Intro to Organic Chemistry Pretty much most fun























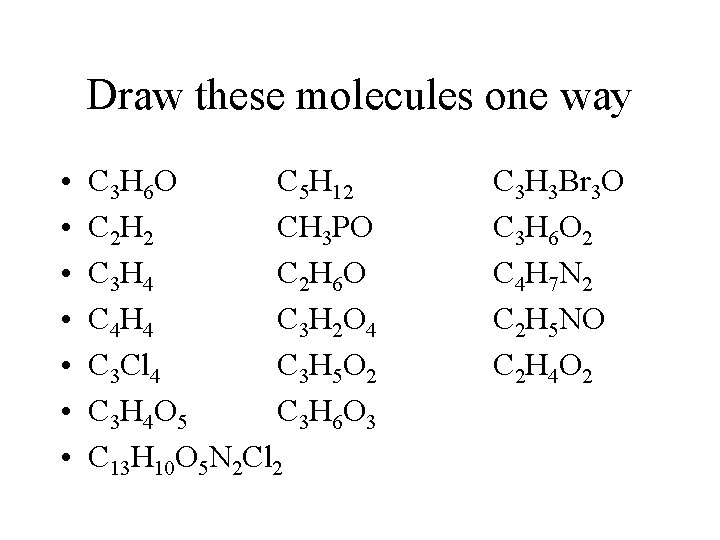

- Slides: 31

Intro to Organic Chemistry Pretty much most fun you’ll ever have

What’s Different about Chem II? • • Notes are important! Lots of practice, groups, board work less “busy work” longer labs, more dangerous subject is relevant to everyday life no math lots of stories-yours & mine

Classification thing Classify each of the following broad groups into 2 sub-groups: Students in this class BJHS Teachers

In the olden days (early 1800’s) • Everything in the world was classified into 2 groups • What do you think they were called?

• Yep! – Living and Nonliving • Now called Organic and Inorganic • Organic originally referred to 1. part of something living or dead or 2. a substance produced by something living or dead *A new def is coming later

• Everything classified as organic had a special quality scientists called • The Vital Force • Def: a special (mythical) characteristic of all organic things • Ex: Baywatch story

• http: //www. youtube. com/watch? v=lq. NXE 0 7 f. Kuw

• Big problem back then: there was no way to create vital force compounds in the lab! • BUT, in the early 1800’s, 2 events changed chemistry forever. .

The st 1 one – 1. 1828 -German chemist named Friedrich Wohler accidentally produced urea in the lab. – Don’t write this: Wohler, Friedrich (1800 -1882), a German chemist. He was the first to create an organic substance from inorganic chemicals; in 1828 he heated ammonium cyanate, an inorganic compound, and created urea, a compound found in animal urine. Thus he disproved the belief that organic substances could be formed only in living things. Wohler experimented with almost every chemical element known in his day. He isolated the elements aluminum and beryllium, and discovered calcium carbide. – He looks so happy – Note-Urea is a constituent of urine, but the 2 are not the same

• What did Wohler do? • NH 4 Cl + Ag. NCO --> NH 4 NCO -->NH 2 CONH 2 + Ag. Cl • What’s weird about this equation? • Joke #1 http: //chemistry. about. com/od/moleculescompounds/ ig/Molecules-with-Strange-Names/ Lord of the Carbon Rings • http: //www. youtube. com/watch? v=BDXJTh Yw 4_4 • When Wohler presented his findings people laughed at him-he died sad, ugly, and alone

Amazing info on urea • When proteins are metabolized, N (nitrogen) compds are produced • But N compds can’t be stored in the body • They must be excreted in a nontoxic & HOH-soluble form • http: //www. youtube. com/watch? v=a. BVKa. Tot. KBk • NH 3 is one possibility, it’s soluble, but it’s also toxic to humans. (not toxic to snakes & birds) • So, we excrete N compds as urea-it’s both soluble and nontoxic : ) • Fun urine stories • http: //www. youtube. com/watch? v=4 U_xmf. Sw. YSw&feature=Play. List &p=7 CBCB 29 FD 596 FD 12&index=5 • Fish not supposed to go there. . . http: //www. youtube. com/watch? v=o. Du 1 E 4 End. Jw

• The 2 nd One (one what? ) 1845 -Herman Kolbe synthesized trichloroacetic acid (an organic thing) from inorganic substances-again creating the a vital force compound! • Structure? • Uses?

• Eventually, chemists made lots of vital force compounds-today there are millions • Here’s an ex of something created in the lab that’s not even found in nature! • CH 4 + Cl 2 CHCl 3 + CCl 4 • methane + chlorine chloroform + carbon tetrachloride • The products (things after the arrows) aren’t found in nature-that’s significant b/c things like this had never been made! • There are even patents on some life formsex: oil-eating bacteria

Let’s talk about methane • Methane-CH 4 also called swamp/marsh gas • Also on other planets • http: //videos. howstuffworks. com/discovery/31001 methane-discovery-on-distant-planet-video. htm 2 min • methane stories, gas detector, church, etc. • apples give off methane, that’s why they’re not stored with nanners at Kroger-they would ripen too fast • http: //www. youtube. com/watch? v=Oh-1 w. OSTi. P 0 2 min

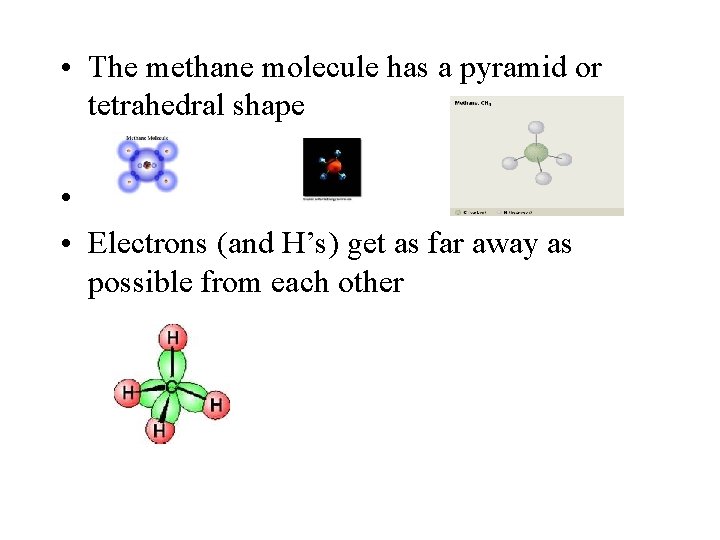
• The methane molecule has a pyramid or tetrahedral shape • • Electrons (and H’s) get as far away as possible from each other

Methane & the Bermuda Triangle • • • Methane Gas Hydrates This theory appears to hold promise for at least some of the disappearances in the Bermuda Triangle. Scientists at Cardiff University have discovered the presence of large concentrations of methane gas trapped in the ocean floor. This gas is due to dying and decomposing sea organisms. The sediment contains bacteria that produce methane, which accumulates as super concentrated methane ice, called gas hydrates. The layer of ice traps the methane gas, and scientists are studying it as a potential energy source. Image courtesy Office of Naval Research Within seconds of a methane gas pocket rupturing, the gas surges up and erupts on the surface without warning. If a ship is in the area of the blowout, the water beneath it would suddenly become much less dense. The vessel could sink and sediment could quickly cover it as it settles onto the sea floor. Even planes flying overhead could catch fire during such a blowout. Although he doesn't agree with the methane hydrate theory as an explanation for the Bermuda Triangle, Bill Dillon, a research geologist with the United States Geological Survey said that, "On several occasions, oil drilling rigs have sunk as the result of [methane] gas escape. " • Simulation http: //www. youtube. com/watch? v=Dk. IIMEVn. NDg&featu re=Play. List&p=4 BBEE 997 F 92 D 7079&playnext=1&playn ext_from=PL&index=7 3 min

Cows and methane • Cows excrete methane • methane depletes the ozone layer • So, are cows are bad? maybe • http: //www. youtube. com/watch? v=zb. KRSY Au. SNg 4 min • Our govt comes to the rescue! The Ruminant Livestock Methane Program is formed-consuming millions of taxpayer dollars in the study of reducing bovine methane emissions • http: //videos. howstuffworks. com/planet-green/33432 -stuffhappens-wheres-the-beef-video. htm 4 min


New Def of Organic • Obviously, the old def of organic needed to be changed • New def: organic things contain Carbon • BUT. . . • not all carbon compds are organic – ex: CO (carbon monoxide) – CO 2 (carbon dioxide) – CO 3’s (carbonates) – CN’s (cyanides)

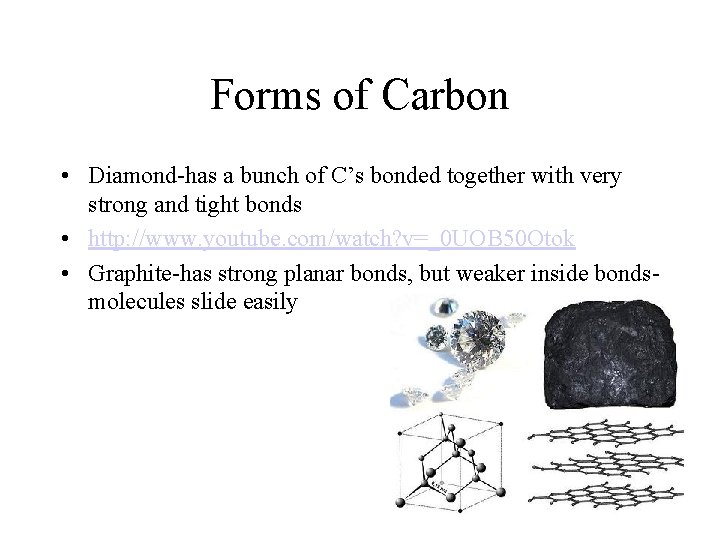
Forms of Carbon • Diamond-has a bunch of C’s bonded together with very strong and tight bonds • http: //www. youtube. com/watch? v=_0 UOB 50 Otok • Graphite-has strong planar bonds, but weaker inside bondsmolecules slide easily

• Q: are there more organic compounds or inorganic compounds in the world? • A: Organic! • Q: why? • A: Reason #1 -the C atom is very versatile! • Let’s look at it

The Carbon Atom • • • Atomic # =6 # electrons = 6 # neutrons = 6 666=and all life is based on carbon C has 4 valence electrons-what does this mean? • electron configuration of carbon

Carbon cont’d • C would like to gain or lose 4 e-s, but that’s unlikely • Looking at the EN (? ) of C, it’s kind of in the middle-has no strong desire to give up or gain e-s • If C stayed like this, it wouldn’t bond w/anything • So, it decides to hybridize!

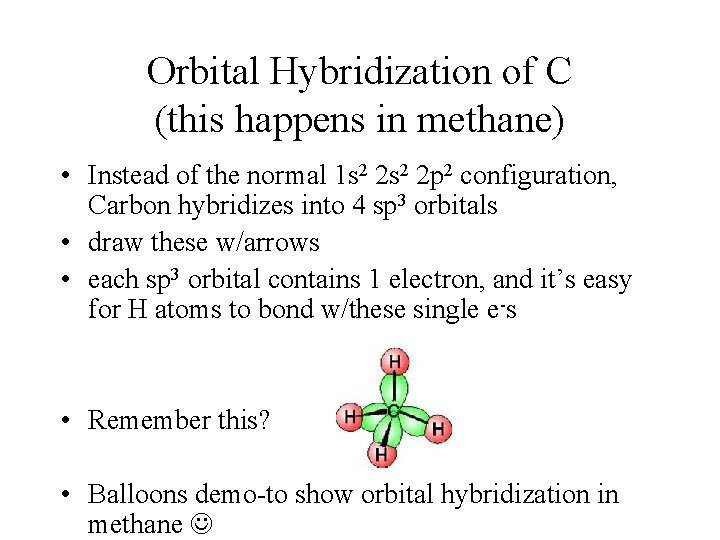
Orbital Hybridization of C (this happens in methane) • Instead of the normal 1 s 2 2 p 2 configuration, Carbon hybridizes into 4 sp 3 orbitals • draw these w/arrows • each sp 3 orbital contains 1 electron, and it’s easy for H atoms to bond w/these single e-s • Remember this? • Balloons demo-to show orbital hybridization in methane

Bond #’s in Organic Compds • • 8 is the happy number, but 2 is also OK CHONPS halogens don’t forget your diatomics! Draw these: NH 3 H 2 O CH 2 O HCl Let’s build them C 3 H 8 HCN

Organic Families • • • • Alkanes Alkyl halides Alkynes Alkenes Alcohols Aromatics Aldehydes Ketones Ethers Esters Carboxylic acids Amines Amides

• Organic families song-cheesy 3 min http: //www. youtube. com/watch? v=m. Ajrn. Zznk. Y&feature=related • Bonding video 3 min http: //www. youtube. com/watch? v=o. NBzy M 6 Tc. K 8&feature=related

Diffs Bet Orgs and Inorgs • • • • Elements present mpt/freezg pt boiling pt solublity in HOH solubility in organic solvents flammability bond type electrical conductivity rxn rate side rxns product yield structure ions/molecules in solution

Isomerism • • • Fun with Tinker Toys Draw C 2 H 6 O names & properties Def: Isomers The existence of isomers is one reason for the large # of organic compds • What was the other reason? Be able to list both.
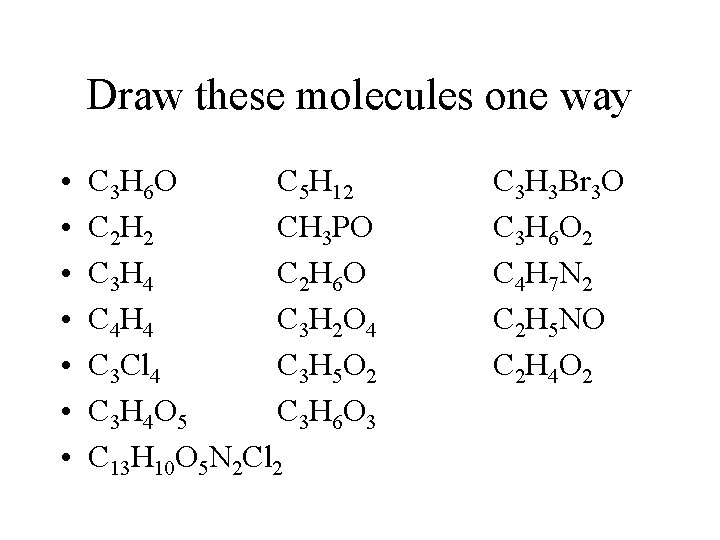
Draw these molecules one way • • C 3 H 6 O C 5 H 12 C 2 H 2 CH 3 PO C 3 H 4 C 2 H 6 O C 4 H 4 C 3 H 2 O 4 C 3 Cl 4 C 3 H 5 O 2 C 3 H 4 O 5 C 3 H 6 O 3 C 13 H 10 O 5 N 2 Cl 2 C 3 H 3 Br 3 O C 3 H 6 O 2 C 4 H 7 N 2 C 2 H 5 NO C 2 H 4 O 2

The end
 Importance of organic chemistry
Importance of organic chemistry Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Much ado about nothing fun facts
Much ado about nothing fun facts How much caffeine in a snickers bar
How much caffeine in a snickers bar To whom much is given much is expected meaning
To whom much is given much is expected meaning How much is too much plagiarism
How much is too much plagiarism Cycloalkanes
Cycloalkanes Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Displayed formula
Displayed formula Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Pericyclic
Pericyclic David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Is alkane an organic compound
Is alkane an organic compound Leveling effect organic chemistry
Leveling effect organic chemistry Iupac nomenclature
Iupac nomenclature Organic chemistry lab report example
Organic chemistry lab report example Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Grade 10 organic chemistry
Grade 10 organic chemistry Cyclo organic chemistry
Cyclo organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis Organic chemistry meth eth prop but
Organic chemistry meth eth prop but How is cracking done
How is cracking done Organic and biochemistry
Organic and biochemistry Organic chemistry myanmar
Organic chemistry myanmar Electrophilic addition hbr
Electrophilic addition hbr Gc organic chemistry
Gc organic chemistry Hono organic chemistry
Hono organic chemistry Father of organic chemistry
Father of organic chemistry





















































