Chapter 12 SOLUTIONS Solution homogeneous mixture of two




























- Slides: 31

Chapter 12 SOLUTIONS!

• Solution: homogeneous mixture of two or more substances • Solute: the part being dissolved • Solvent: the part doing the dissolving • Think: YOU dissolve the sol. Ute! • Ok…now… knowing that salt water is a solution, why shouldn’t you drink it? (THINK about the concentration of SOLUTE)

Types of solutions • Aqueous solutions: water = solvent • Why is water often called ‘the universal solvent’? • Solubility = the amount of substance that will dissolve in a given amount of solvent at a given TEMPERATURE!

Why mix? • Entropy: a measure of energy randomization or energy dispersal in a system – Basically, energy wants to be evenly distributed – Think about what will happen if you hold one end of a metal rod over an open flame. Why? – Because energy wants to be evenly distributed, particles (with kinetic energy) want to be evenly distributed. THIS IS MIXING!

What about IMF? • In the absence of IMF, entropy dictates behavior = things will spread as much as possible • BUT, we know that there are ALWAYS IMF, SO…… • We look at the IMF between: • Solute and solvent • Solvent and itself • Solute and itself

• What will happen if the solvent-solvent interaction or the solute-solute interaction is much greater than the solute-solvent interaction? • WHY? • This is WHY ‘like dissolves like’

Energetics of solution formation • The process of forming a solution can be endothermic or exothermic. WHY? • Solvation occurs in three parts, you must consider the energy flow in each of the three parts to get the net energy flow

• 1. separating solute into its particles= always endothermic. WHY? • 2. Separating solvent particles from each other= always endothermic. WHY? • 3. Mixing solute with the solvent = exothermic. WHY? • You can calculate the change in enthalpy!

Hess’s Law • Enthalpy = measure of energy of a system • Change in enthalpy of solution = change of enthalpy of solute + change of enthalpy of solvent + change of enthalpy of mix

• • Describe the reaction in terms of energy flow if: Change in enthalpy of solution = about 0 Change in enthalpy of solution is negative Change in enthalpy of solution is positive • Why would a solution form at all if the change in enthalpy was negative? • ENTROPY!

Homework • Pg 561, 1 -9 • Pg 562, 29 -36 (for 35 and 36, only a + b)

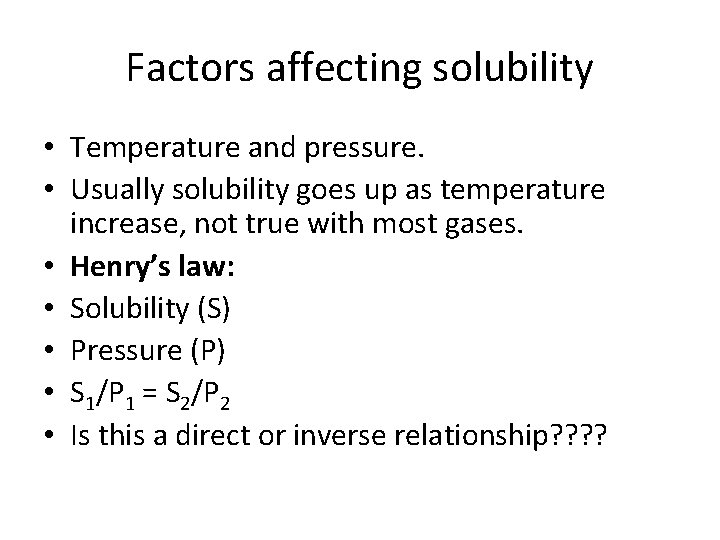
Factors affecting solubility • Temperature and pressure. • Usually solubility goes up as temperature increase, not true with most gases. • Henry’s law: • Solubility (S) • Pressure (P) • S 1/P 1 = S 2/P 2 • Is this a direct or inverse relationship? ?

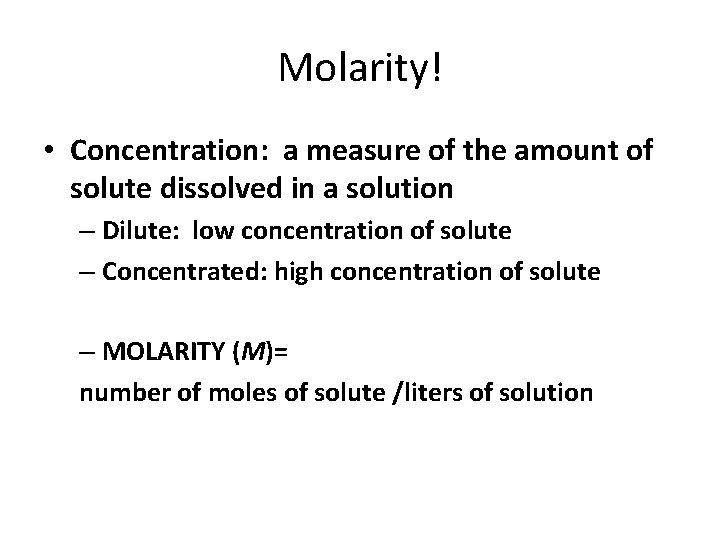
Molarity! • Concentration: a measure of the amount of solute dissolved in a solution – Dilute: low concentration of solute – Concentrated: high concentration of solute – MOLARITY (M)= number of moles of solute /liters of solution

• A saline solution contains 0. 90 g Na. Cl in exactly 100 ml of solution. What is the molarity of the solution? • What do you know? • 0. 90 g Na. Cl • 100 ml solution • Molarity = moles of solute / liters solution • YOU MUST CONVERT!

• How many moles of solute are present in 1. 5 L of a 0. 24 M Na 2 SO 4? • What do you know? • 1. 5 L of solvent • Molarity = 0. 24 M • Molarity = moles of solute/Liters of solvent

You can make DILUTIONS! • Sometimes you need to dilute a solution to use it. (ex. ACID in the lab) • M 1 x V 1 = M 2 x V 2 • volume must be in liters! (because molarity is moles/liter)

• How many milliliters of stock solution of 2. 00 M Mg. SO 4 would you need to prepare 100. 00 ml of 0. 400 M Mg. SO 4 M 1 x V 1 = M 2 x V 2 M 1 = 2. 00 M V 1 = ? M 2= 0. 400 M V 2 = 100. 00 m. L (CONVERT)

Percent Solutions! • If both the solvent and solute are liquids, it’s easier to make a solution by using volume. You would express concentration as a percent volume • Percent volume (%v/v) = volume of solute/volume of solvent x 100% • Percent (mass/volume)(%(m/v)= mass solute (g)/ solution volume (m. L) x 100

Let’s try it • What is the percent by volume of ethanol in the final solution when 85 m. L of ethanol is diluted to a volume of 250 m. L with water? • How many grams of glucose would you need to prepare 2, 000 ml of 2. 8% glucose (m/v) solution

Molality • Molarity depends on volume, but is volume constant? ? ? • What happens to volume with temperature changes? • We need something that is temperature independent!

• Molality (m): amount of solute(mol)/ mass of solvent (kg) NOTE: molality is defined in terms of kg of SOLVENT, not SOLUTION!!!! Molality is very useful when one must compare concentrations over a range of temperatures

• If molality is so great, why do we learn about molarity at all? ? ?

Parts by mass and parts by volume • We touched on something like this with percentages, but you will often see concentration as a ratio of masses • Percent = per hundred, so mass of solute/mass of solution x 100 = part per hundred Mass of solute/mass of solution x 106= parts per million (ppm) Mass of solute/mass of solution x 109 = parts per billion (ppb)

• You can also represent concentrations in ratios of volumes (just do it the same way, but use volume instead of mass) • Representing concentrations in this way is particularly useful in biology applications

Let’s try it • What volume (in m. L) of a soft drink that is 10. 5% sucrose (C 12 H 22 O 11) by mass contains 78. 5 g of sucrose? (the density of the solution is 1. 04 g/m. L) • Start with what you know: 78. 5 g sucrose • You want to know m. L • CONVERT!

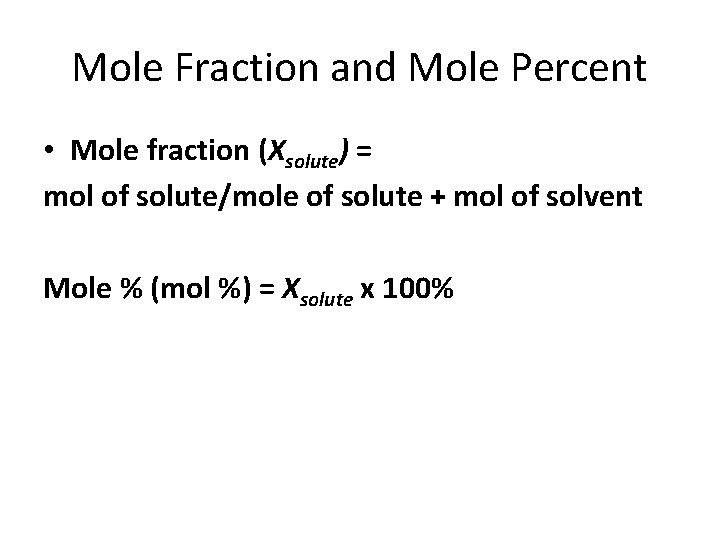
Mole Fraction and Mole Percent • Mole fraction (Xsolute) = mol of solute/mole of solute + mol of solvent Mole % (mol %) = Xsolute x 100%

Let’s TRY it! • A solution is prepared by dissolving 17. 2 g of ethylene glycol (C 2 H 6 O 2) in 0. 500 kg of water. The final volume of the solution is 515 m. L. Find the following A) molarity= amount of solute(mol)/L of solution B) molality= mol of solute/ kg of solvent C) percent by mass= mass solute/mass solution x 100 D) mole fraction = mole solute/ mol solute + mol solvent E) mole percent= mole fraction x 100

Homework • Pg 537 practice + more practice 12. 3 • Pg 540 practice 12. 4 • Pg 563 # 51 -54

Boiling Point Elevation and Freezing Point Depression • Boiling Point Elevation: Is the boiling point of the solution minus the boiling point of the pure solvent • Boiling point elevation is proportionate to molal concentration, so we add a proportionality constant (Kb) to make an equality • Change in boiling point = Kb x m

• Same thing for freezing point depression, we just use a molal freezing-point depression constant (Kf) • Change in freezing point = Kf x m

Let’s try it! • A solution of 7. 50 g of a nonvolatile compound in 22. 60 g of water boils at 100. 78 degrees Celcius at 760 mm. Hg. Wht is the molar mass of the solute? Boiling point constant for water = 0. 512 degrees/molal