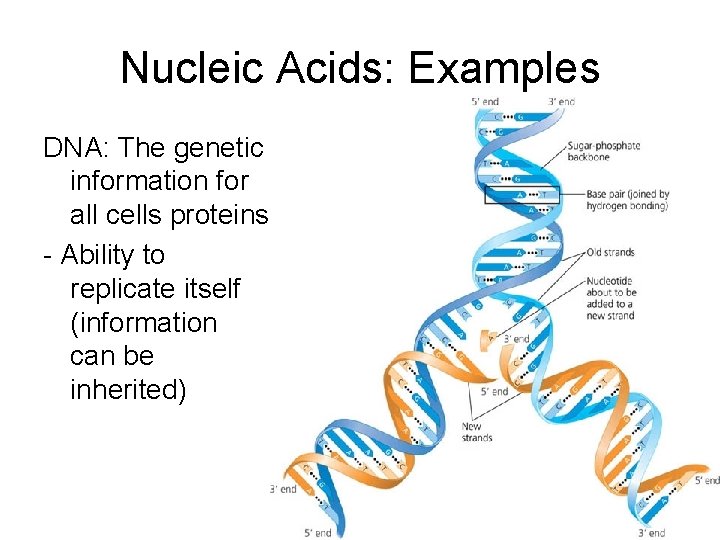
Nucleic Acids: Examples DNA: The genetic information for all cells proteins. - Ability to replicate itself (information can be inherited)

Nucleic Acids Examples • RNA • ATP

Indicator demo 3) Why is there not a test for nucleic acids in food items?
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Chapter 6 organic macromolecules
Chapter 6 organic macromolecules Macromolecule chart
Macromolecule chart Whats an organic molecule
Whats an organic molecule Enzymes are composed of what organic molecule
Enzymes are composed of what organic molecule Whats an organic molecule
Whats an organic molecule The four types of organic molecules
The four types of organic molecules Is ch4o organic or inorganic
Is ch4o organic or inorganic Biochemistry
Biochemistry Macromolecule
Macromolecule What are macromolecules
What are macromolecules Macromolecules in cellular respiration
Macromolecules in cellular respiration What are macromolecules
What are macromolecules What are macromolecules
What are macromolecules Chapter 5 the structure and function of macromolecules
Chapter 5 the structure and function of macromolecules Cell membrane
Cell membrane What macromolecule is this
What macromolecule is this Whats a macromolecule
Whats a macromolecule Macromolecules poem
Macromolecules poem Site:slidetodoc.com
Site:slidetodoc.com Digestive organelle where macromolecules are hydrolyzed
Digestive organelle where macromolecules are hydrolyzed Proteins structure
Proteins structure Macromolecules
Macromolecules Macromolecules you are what you eat
Macromolecules you are what you eat Macromolecules superheroes
Macromolecules superheroes Pizza macromolecules
Pizza macromolecules How is nitrogen important
How is nitrogen important Macromolecules foldable
Macromolecules foldable You are what you eat macromolecules
You are what you eat macromolecules