I PROPERTIES OF SOLUTIONS SOLUTION a homogeneous mixture




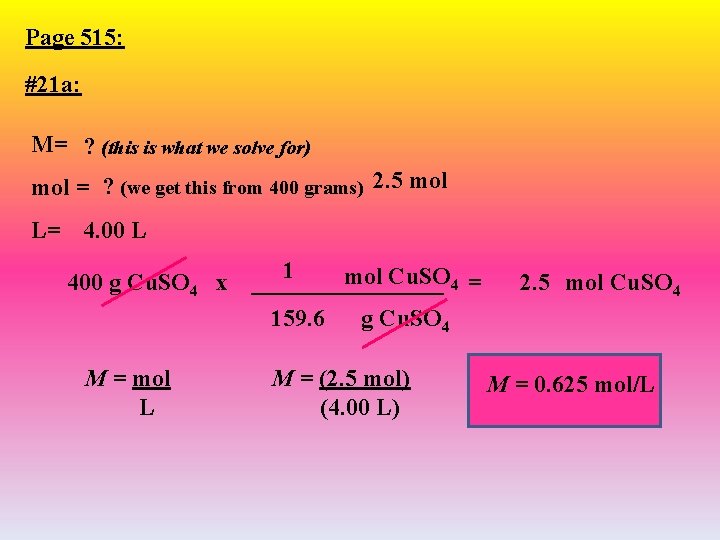






- Slides: 42

I. PROPERTIES OF SOLUTIONS • SOLUTION: - a homogeneous mixture - looks the same throughout • SOLUBILITY: the amount of solute (dissolved substance) that dissolves in an of solvent (substance that does the dissolving) at a specific temperature • SATURATED: a solution containing the maximum amount of dissolved solute • UNSATURATED: a solution containing less than the maximum amount of dissolved solute

• MISCIBLE: describes liquids that DO dissolve in each other Ex. Water and vinegar • IMMISCIBLE: describes liquids that DO NOT dissolve in each other Ex. Water and oil QUESTION: Why would it be wrong to say that sugar and water are miscible?

FACTORS AFFECTING SOLUBILITY: 1. Temperature: - SOLIDS are MORE soluble in liquids at HIGHER temps. - GASES are LESS soluble in liquids at HIGHER temps. Why does warm soda go “flat? ” Soda “goes flat” when the CO 2 gas escapes from the liquid; At a warm temp. the CO 2 gas is less soluble, so the gas escapes the liquid faster 2. Particle size: - SMALLER particles dissolve FASTER 3. Pressure: - GASES are MORE soluble in liquids at HIGHER pressures **4. Agitation: - Particles dissolve faster when stirred, mixed, shaken, etc.

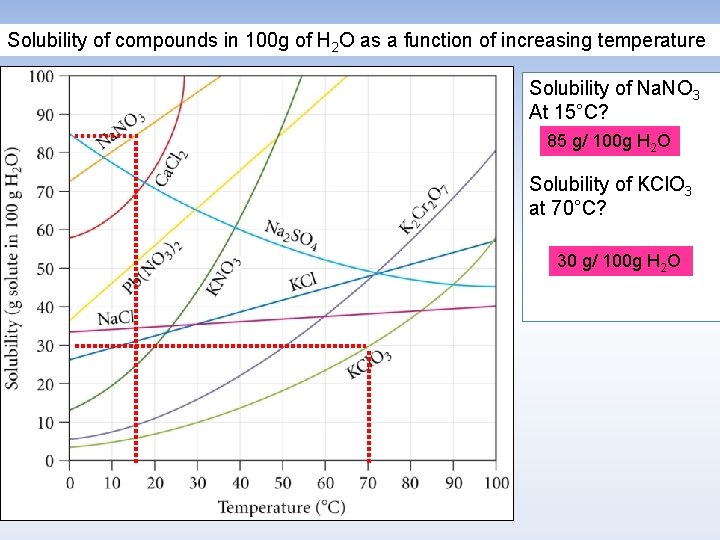
Solubility of compounds in 100 g of H 2 O as a function of increasing temperature Solubility of Na. NO 3 At 15°C? 85 g/ 100 g H 2 O Solubility of KCl. O 3 at 70°C? 30 g/ 100 g H 2 O


Use Figure 18. 4 on pg. 504 to answer the following questions: 1. ) What is the solubility of NH 4 Cl in water at 35°C? 40 g / 100 g H 2 O 2. ) What is the solubility of Na. NO 3 in water at 10°C? 80 g / 100 g H 2 O 3. ) What is the solubility of KBr in water at 65°C? 90 g / 100 g H 2 O 4. ) What is the solubility of KNO 3 in water at 70°C? 120 g / 100 g H O 2 5. ) Which compound’s solubility is relatively unaffected by increasing temperature? How do you know? Na. Cl, b/c its solubility curve remains relatively flat 6. ) Which compound’s solubility follows a trend opposite of the others? How do you know? Yb 2(SO 4)3 ; its solubility decreases with increasing temp.

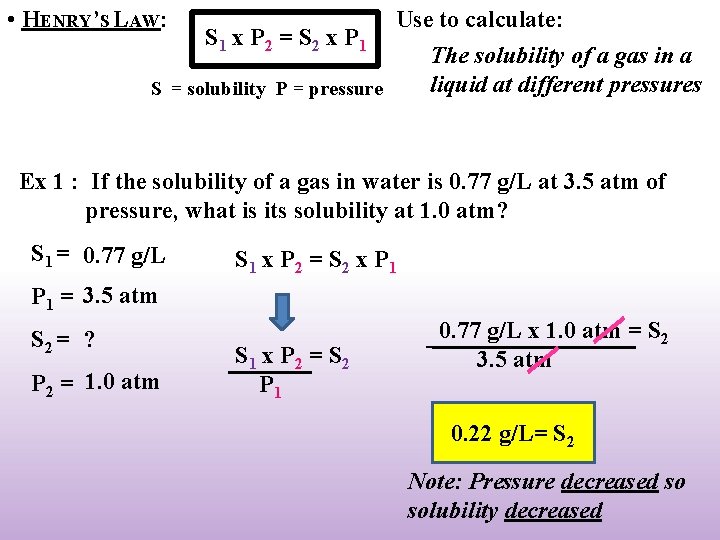
• HENRY’S LAW: Use to calculate: S 1 x P 2 = S 2 x P 1 The solubility of a gas in a liquid at different pressures S = solubility P = pressure Ex 1 : If the solubility of a gas in water is 0. 77 g/L at 3. 5 atm of pressure, what is its solubility at 1. 0 atm? S 1 = 0. 77 g/L S 1 x P 2 = S 2 x P 1 = 3. 5 atm S 2 = ? P 2 = 1. 0 atm S 1 x P 2 = S 2 P 1 0. 77 g/L x 1. 0 atm = S 2 3. 5 atm 0. 22 g/L= S 2 Note: Pressure decreased so solubility decreased

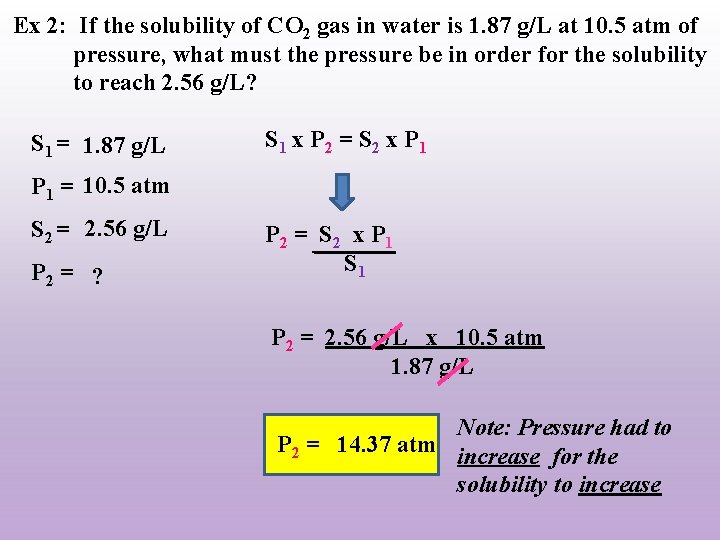
Ex 2: If the solubility of CO 2 gas in water is 1. 87 g/L at 10. 5 atm of pressure, what must the pressure be in order for the solubility to reach 2. 56 g/L? S 1 = 1. 87 g/L S 1 x P 2 = S 2 x P 1 = 10. 5 atm S 2 = 2. 56 g/L P 2 = ? P 2 = S 2 x P 1 S 1 P 2 = 2. 56 g/L x 10. 5 atm 1. 87 g/L Note: Pressure had to P 2 = 14. 37 atm increase for the solubility to increase

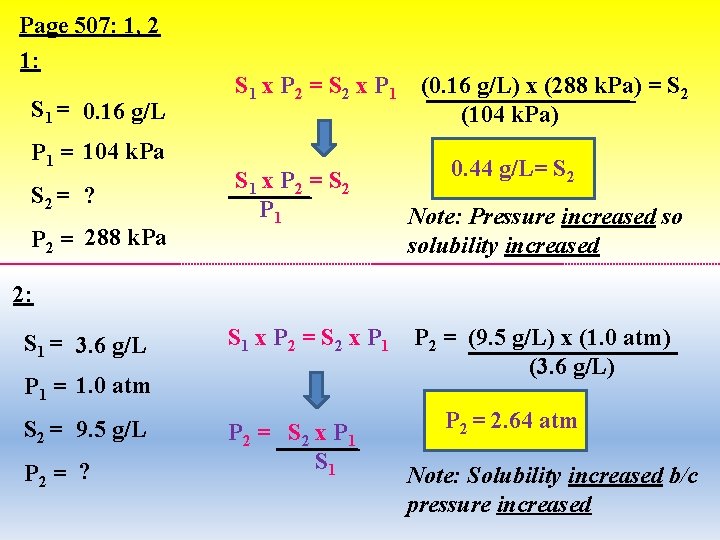
Page 507: 1, 2 1: S 1 = 0. 16 g/L P 1 = 104 k. Pa S 2 = ? P 2 = 288 k. Pa S 1 x P 2 = S 2 x P 1 S 1 x P 2 = S 2 P 1 (0. 16 g/L) x (288 k. Pa) = S 2 (104 k. Pa) 0. 44 g/L= S 2 Note: Pressure increased so solubility increased 2: S 1 = 3. 6 g/L S 1 x P 2 = S 2 x P 1 = 1. 0 atm S 2 = 9. 5 g/L P 2 = ? P 2 = S 2 x P 1 S 1 P 2 = (9. 5 g/L) x (1. 0 atm) (3. 6 g/L) P 2 = 2. 64 atm Note: Solubility increased b/c pressure increased

Page 508: Section Review 18. 1 (3, 4, 6) 3. temperature, particle size, and pressure 4. By using Henry’s Law : S 1 x P 2 = S 2 x P 1 6. a. Add more solvent 6. b. Add more solute

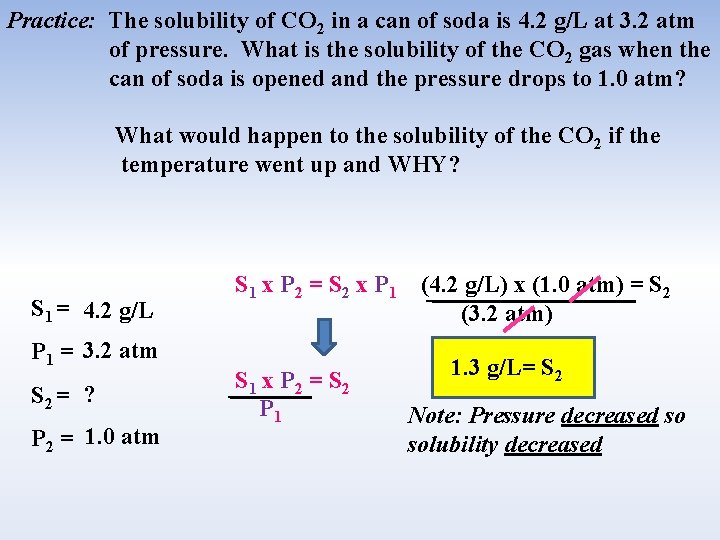
Practice: The solubility of CO 2 in a can of soda is 4. 2 g/L at 3. 2 atm of pressure. What is the solubility of the CO 2 gas when the can of soda is opened and the pressure drops to 1. 0 atm? What would happen to the solubility of the CO 2 if the temperature went up and WHY? S 1 = 4. 2 g/L P 1 = 3. 2 atm S 2 = ? P 2 = 1. 0 atm S 1 x P 2 = S 2 x P 1 S 1 x P 2 = S 2 P 1 (4. 2 g/L) x (1. 0 atm) = S 2 (3. 2 atm) 1. 3 g/L= S 2 Note: Pressure decreased so solubility decreased

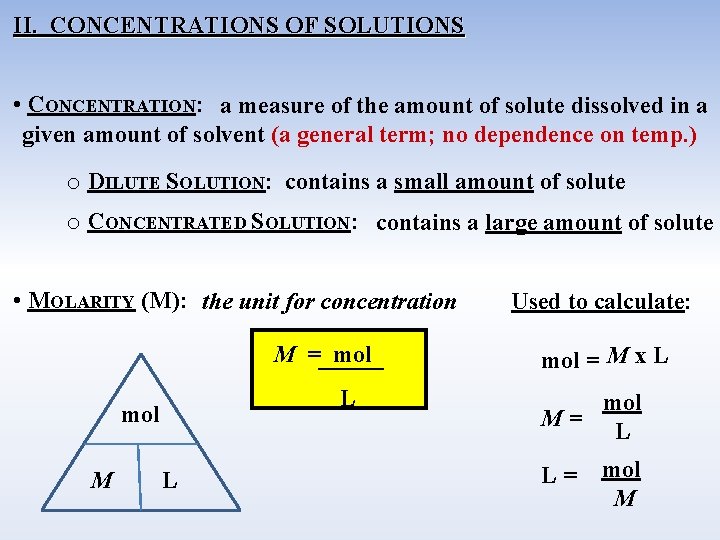
II. CONCENTRATIONS OF SOLUTIONS • CONCENTRATION: a measure of the amount of solute dissolved in a given amount of solvent (a general term; no dependence on temp. ) o DILUTE SOLUTION: contains a small amount of solute o CONCENTRATED SOLUTION: contains a large amount of solute • MOLARITY (M): the unit for concentration M = mol L mol M L Used to calculate: mol = M x L mol M= L L= mol M

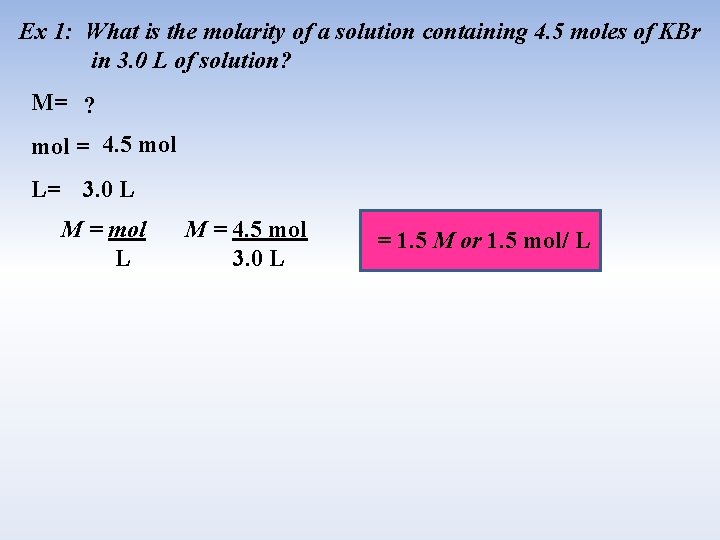
Ex 1: What is the molarity of a solution containing 4. 5 moles of KBr in 3. 0 L of solution? M= ? mol = 4. 5 mol L= 3. 0 L M = mol L M = 4. 5 mol 3. 0 L = 1. 5 M or 1. 5 mol/ L

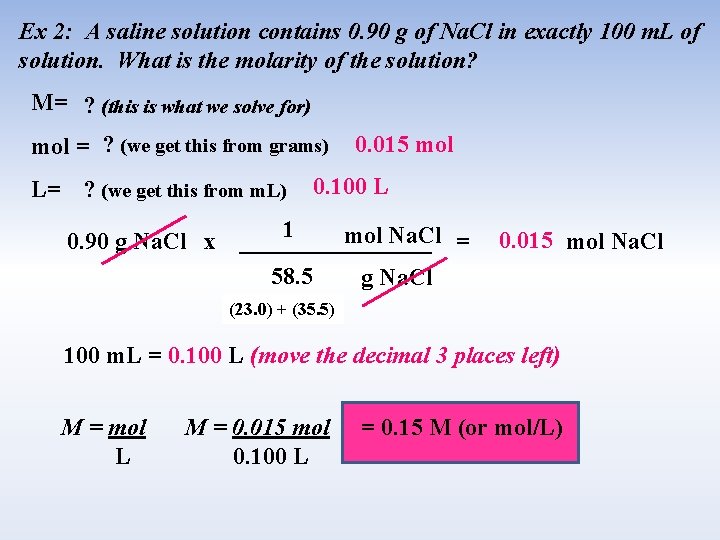
Ex 2: A saline solution contains 0. 90 g of Na. Cl in exactly 100 m. L of solution. What is the molarity of the solution? M= ? (this is what we solve for) mol = ? (we get this from grams) L= ? (we get this from m. L) 0. 90 g Na. Cl x 0. 015 mol 0. 100 L 1 mol Na. Cl = ________ 58. 5 g Na. Cl 0. 015 mol Na. Cl (23. 0) + (35. 5) 100 m. L = 0. 100 L (move the decimal 3 places left) M = mol L M = 0. 015 mol 0. 100 L = 0. 15 M (or mol/L)

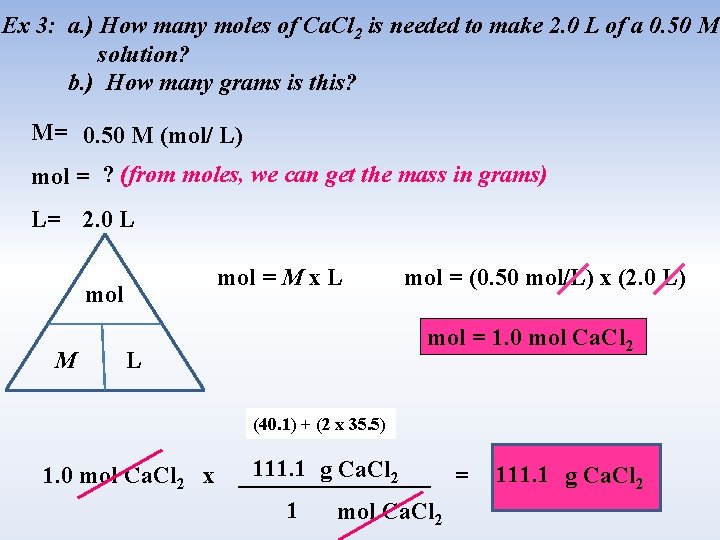
Ex 3: a. ) How many moles of Ca. Cl 2 is needed to make 2. 0 L of a 0. 50 M solution? b. ) How many grams is this? M= 0. 50 M (mol/ L) mol = ? (from moles, we can get the mass in grams) L= 2. 0 L mol = M x L mol M mol = (0. 50 mol/L) x (2. 0 L) mol = 1. 0 mol Ca. Cl 2 L (40. 1) + (2 x 35. 5) 1. 0 mol Ca. Cl 2 x 111. 1 g Ca. Cl 2 ________ = 1 mol Ca. Cl 2 111. 1 g Ca. Cl 2

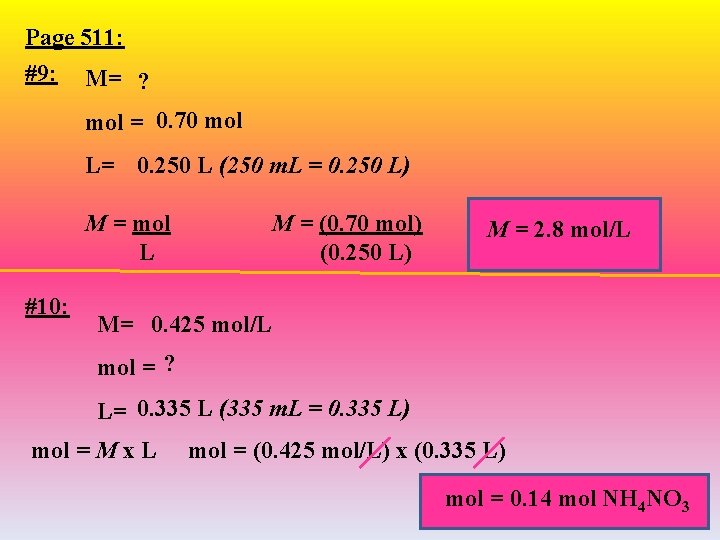
Page 511: #9: M= ? mol = 0. 70 mol L= 0. 250 L (250 m. L = 0. 250 L) M = mol L #10: M = (0. 70 mol) (0. 250 L) M = 2. 8 mol/L M= 0. 425 mol/L mol = ? L= 0. 335 L (335 m. L = 0. 335 L) mol = M x L mol = (0. 425 mol/L) x (0. 335 L) mol = 0. 14 mol NH 4 NO 3

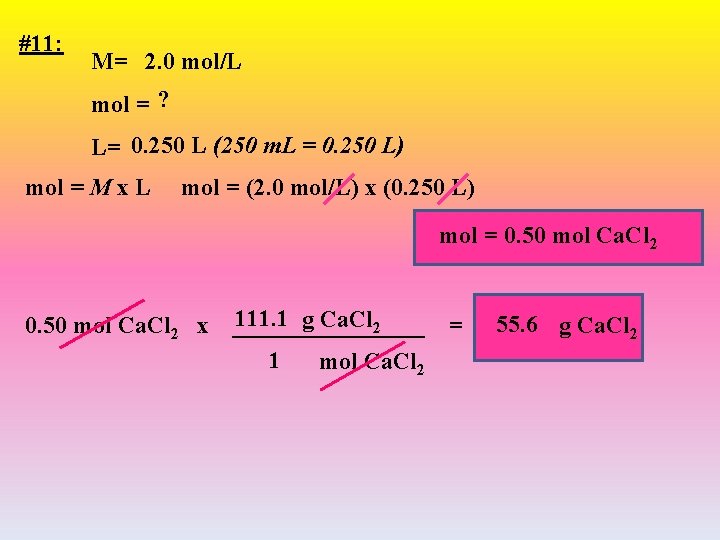
#11: M= 2. 0 mol/L mol = ? L= 0. 250 L (250 m. L = 0. 250 L) mol = M x L mol = (2. 0 mol/L) x (0. 250 L) mol = 0. 50 mol Ca. Cl 2 x 111. 1 g Ca. Cl 2 ________ = 1 mol Ca. Cl 2 55. 6 g Ca. Cl 2
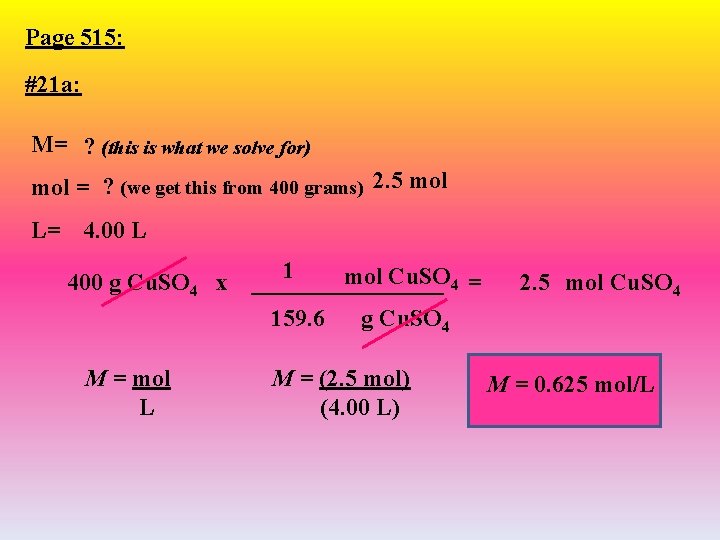
Page 515: #21 a: M= ? (this is what we solve for) mol = ? (we get this from 400 grams) 2. 5 mol L= 4. 00 L 400 g Cu. SO 4 x M = mol L 1 mol Cu. SO 4 = ________ 159. 6 g Cu. SO 4 M = (2. 5 mol) (4. 00 L) 2. 5 mol Cu. SO 4 M = 0. 625 mol/L

Page 515: #21 b: M= ? mol = 0. 06 mol L= 1. 500 L (1500 m. L = 1. 500 L) M = mol L M = (0. 06 mol) (1. 500 L) M = 0. 04 mol/L

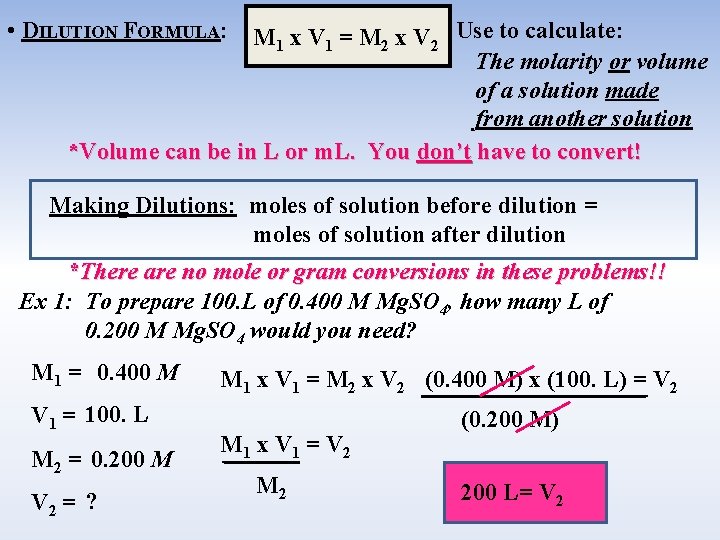
• DILUTION FORMULA: M 1 x V 1 = M 2 x V 2 Use to calculate: The molarity or volume of a solution made from another solution *Volume can be in L or m. L. You don’t have to convert! Making Dilutions: moles of solution before dilution = moles of solution after dilution *There are no mole or gram conversions in these problems!! Ex 1: To prepare 100. L of 0. 400 M Mg. SO 4, how many L of 0. 200 M Mg. SO 4 would you need? M 1 = 0. 400 M V 1 = 100. L M 2 = 0. 200 M V 2 = ? M 1 x V 1 = M 2 x V 2 (0. 400 M) x (100. L) = V 2 M 1 x V 1 = V 2 M 2 (0. 200 M) 200 L= V 2

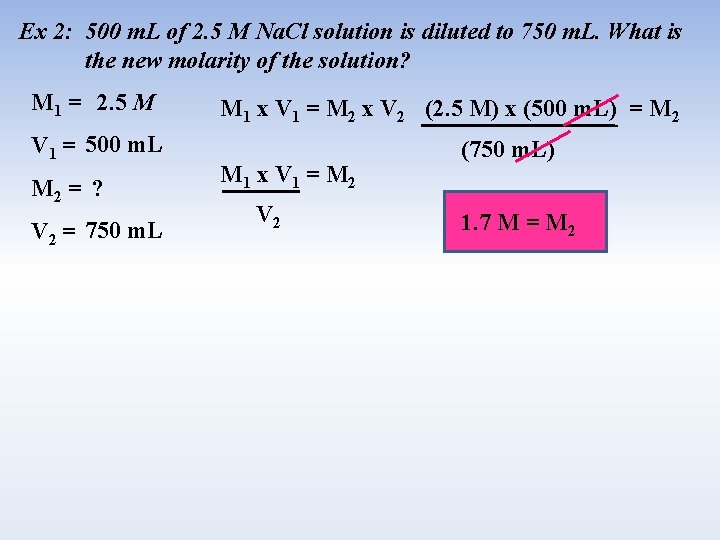
Ex 2: 500 m. L of 2. 5 M Na. Cl solution is diluted to 750 m. L. What is the new molarity of the solution? M 1 = 2. 5 M V 1 = 500 m. L M 2 = ? V 2 = 750 m. L M 1 x V 1 = M 2 x V 2 (2. 5 M) x (500 m. L) = M 2 M 1 x V 1 = M 2 V 2 (750 m. L) 1. 7 M = M 2

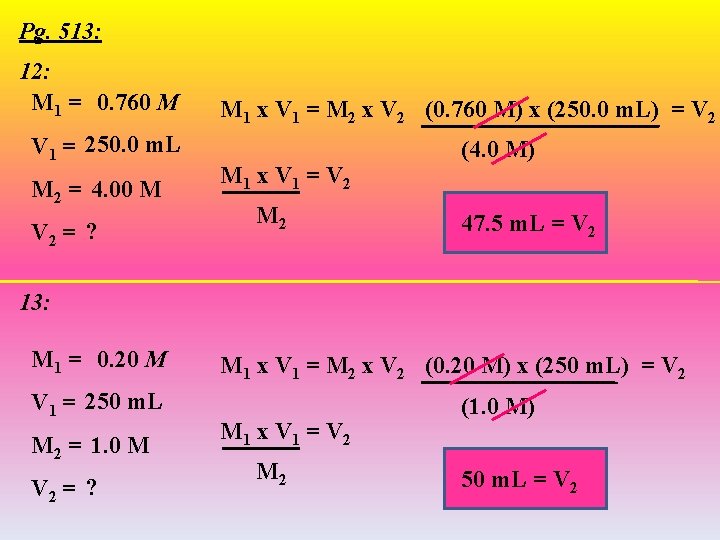
Pg. 513: 12: M 1 = 0. 760 M V 1 = 250. 0 m. L M 2 = 4. 00 M V 2 = ? M 1 x V 1 = M 2 x V 2 (0. 760 M) x (250. 0 m. L) = V 2 M 1 x V 1 = V 2 M 2 (4. 0 M) 47. 5 m. L = V 2 13: M 1 = 0. 20 M V 1 = 250 m. L M 2 = 1. 0 M V 2 = ? M 1 x V 1 = M 2 x V 2 (0. 20 M) x (250 m. L) = V 2 M 1 x V 1 = V 2 M 2 (1. 0 M) 50 m. L = V 2

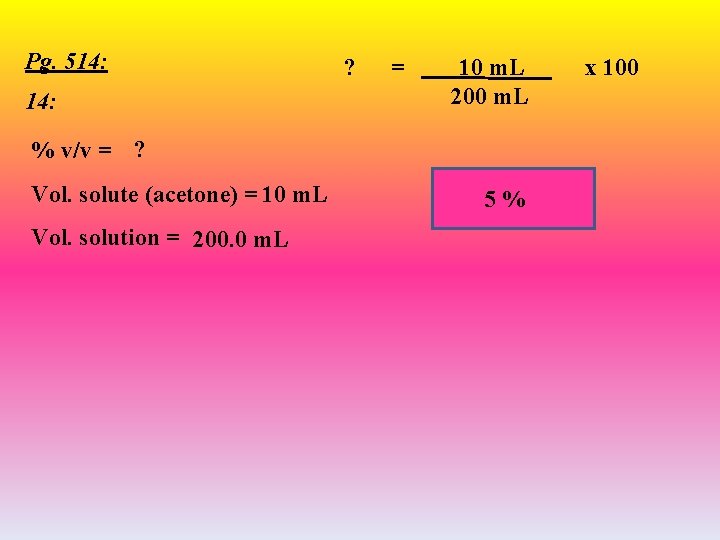
Pg. 514: ? = 10 m. L 200 m. L % v/v = ? Vol. solute (acetone) = 10 m. L Vol. solution = 200. 0 m. L 5% x 100

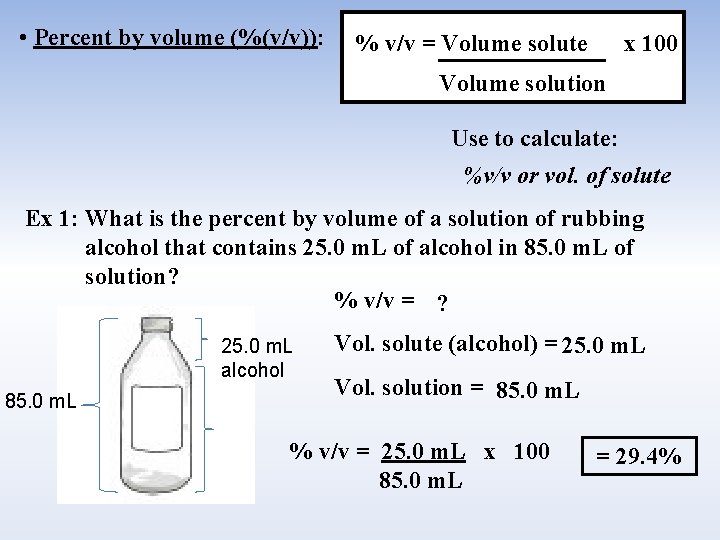
• Percent by volume (%(v/v)): % v/v = Volume solute x 100 Volume solution Use to calculate: %v/v or vol. of solute Ex 1: What is the percent by volume of a solution of rubbing alcohol that contains 25. 0 m. L of alcohol in 85. 0 m. L of solution? % v/v = ? 25. 0 m. L alcohol 85. 0 m. L Vol. solute (alcohol) = 25. 0 m. L Vol. solution = 85. 0 m. L % v/v = 25. 0 m. L x 100 85. 0 m. L = 29. 4%

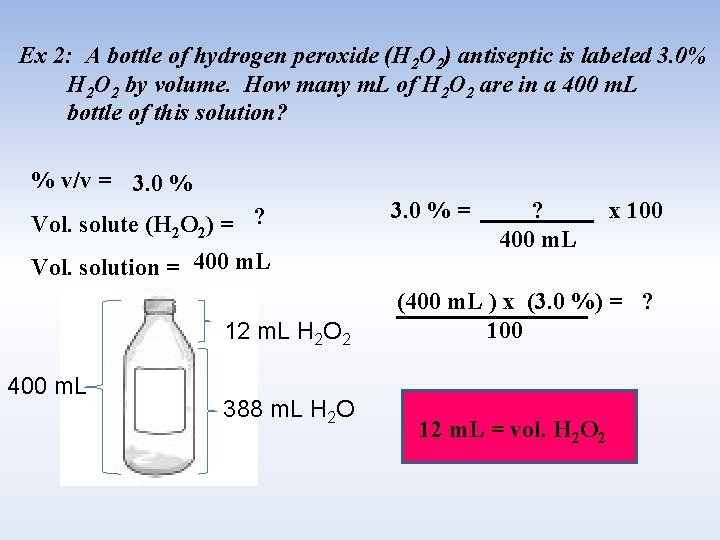
Ex 2: A bottle of hydrogen peroxide (H 2 O 2) antiseptic is labeled 3. 0% H 2 O 2 by volume. How many m. L of H 2 O 2 are in a 400 m. L bottle of this solution? % v/v = 3. 0 % Vol. solute (H 2 O 2) = ? Vol. solution = 400 m. L 12 m. L H 2 O 2 400 m. L 388 m. L H 2 O 3. 0 % = ? 400 m. L x 100 (400 m. L ) x (3. 0 %) = ? 100 12 m. L = vol. H 2 O 2

IV. CALCULATIONS INVOLVING COLLIGATIVE PROPERTIES Because volume depends on temperature and molarity depends on Volume, we need a way of expressing solution concentration when Temperature changes. To do that, we use molality: • MOLALITY (m): m = mol solute kg solvent Although we could use this formula to solve for m, mol, or kg, we will focus on using it only to find molality (m)

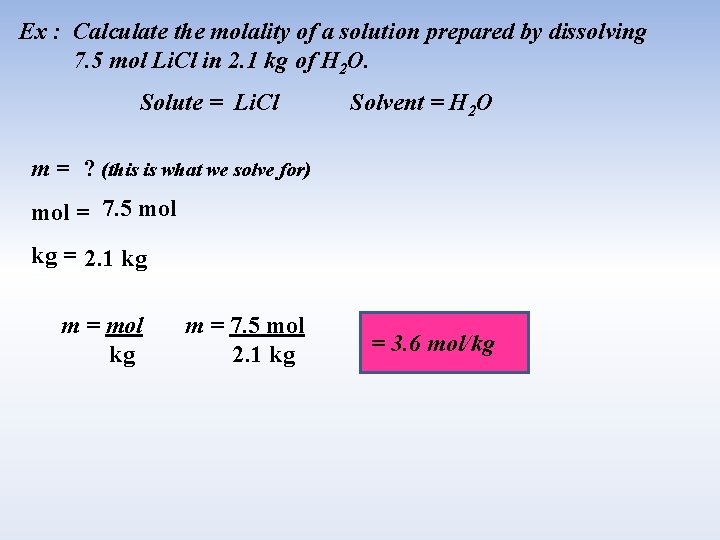
Ex : Calculate the molality of a solution prepared by dissolving 7. 5 mol Li. Cl in 2. 1 kg of H 2 O. Solute = Li. Cl Solvent = H 2 O m = ? (this is what we solve for) mol = 7. 5 mol kg = 2. 1 kg m = mol kg m = 7. 5 mol 2. 1 kg = 3. 6 mol/kg

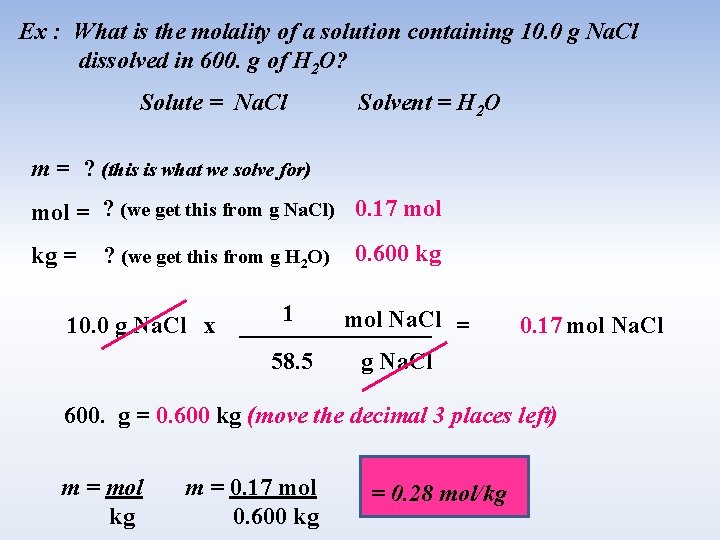
Ex : What is the molality of a solution containing 10. 0 g Na. Cl dissolved in 600. g of H 2 O? Solute = Na. Cl Solvent = H 2 O m = ? (this is what we solve for) mol = ? (we get this from g Na. Cl) 0. 17 mol kg = ? (we get this from g H 2 O) 10. 0 g Na. Cl x 0. 600 kg 1 mol Na. Cl = ________ 58. 5 g Na. Cl 0. 17 mol Na. Cl 600. g = 0. 600 kg (move the decimal 3 places left) m = mol kg m = 0. 17 mol 0. 600 kg = 0. 28 mol/kg

III. COLLIGATIVE PROPERTIES OF SOLUTIONS • COLLIGATIVE PROPERTIES: - depend only on the number of particles dissolved in a solution NOT on what they are → Remember, only IONIC compounds (metal & nonmetal) break apart in H 2 O, MOLECULAR do NOT Ex. How many particles are released when each compound dissolves in water? Na. Cl 2 Ca. F 2 3 Mg. O 2 Li. Br 2 K 2 S 3

• BOILING POINT ELEVATION: the increase in temperature between the boiling point of a solution & the boiling point of a pure solvent Ex. Salt water boils ABOVE 100°C • FREEZING POINT DEPRESSION: the decrease in temperature between the freezing point of a solution & the freezing point of a pure solvent Ex. Salt water freezes BELOW 0°C

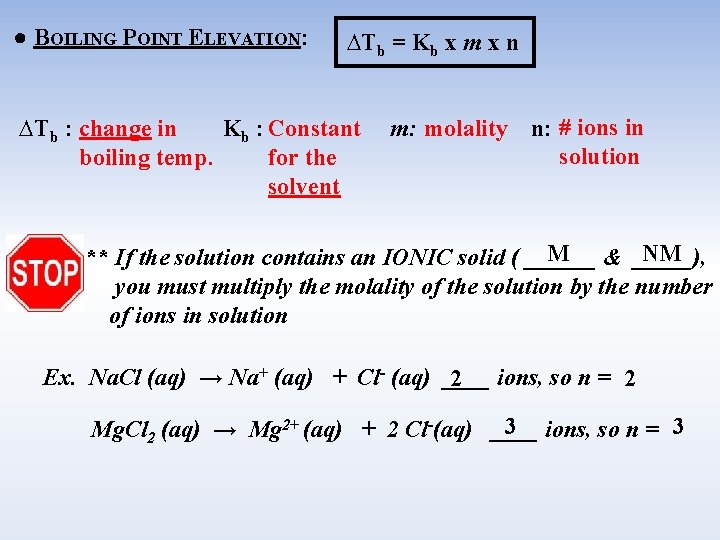
● BOILING POINT ELEVATION: ∆Tb = Kb x m x n ∆Tb : change in Kb : Constant boiling temp. for the solvent m: molality n: # ions in solution M & _____), NM ** If the solution contains an IONIC solid ( ______ you must multiply the molality of the solution by the number of ions in solution Ex. Na. Cl (aq) → Na+ (aq) + Cl- (aq) ____ 2 ions, so n = 2 3 ions, so n = 3 Mg. Cl 2 (aq) → Mg 2+ (aq) + 2 Cl-(aq) ____

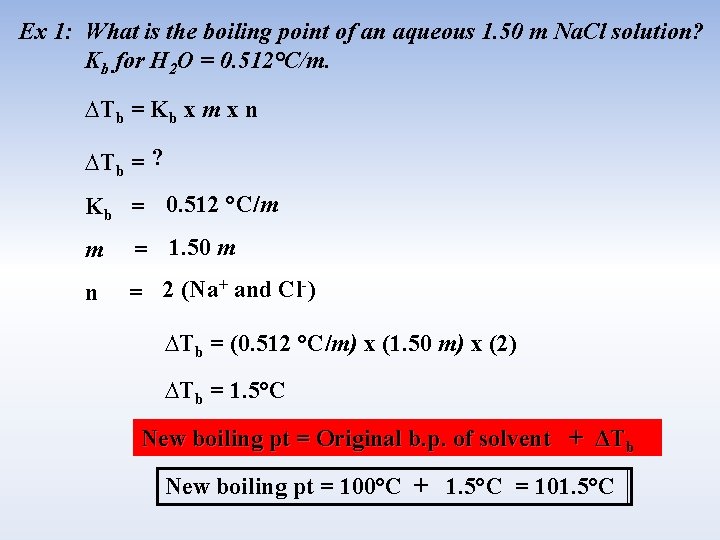
Ex 1: What is the boiling point of an aqueous 1. 50 m Na. Cl solution? Kb for H 2 O = 0. 512°C/m. ∆Tb = Kb x m x n ∆Tb = ? Kb = 0. 512 °C/m m = 1. 50 m n = 2 (Na+ and Cl-) ∆Tb = (0. 512 °C/m) x (1. 50 m) x (2) ∆Tb = 1. 5°C New boiling pt = Original b. p. of solvent + ΔTb New boiling pt = 100°C + 1. 5°C = 101. 5°C

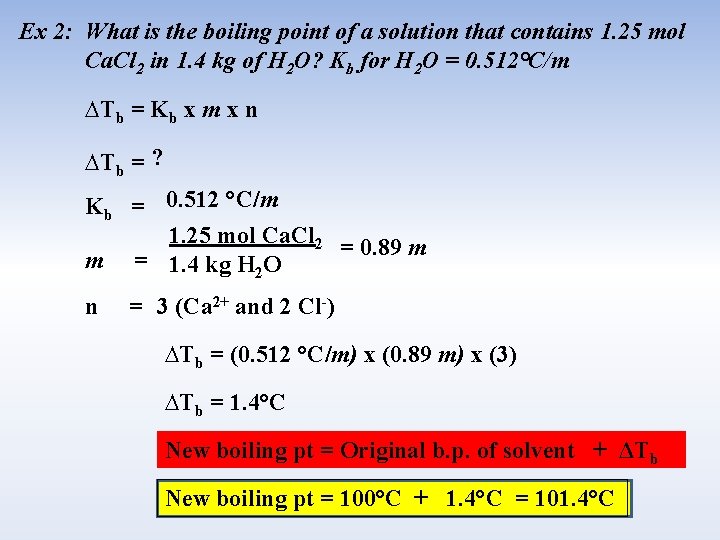
Ex 2: What is the boiling point of a solution that contains 1. 25 mol Ca. Cl 2 in 1. 4 kg of H 2 O? Kb for H 2 O = 0. 512°C/m ∆Tb = Kb x m x n ∆Tb = ? Kb = 0. 512 °C/m 1. 25 mol Ca. Cl 2 = 0. 89 m m = 1. 4 kg H 2 O n = 3 (Ca 2+ and 2 Cl-) ∆Tb = (0. 512 °C/m) x (0. 89 m) x (3) ∆Tb = 1. 4°C New boiling pt = Original b. p. of solvent + ΔTb New boiling pt = 100°C + 1. 4°C = 101. 4°C

● FREEZING POINT DEPRESSION: ∆Tf = Kf x m x n ∆Tf : change in Kf : Constant m: molality freezing temp. for the solvent n: # ions in solution ** These problems are solved the same way as boiling point elevation problems except Kf is used and the ∆T tells you how much the temperature goes down

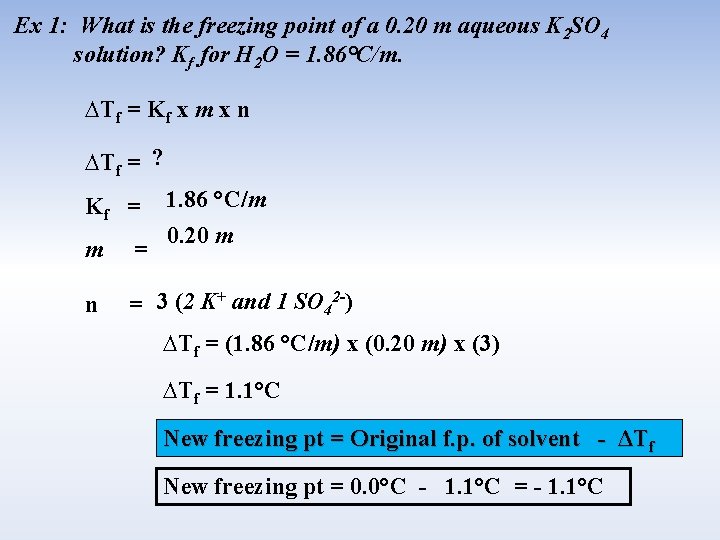
Ex 1: What is the freezing point of a 0. 20 m aqueous K 2 SO 4 solution? Kf for H 2 O = 1. 86°C/m. ∆Tf = Kf x m x n ∆Tf = ? Kf = 1. 86 °C/m 0. 20 m m = n = 3 (2 K+ and 1 SO 42 -) ∆Tf = (1. 86 °C/m) x (0. 20 m) x (3) ∆Tf = 1. 1°C New freezing pt = Original f. p. of solvent - ΔTf New freezing pt = 0. 0°C - 1. 1°C = - 1. 1°C

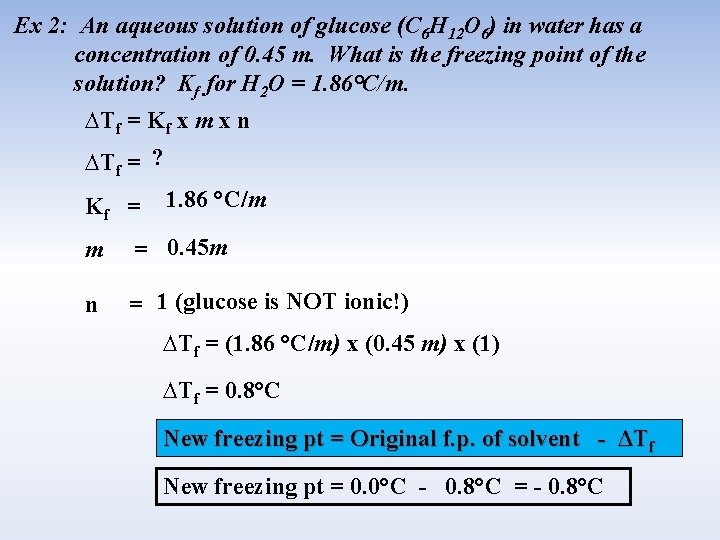
Ex 2: An aqueous solution of glucose (C 6 H 12 O 6) in water has a concentration of 0. 45 m. What is the freezing point of the solution? Kf for H 2 O = 1. 86°C/m. ∆Tf = Kf x m x n ∆Tf = ? Kf = 1. 86 °C/m m = 0. 45 m n = 1 (glucose is NOT ionic!) ∆Tf = (1. 86 °C/m) x (0. 45 m) x (1) ∆Tf = 0. 8°C New freezing pt = Original f. p. of solvent - ΔTf New freezing pt = 0. 0°C - 0. 8°C = - 0. 8°C

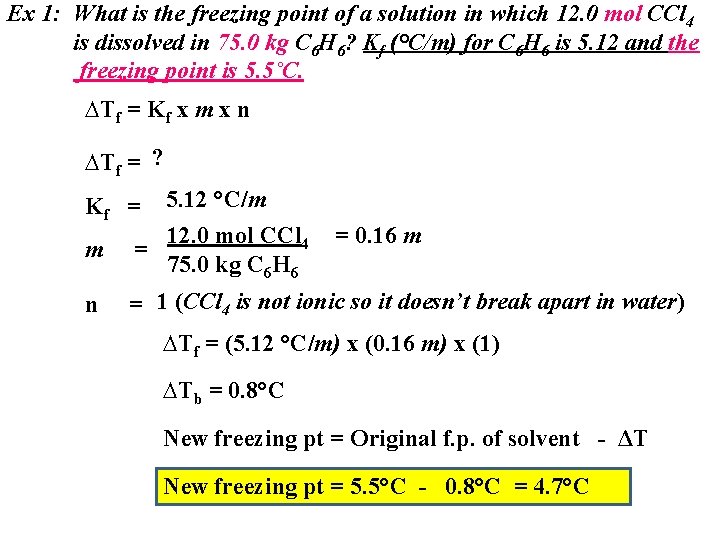
Ex 1: What is the freezing point of a solution in which 12. 0 mol CCl 4 is dissolved in 75. 0 kg C 6 H 6? Kf (°C/m) for C 6 H 6 is 5. 12 and the freezing point is 5. 5˚C. ∆Tf = Kf x m x n ∆Tf = ? Kf = 5. 12 °C/m = 0. 16 m m 12. 0 mol CCl 4 = 75. 0 kg C 6 H 6 n = 1 (CCl 4 is not ionic so it doesn’t break apart in water) ∆Tf = (5. 12 °C/m) x (0. 16 m) x (1) ∆Tb = 0. 8°C New freezing pt = Original f. p. of solvent - ΔT New freezing pt = 5. 5°C - 0. 8°C = 4. 7°C

Pg. 528: 55. a. What is the boiling point of a solution that contains: 0. 50 mol glucose (molecular) molecular in 1000 g of H 2 O? (Kb (°C/m) for H 2 O is 0. 512. ) ∆T = K x m x n b b ∆Tb = ? Kb = 0. 512 °C/m 0. 50 mol glucose = 0. 50 m m = 1. 000 g H 2 O n = 1 (b/c glucose is not ionic, its doesn’t break apart) ∆Tb = (0. 512 °C/m) x (0. 50 m) x (1) ∆Tb = 0. 3°C New boiling pt = Original b. p. of solvent + ΔT New boiling pt = 100°C + 0. 3°C = 100. 3°C

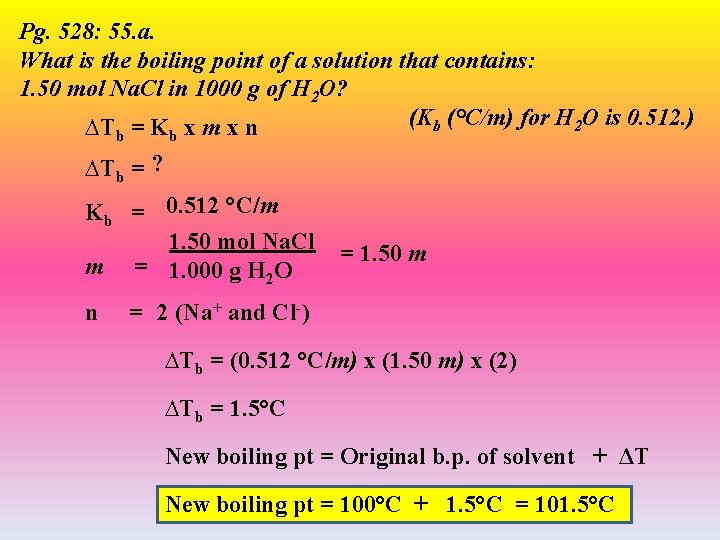
Pg. 528: 55. a. What is the boiling point of a solution that contains: 1. 50 mol Na. Cl in 1000 g of H 2 O? (Kb (°C/m) for H 2 O is 0. 512. ) ∆T = K x m x n b b ∆Tb = ? Kb = 0. 512 °C/m 1. 50 mol Na. Cl m = 1. 000 g H 2 O n = 1. 50 m = 2 (Na+ and Cl-) ∆Tb = (0. 512 °C/m) x (1. 50 m) x (2) ∆Tb = 1. 5°C New boiling pt = Original b. p. of solvent + ΔT New boiling pt = 100°C + 1. 5°C = 101. 5°C

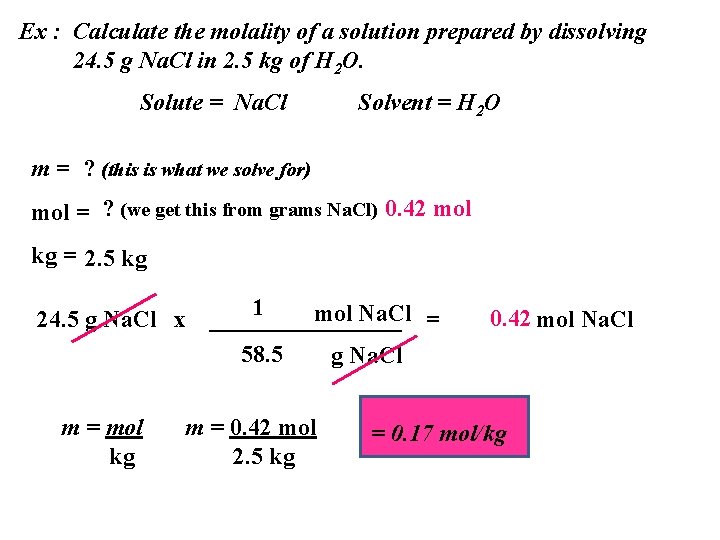
Ex : Calculate the molality of a solution prepared by dissolving 24. 5 g Na. Cl in 2. 5 kg of H 2 O. Solute = Na. Cl Solvent = H 2 O m = ? (this is what we solve for) mol = ? (we get this from grams Na. Cl) 0. 42 mol kg = 2. 5 kg 24. 5 g Na. Cl x m = mol kg 1 mol Na. Cl = ________ 58. 5 g Na. Cl m = 0. 42 mol 2. 5 kg 0. 42 mol Na. Cl = 0. 17 mol/kg

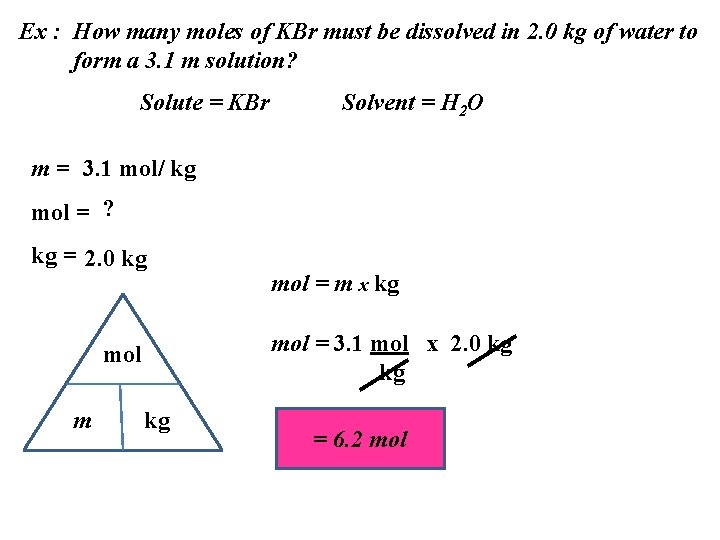
Ex : How many moles of KBr must be dissolved in 2. 0 kg of water to form a 3. 1 m solution? Solute = KBr Solvent = H 2 O m = 3. 1 mol/ kg mol = ? kg = 2. 0 kg mol = 3. 1 mol x 2. 0 kg kg mol m mol = m x kg kg = 6. 2 mol

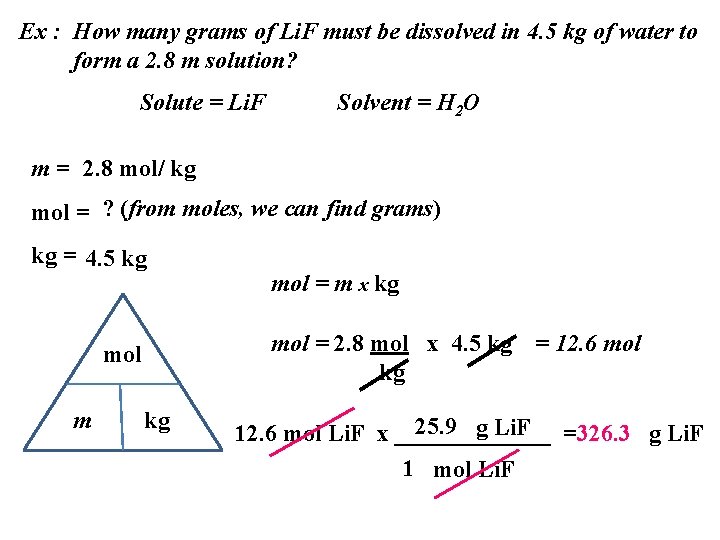
Ex : How many grams of Li. F must be dissolved in 4. 5 kg of water to form a 2. 8 m solution? Solute = Li. F Solvent = H 2 O m = 2. 8 mol/ kg mol = ? (from moles, we can find grams) kg = 4. 5 kg mol = 2. 8 mol x 4. 5 kg = 12. 6 mol kg mol m mol = m x kg kg 25. 9 g Li. F =326. 3 g Li. F 12. 6 mol Li. F x _______ 1 mol Li. F