IMMUNOLOGICAL TOLERANCE AND AUTOIMMUNITY Tolerance Specific immunologic unresponsiveness




![• The most important step is activation of self reactive helper [CD 4] • The most important step is activation of self reactive helper [CD 4]](https://slidetodoc.com/presentation_image/90f21ba4039b44bbd1f28034179a4565/image-16.jpg)









- Slides: 54

IMMUNOLOGICAL TOLERANCE AND AUTOIMMUNITY

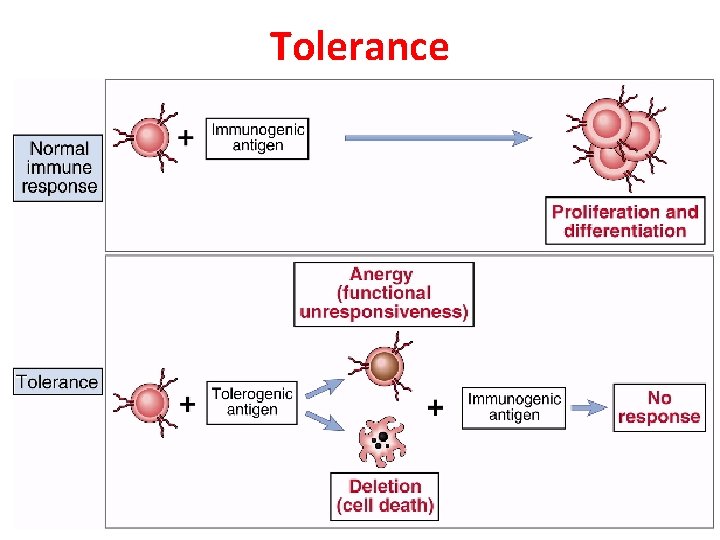
Tolerance • Specific immunologic unresponsiveness i. e. , an immune response to certain antigen does not occur, although the immune system is other wise functioning normally • Unresponsiveness to self antigens is called autotolerance • This is the natural tolerance that is essential for our survival. Loss of self tolerance results in autoimmune diseases.

• In general, antigens that are present during embryonic life are considered “Self” and do not stimulate an immunologic response • The lack of immune response in the fetus is caused by the deletion of self reactive T-cell precursors in the Thymus.

• Ags encountered later when body is immunologically mature are considered as “nonself” • These usually elicit an immunologic response. If there is no immunological response to any antigen it is called as tolerance • Both T & B cells participate in tolerance but T-cell tolerance plays a major role.

Tolerance

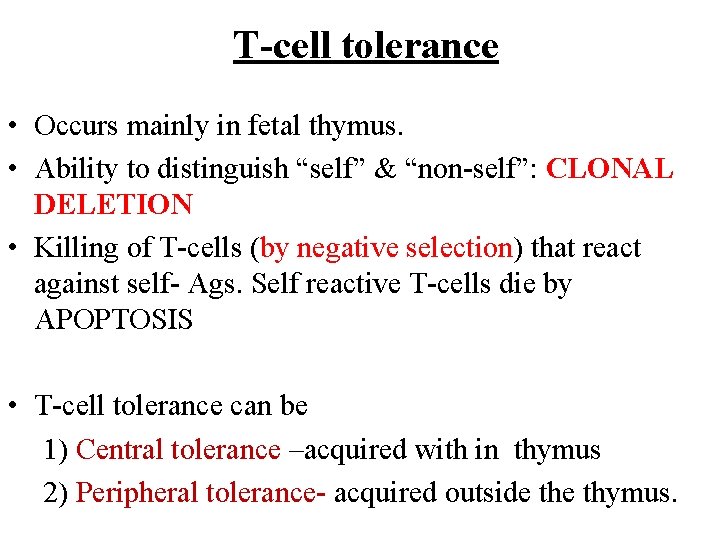
T-cell tolerance • Occurs mainly in fetal thymus. • Ability to distinguish “self” & “non-self”: CLONAL DELETION • Killing of T-cells (by negative selection) that react against self- Ags. Self reactive T-cells die by APOPTOSIS • T-cell tolerance can be 1) Central tolerance –acquired with in thymus 2) Peripheral tolerance- acquired outside thymus.

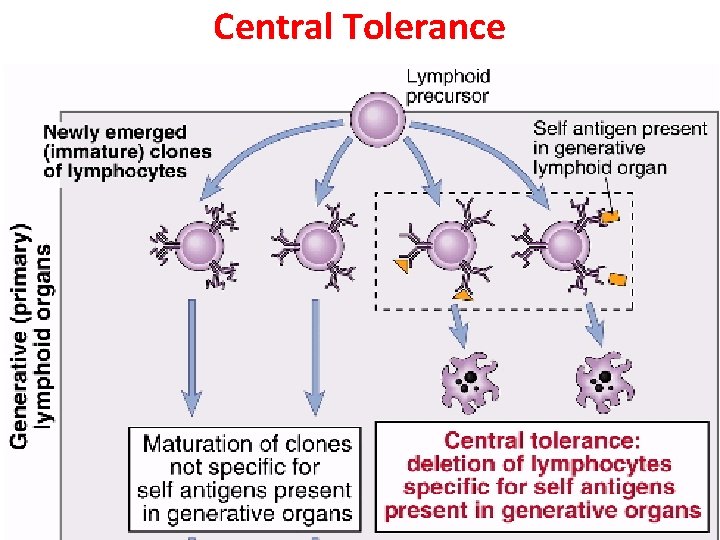
Central Tolerance (Clonal deletion) § Deletion or killing of self reactive T-cells by apoptosis during the embryonic life (Process of ‘Negative selection’) § Clonal deletion of self reactive T cells: thymus § Clonal deletion of self reactive B cells: bone marrow § Likewise, differentiating early B cells become tolerant when they encounter cell-associated or soluble self antigen.

Central Tolerance

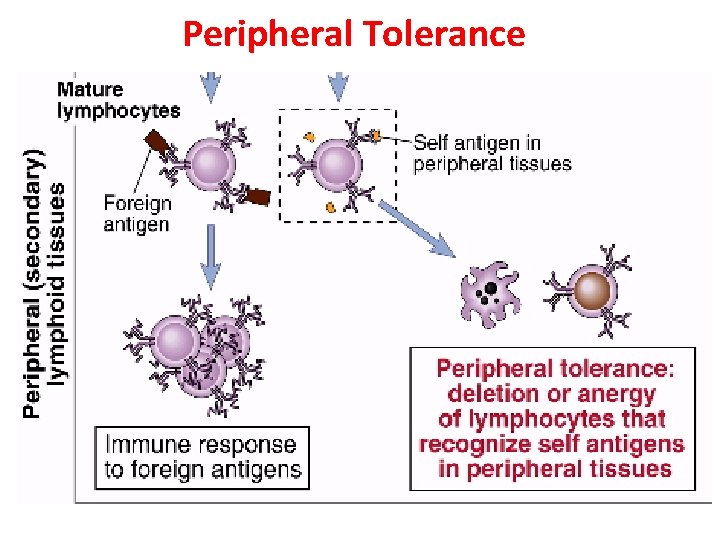
Peripheral Tolerance (Clonal Anergy) § Mature immuno-competent cells recognise the antigen but do not respond to it § Clonal anergy- self reactive T-cells that are not activated due to improper co-stimulation • Clonal ignorance- self reactive T-cells ignore self antigens. These self reactive T-cells are kept ignorant- by physical separation from target antigen e. g. blood brain barrier

Peripheral Tolerance

B cell tolerance B cell becomes tolerant to self by 2 mechanisms. 1. Clonal deletion: probably occur while the B-cell precursors are in the bone marrow 2. Clonal anergy: B cells in the periphery. Tolerance in B cell is less complete than in T cell • Most autoimmune diseases are mediated by Abs

Induction of tolerance • Antigen is administered in a specialized way (as a tolerogen) that will lead to induction of tolerance • Induction of tolerance may be used as a therapeutic approach for preventing harmful immune responses • Tolerance may be induced – 1. In the prevention of graft rejection. 2. In the treatment of autoimmune diseases. 3. In the treatment of allergic conditions.

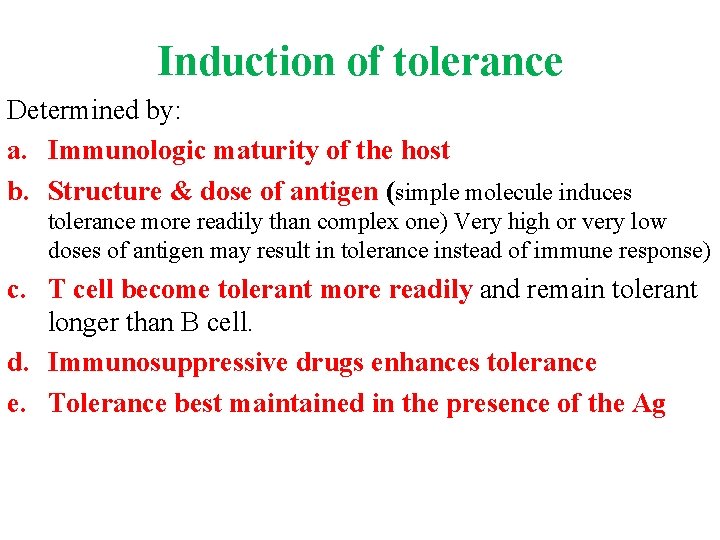
Induction of tolerance Determined by: a. Immunologic maturity of the host b. Structure & dose of antigen (simple molecule induces tolerance more readily than complex one) Very high or very low doses of antigen may result in tolerance instead of immune response) c. T cell become tolerant more readily and remain tolerant longer than B cell. d. Immunosuppressive drugs enhances tolerance e. Tolerance best maintained in the presence of the Ag

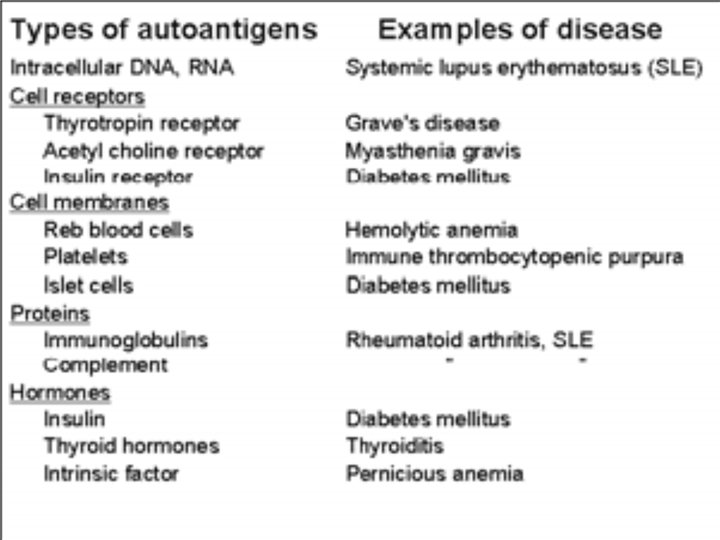
Autoimmune diseases are the diseases resulting from immune response against host’s own cells or tissues.

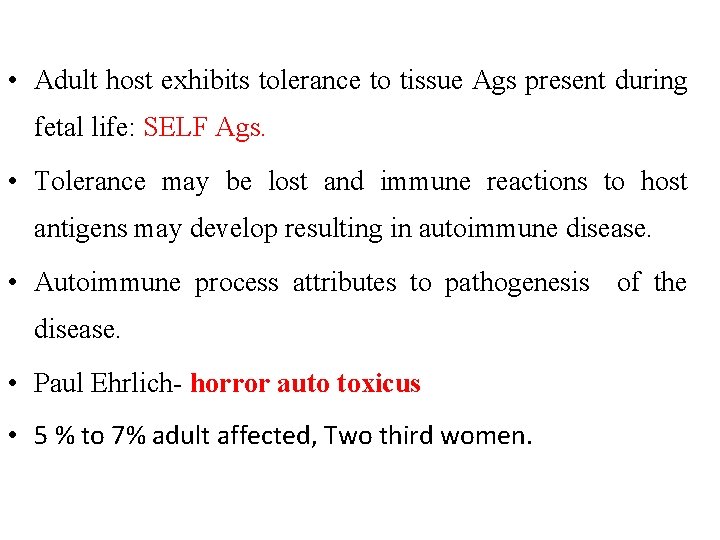
• Adult host exhibits tolerance to tissue Ags present during fetal life: SELF Ags. • Tolerance may be lost and immune reactions to host antigens may develop resulting in autoimmune disease. • Autoimmune process attributes to pathogenesis of the disease. • Paul Ehrlich- horror auto toxicus • 5 % to 7% adult affected, Two third women.
![The most important step is activation of self reactive helper CD 4 • The most important step is activation of self reactive helper [CD 4]](https://slidetodoc.com/presentation_image/90f21ba4039b44bbd1f28034179a4565/image-16.jpg)
• The most important step is activation of self reactive helper [CD 4] T cells • These Th-1 or Th-2 cells can induce either CMI or AMI autoimmune reaction • Most autoimmune diseases are antibody mediated.

Mechanism of Autoimmunity Break down of self tolerance due to: 1. Altered antigens (Neo-antigens) 2. Release of Hidden (Sequestered antigens) 3. Cross reacting foreign antigens and Molecular mimicry 4. Epitope spreading

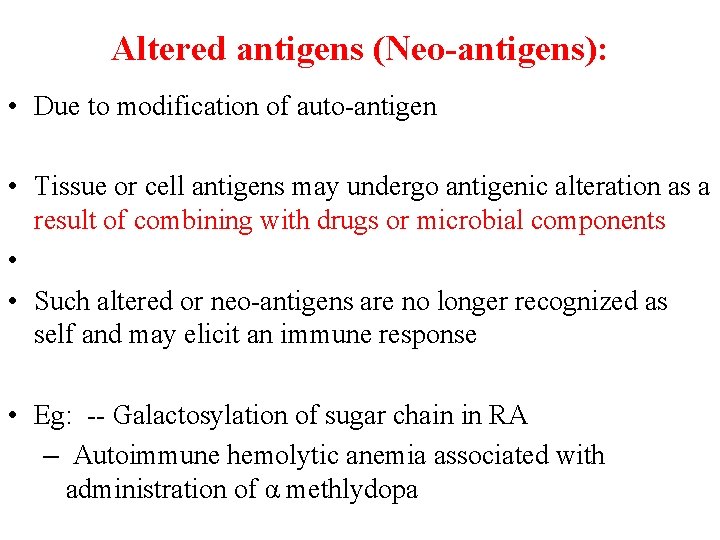
Altered antigens (Neo-antigens): • Due to modification of auto-antigen • Tissue or cell antigens may undergo antigenic alteration as a result of combining with drugs or microbial components • • Such altered or neo-antigens are no longer recognized as self and may elicit an immune response • Eg: -- Galactosylation of sugar chain in RA – Autoimmune hemolytic anemia associated with administration of α methlydopa

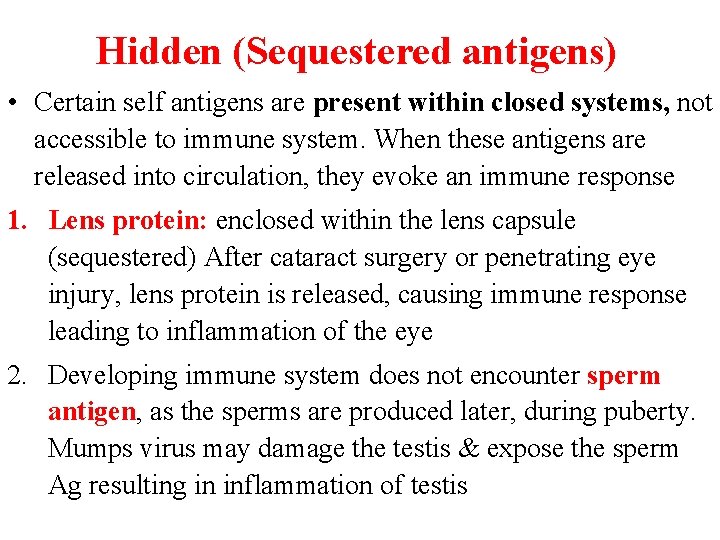
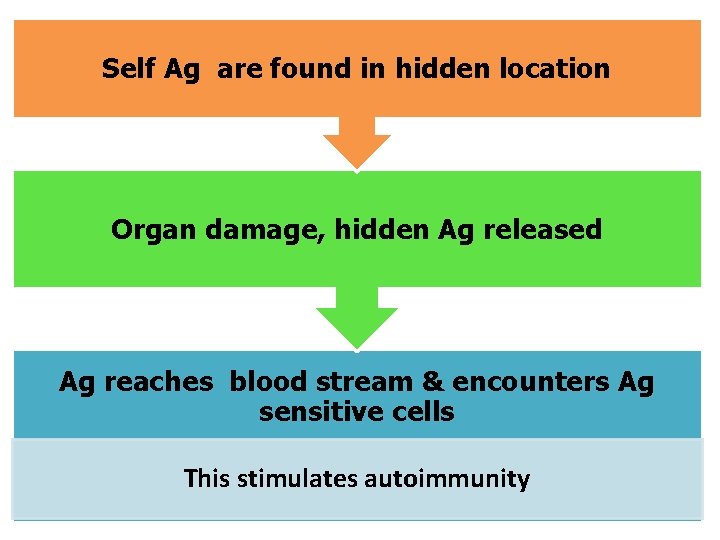
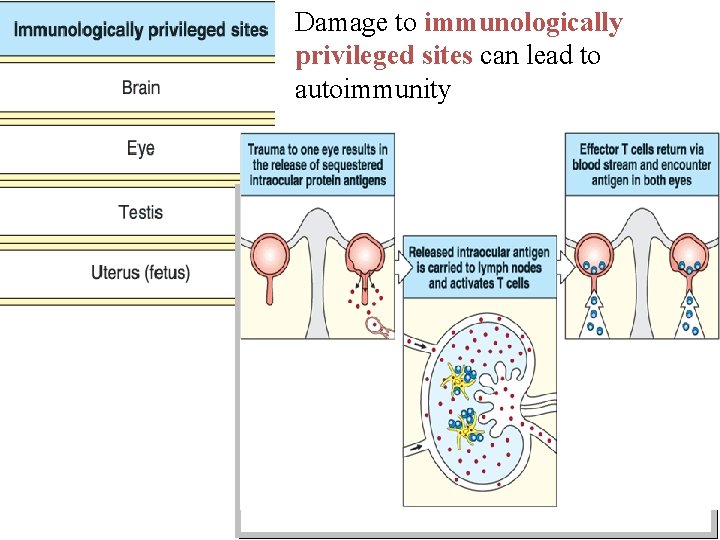
Hidden (Sequestered antigens) • Certain self antigens are present within closed systems, not accessible to immune system. When these antigens are released into circulation, they evoke an immune response 1. Lens protein: enclosed within the lens capsule (sequestered) After cataract surgery or penetrating eye injury, lens protein is released, causing immune response leading to inflammation of the eye 2. Developing immune system does not encounter sperm antigen, as the sperms are produced later, during puberty. Mumps virus may damage the testis & expose the sperm Ag resulting in inflammation of testis

Self Ag are found in hidden location Organ damage, hidden Ag released Ag reaches blood stream & encounters Ag sensitive cells This stimulates autoimmunity

Damage to immunologically privileged sites can lead to autoimmunity

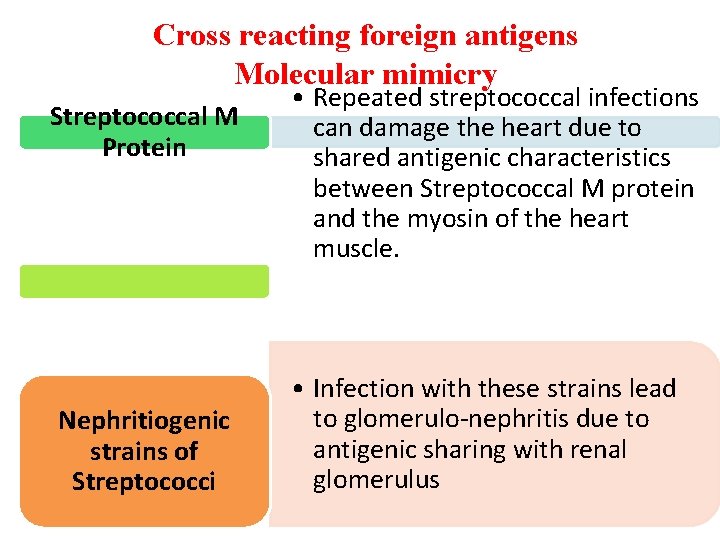
Cross reacting foreign antigens Molecular mimicry Streptococcal M Protein Nephritiogenic strains of Streptococci • Repeated streptococcal infections can damage the heart due to shared antigenic characteristics between Streptococcal M protein and the myosin of the heart muscle. • Infection with these strains lead to glomerulo-nephritis due to antigenic sharing with renal glomerulus

Epitope spreading • A new helper determinant may arise due to drug modification or insertion of viral antigen into membrane of infected cell • These exposed auto antigens stimulate auto reactive T cells and auto immune disease results • The self reactive T cells here are directed not against the virus but against the cellular antigen

Other mechanisms • • • Imbalance of Tc & Th cells Polyclonal activation of lymphocytes Defects in regulatory cells Up-regulation of T cell interaction molecules Cytokine imbalance Autoimmune disorders are multifactorial

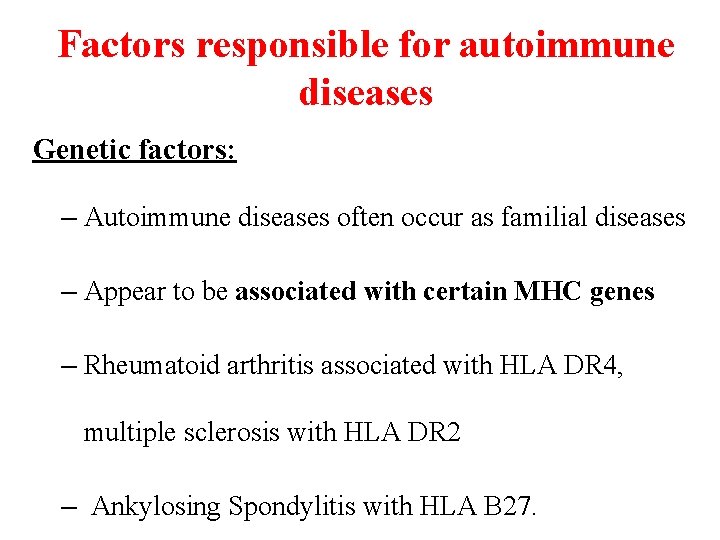
Factors responsible for autoimmune diseases Genetic factors: – Autoimmune diseases often occur as familial diseases – Appear to be associated with certain MHC genes – Rheumatoid arthritis associated with HLA DR 4, multiple sclerosis with HLA DR 2 – Ankylosing Spondylitis with HLA B 27.

Hormonal factors: • Approx 90% of all autoimmune diseases occur in women. • Systemic lupus erythomatosus (SLE) is ten times more common in females than in males Environmental factors: ? ? ?

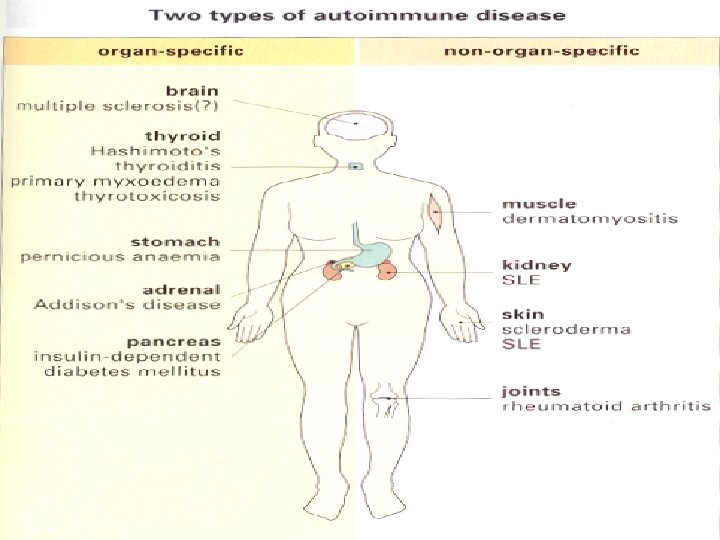
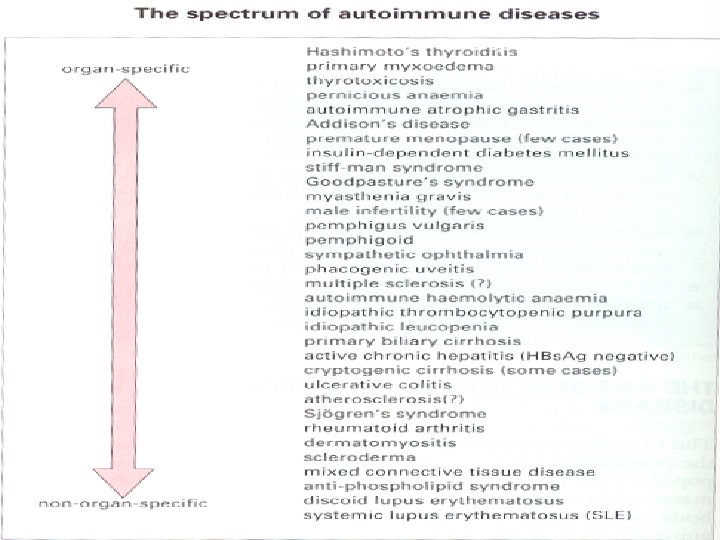
Spectrum of Autoimmune diseases may be classified as: • Organ-specific – In these diseases the target is a single organ or tissue (autoimmune thyroiditis) • Non-organ specific – In these diseases the target is spread over many different organs or tissues (E. g. Systemic lupus erythomatosus).

Organ-specific Non organ-specific • Hashimoto thyroiditis • Systemic lupus (SLE) • Thyrotoxicosis • Rheumatoid arthritis (RA) • Addison’s disease • Scleroderma • Atrophic gastritis • Dermato-myositis • Juvenile diabetes mellitus • Mixed connective tissue • Multiple sclerosis disease (MCTD) • Sjögren’s syndrome




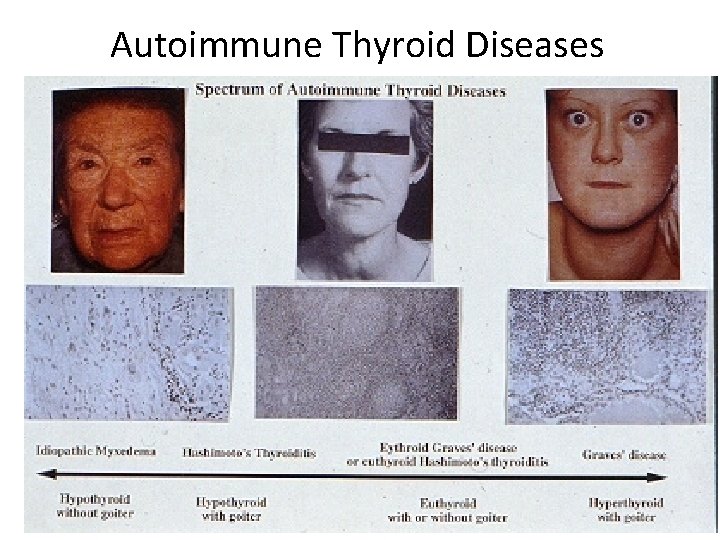
Autoimmune Thyroid Diseases

Hashimoto’s Thyroiditis Abs against Thyroglobulin, these antibodies provoke an inflammatory process that leads to fibrosis of thyroid gland.

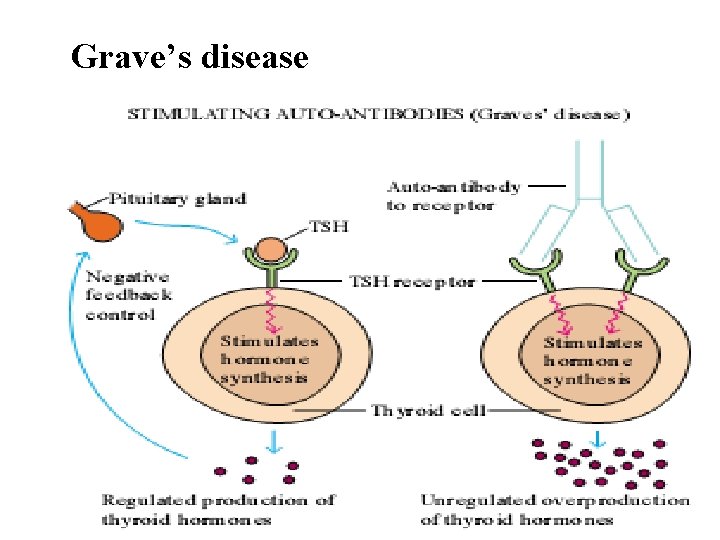
Grave’s disease

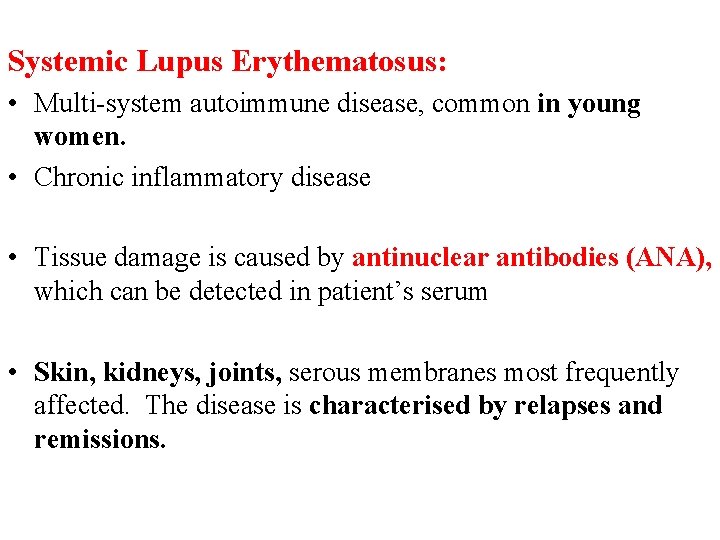
Systemic Lupus Erythematosus: • Multi-system autoimmune disease, common in young women. • Chronic inflammatory disease • Tissue damage is caused by antinuclear antibodies (ANA), which can be detected in patient’s serum • Skin, kidneys, joints, serous membranes most frequently affected. The disease is characterised by relapses and remissions.

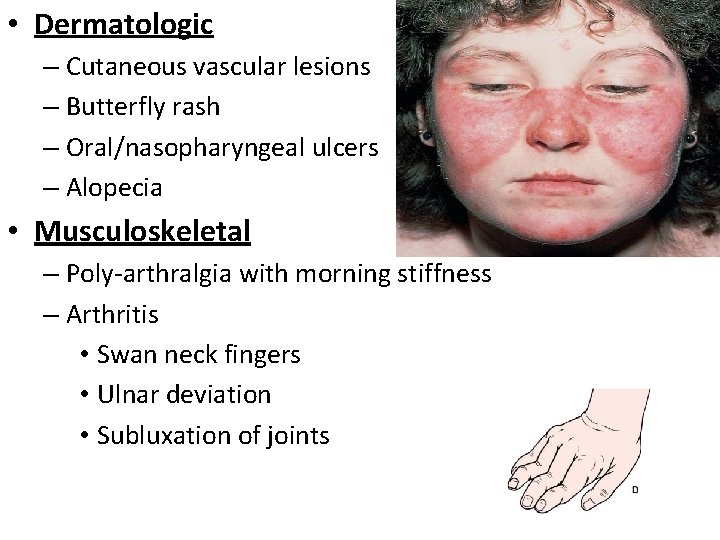
• Dermatologic – Cutaneous vascular lesions – Butterfly rash – Oral/nasopharyngeal ulcers – Alopecia • Musculoskeletal – Poly-arthralgia with morning stiffness – Arthritis • Swan neck fingers • Ulnar deviation • Subluxation of joints

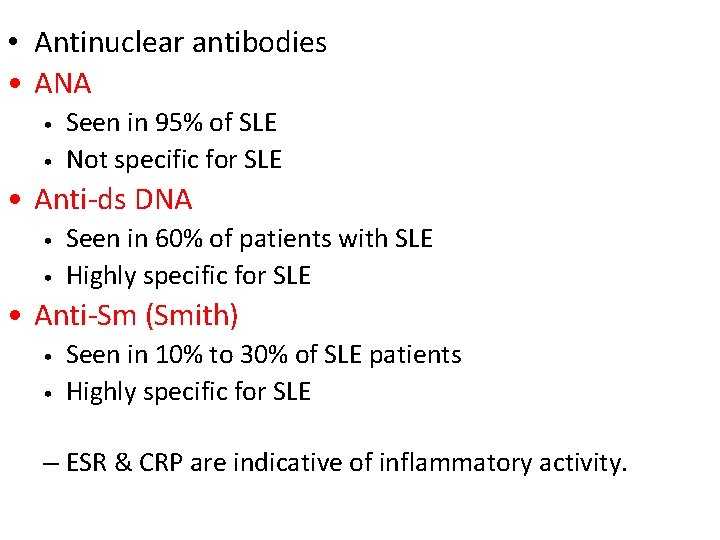
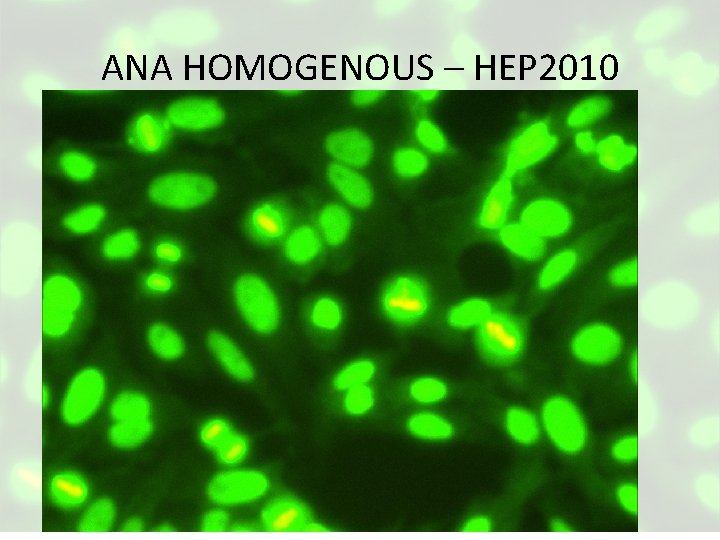
• Antinuclear antibodies • ANA • • Seen in 95% of SLE Not specific for SLE • Anti-ds DNA • • Seen in 60% of patients with SLE Highly specific for SLE • Anti-Sm (Smith) • • Seen in 10% to 30% of SLE patients Highly specific for SLE – ESR & CRP are indicative of inflammatory activity.

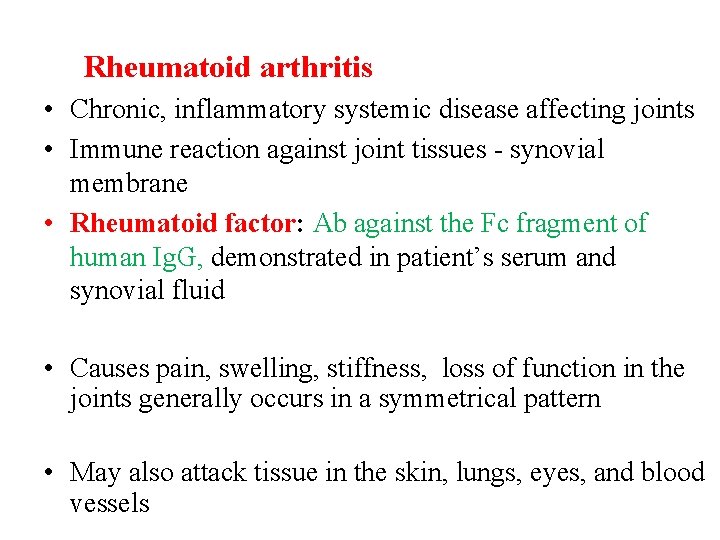
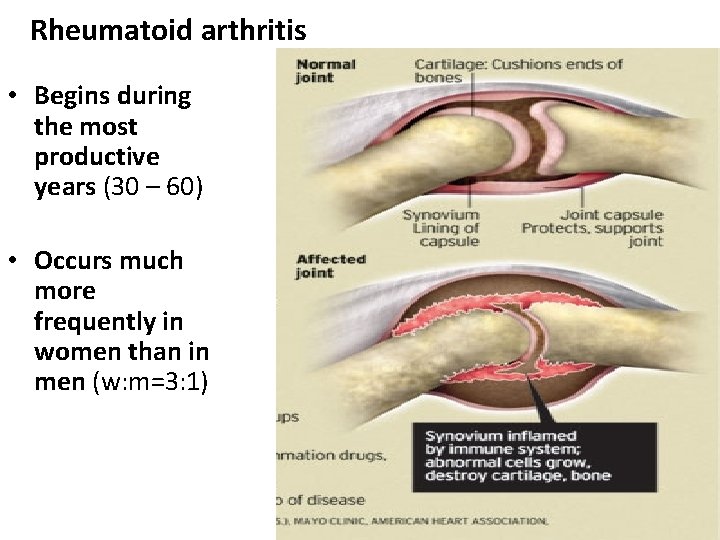
Rheumatoid arthritis • Chronic, inflammatory systemic disease affecting joints • Immune reaction against joint tissues - synovial membrane • Rheumatoid factor: Ab against the Fc fragment of human Ig. G, demonstrated in patient’s serum and synovial fluid • Causes pain, swelling, stiffness, loss of function in the joints generally occurs in a symmetrical pattern • May also attack tissue in the skin, lungs, eyes, and blood vessels

Rheumatoid arthritis • small joints of hands and feet affected first, larger joints later

Rheumatoid arthritis • Begins during the most productive years (30 – 60) • Occurs much more frequently in women than in men (w: m=3: 1)


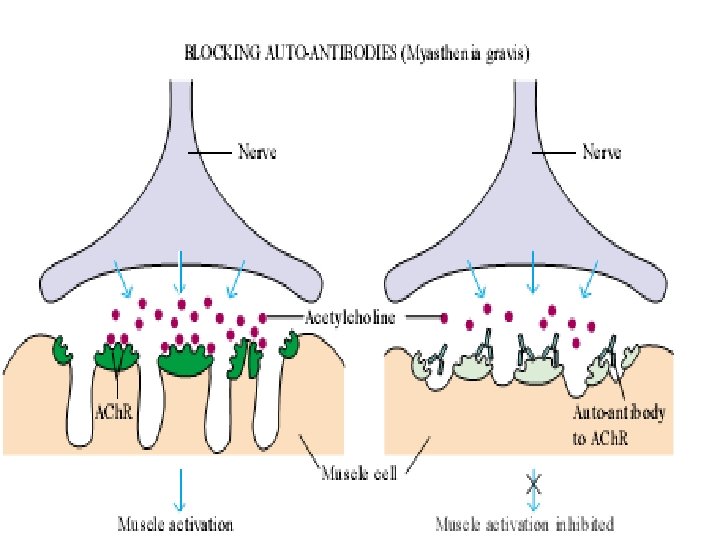
Myasthenia gravis • Normally impulses travel along the nerve to the ending and release the neurotransmitter substance acetylcholine • Acetylcholine travels through the NMJ and binds to Ach receptors which are activated, and generate a muscle contraction • In myasthenia gravis, person’s own antibodies block, alter, or destroy the receptors for acetylcholine at the neuromuscular junction, preventing muscle contraction


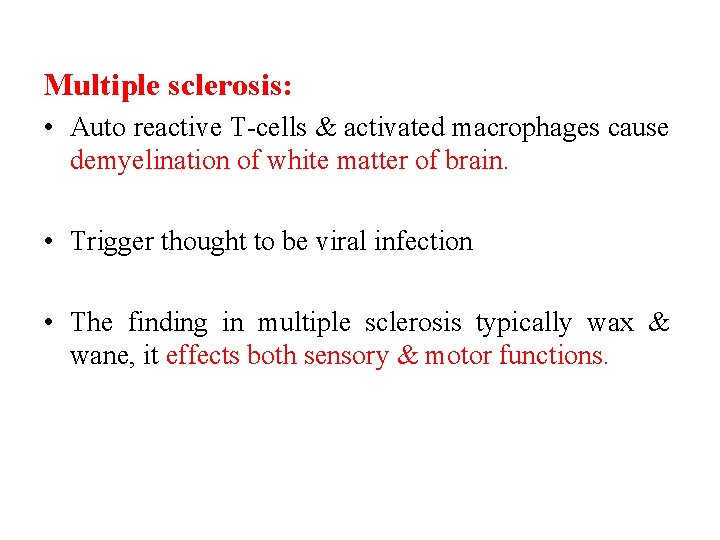
Multiple sclerosis: • Auto reactive T-cells & activated macrophages cause demyelination of white matter of brain. • Trigger thought to be viral infection • The finding in multiple sclerosis typically wax & wane, it effects both sensory & motor functions.

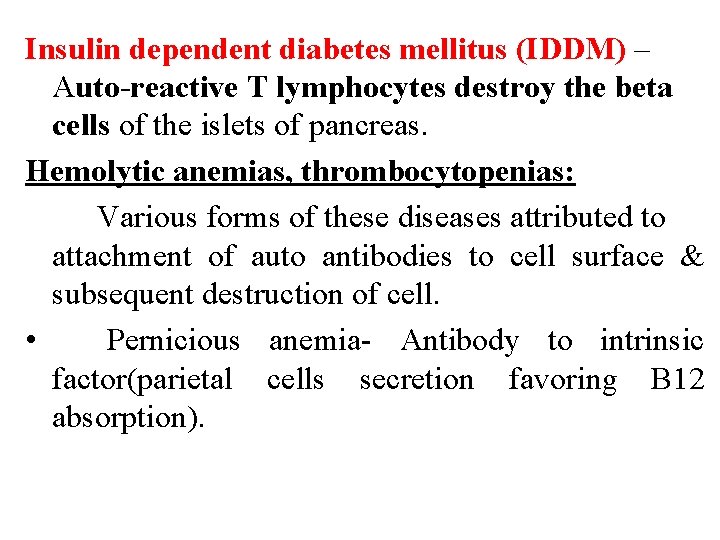
Insulin dependent diabetes mellitus (IDDM) – Auto-reactive T lymphocytes destroy the beta cells of the islets of pancreas. Hemolytic anemias, thrombocytopenias: Various forms of these diseases attributed to attachment of auto antibodies to cell surface & subsequent destruction of cell. • Pernicious anemia- Antibody to intrinsic factor(parietal cells secretion favoring B 12 absorption).

ITP : Ab against platelet, Platelets coated with auto antibody is destroyed in spleen • Several drugs acting as haptens bind to platelet membrane & form neo antigens which induces cytotoxic antibodies-platelet destruction. • Penicillins, cephalosporins, tetracycline, sulphonamide INH, rifampicin & other antimicrobials can cause autoimmune hemolytic anemia by same mechanism.

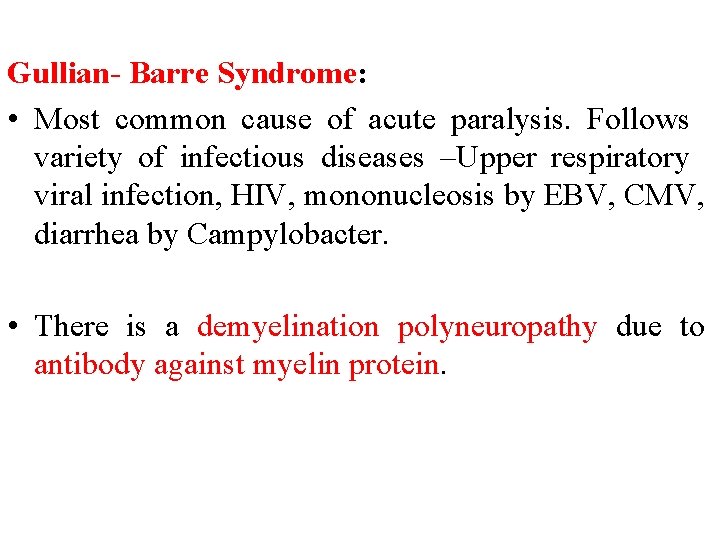
Gullian- Barre Syndrome: • Most common cause of acute paralysis. Follows variety of infectious diseases –Upper respiratory viral infection, HIV, mononucleosis by EBV, CMV, diarrhea by Campylobacter. • There is a demyelination polyneuropathy due to antibody against myelin protein.

Rheumatic fever : Post streptococcal sequelae antibody against M protein cross react withtissue antigen of cardiac muscle and joint tissue. Reiter’s syndrome: • Triad of arthritis, conjunctivitis, urethritis – following infection by Shigella, Salmonella, Yersinia, campylobacter etc. • Patients mostly men with HLA B 27 positive.

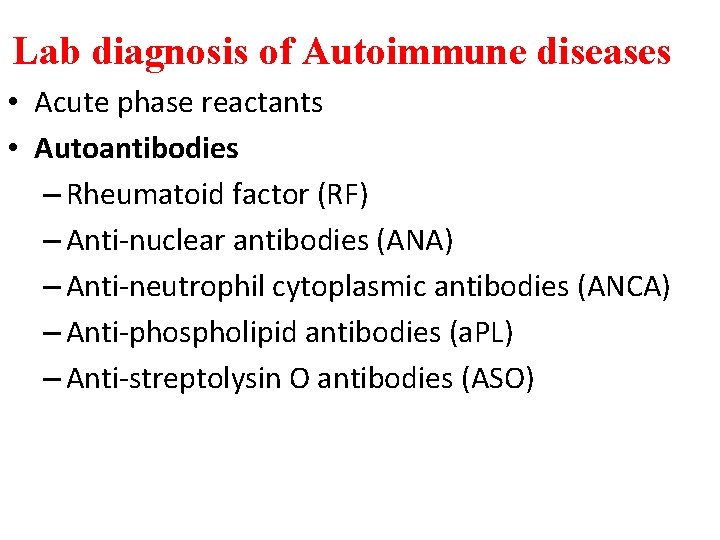
Lab diagnosis of Autoimmune diseases • Acute phase reactants • Autoantibodies – Rheumatoid factor (RF) – Anti-nuclear antibodies (ANA) – Anti-neutrophil cytoplasmic antibodies (ANCA) – Anti-phospholipid antibodies (a. PL) – Anti-streptolysin O antibodies (ASO)

ANA HOMOGENOUS – HEP 2010

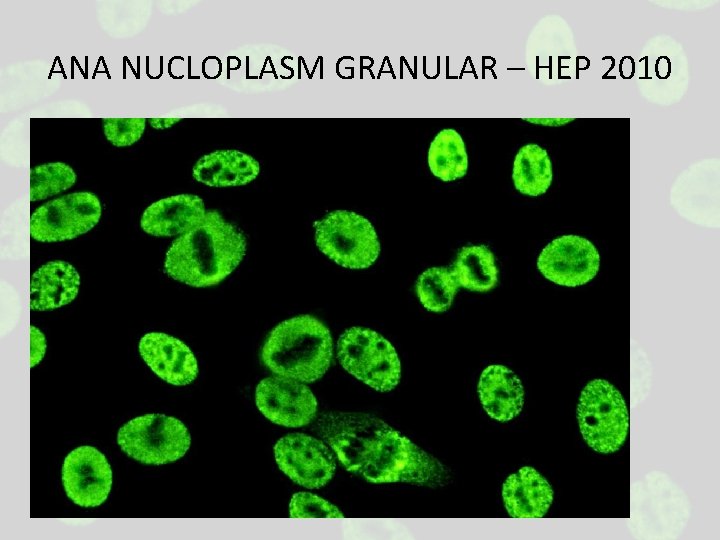
ANA NUCLOPLASM GRANULAR – HEP 2010

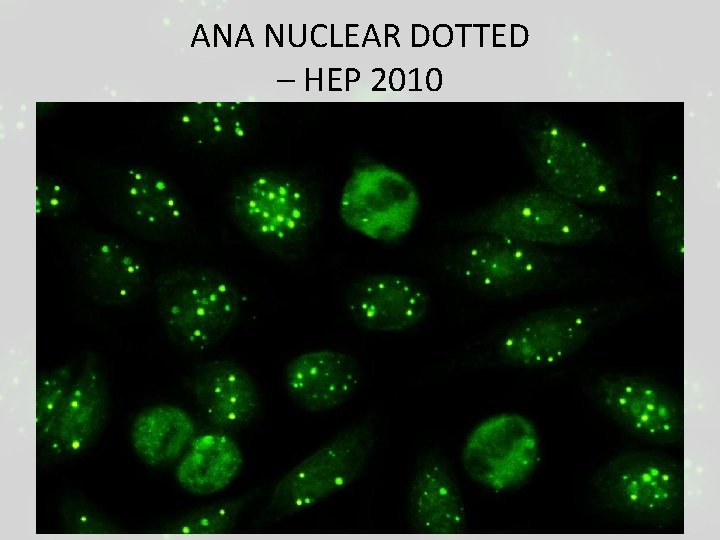
ANA NUCLEAR DOTTED – HEP 2010

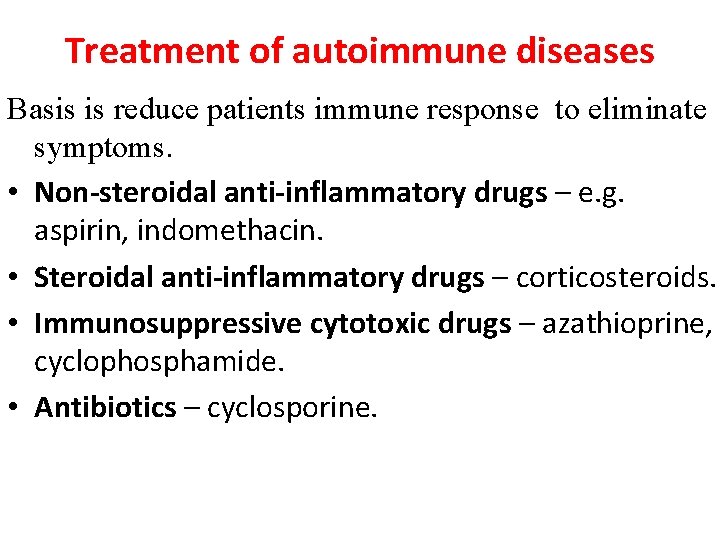
Treatment of autoimmune diseases Basis is reduce patients immune response to eliminate symptoms. • Non-steroidal anti-inflammatory drugs – e. g. aspirin, indomethacin. • Steroidal anti-inflammatory drugs – corticosteroids. • Immunosuppressive cytotoxic drugs – azathioprine, cyclophosphamide. • Antibiotics – cyclosporine.

• Surgery – thymectomy for myasthenia gravis • Plasmapheresis – to remove the offending immune complexes or antibodies • Anti-TNF antibodies or soluble form of TNF receptor may neutralize TNF and are approved for the treatment of rheumatoid arthritis. • Infliximab [antibody to TNF] & Etanercept [TNF receptor] reduce joint inflammation in RA.