General Chemistry An Integrated Approach Hill Petrucci 4


- Slides: 42

General Chemistry: An Integrated Approach Hill, Petrucci, 4 th Edition Chapter 17 Thermodynamics: Spontaneity, Entropy, and Free Energy Mark P. Heitz State University of New York at Brockport © 2005, Prentice Hall, Inc. Chapter 17: Thermodynamics

Introduction • Thermodynamics examines the relationship between heat and work. • Spontaneity is the notion of whether or not a process can take place unassisted. • Entropy is a mathematical concept describing the distribution of energy within a system. • Free energy is a thermodynamic function that relates enthalpy and entropy to spontaneity, and can also be related to equilibrium constants. Chapter 17: Thermodynamics 2

3 Why Study Thermodynamics? • With a knowledge of thermodynamics and by making a few calculations before embarking on a new venture, scientists and engineers can save themselves a great deal of time, money, and frustration. – “To the manufacturing chemist thermodynamics gives information concerning the stability of his substances, the yield which he may hope to attain, the methods of avoiding undesirable substances, the optimum range of temperature and pressure, the proper choice of solvent. …” - from the introduction to Thermodynamics and the Free Energy of Chemical Substances by G. N. Lewis and M. Randall • Thermodynamics tells us what processes are possible. – (Kinetics tells us whether the process is practical. ) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

4 Spontaneous Change • A spontaneous process is one that can occur in a system left to itself; no action from outside the system is necessary to bring it about. • A nonspontaneous process is one that cannot take place in a system left to itself. • If a process is spontaneous, the reverse process is nonspontaneous, and vice versa. • Example: gasoline combines spontaneously with oxygen. • However, “spontaneous” signifies nothing about how fast a process occurs. • A mixture of gasoline and oxygen may remain unreacted for years, or may ignite instantly with a spark. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

5 Spontaneous Change (cont’d) • Thermodynamics determines the equilibrium state of a system. • Thermodynamics is used to predict the proportions of products and reactants at equilibrium. • Kinetics determines the pathway by which equilibrium is reached. • A high activation energy can effectively block a reaction that is thermodynamically favored. • Example: combustion reactions are thermodynamically favored, but (fortunately for life on Earth!) most such reactions also have a high activation energy. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

6 Spontaneous Change (cont’d) • Early chemists proposed that spontaneous chemical reactions should occur in the direction of decreasing energy. • It is true that many exothermic processes are spontaneous and that many endothermic reactions are nonspontaneous. • However, enthalpy change is not a sufficient criterion for predicting spontaneous change … Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

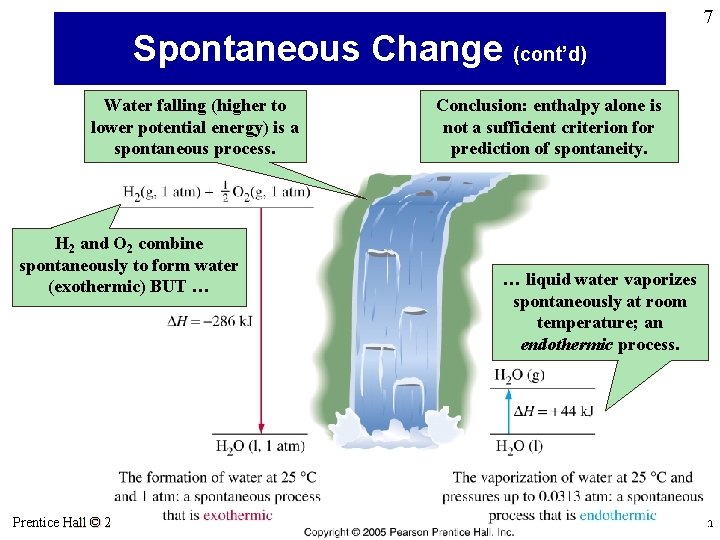
7 Spontaneous Change (cont’d) Water falling (higher to lower potential energy) is a spontaneous process. Conclusion: enthalpy alone is not a sufficient criterion for prediction of spontaneity. H 2 and O 2 combine spontaneously to form water (exothermic) BUT … Prentice Hall © 2005 … liquid water vaporizes spontaneously at room temperature; an endothermic process. General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

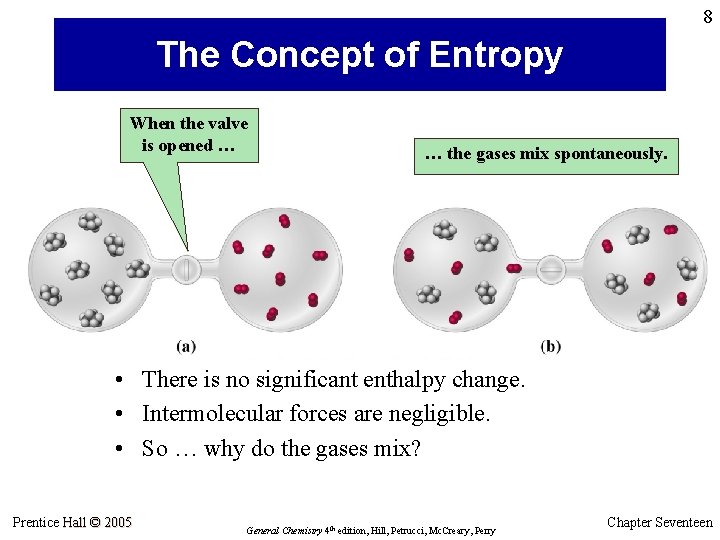
8 The Concept of Entropy When the valve is opened … … the gases mix spontaneously. • There is no significant enthalpy change. • Intermolecular forces are negligible. • So … why do the gases mix? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

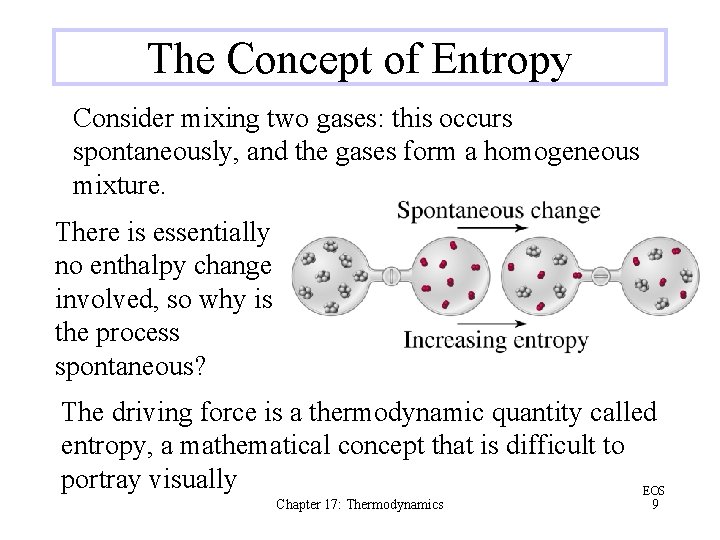
The Concept of Entropy Consider mixing two gases: this occurs spontaneously, and the gases form a homogeneous mixture. There is essentially no enthalpy change involved, so why is the process spontaneous? The driving force is a thermodynamic quantity called entropy, a mathematical concept that is difficult to portray visually EOS Chapter 17: Thermodynamics 9

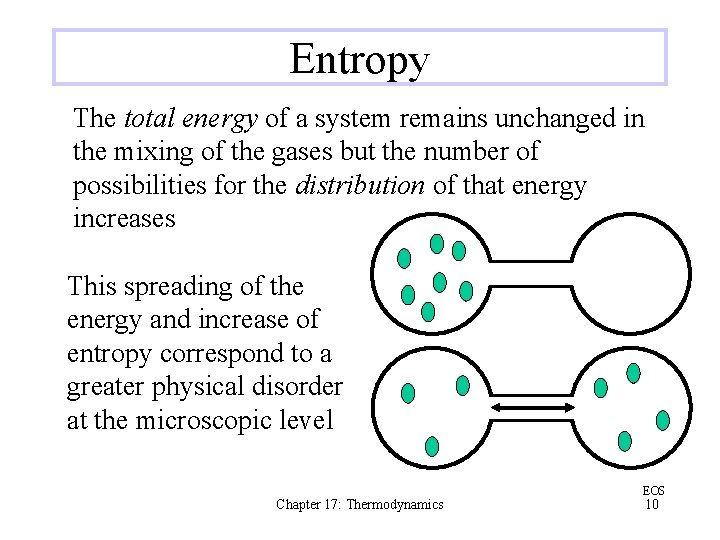
Entropy The total energy of a system remains unchanged in the mixing of the gases but the number of possibilities for the distribution of that energy increases This spreading of the energy and increase of entropy correspond to a greater physical disorder at the microscopic level Chapter 17: Thermodynamics EOS 10

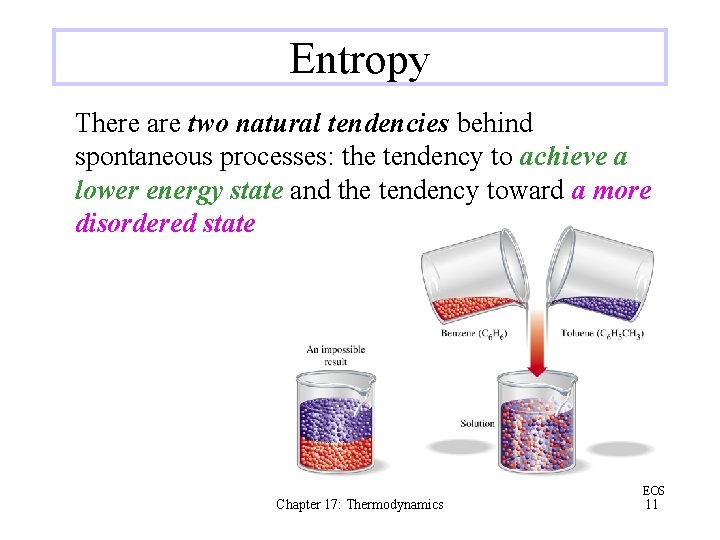
Entropy There are two natural tendencies behind spontaneous processes: the tendency to achieve a lower energy state and the tendency toward a more disordered state Chapter 17: Thermodynamics EOS 11

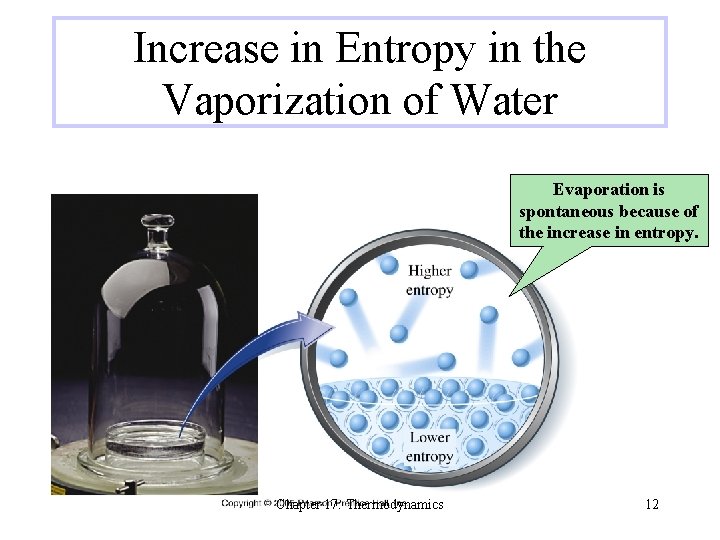
Increase in Entropy in the Vaporization of Water Evaporation is spontaneous because of the increase in entropy. Chapter 17: Thermodynamics 12

13 The Concept of Entropy • The spreading of the energy among states, and increase of entropy, often correspond to a greater physical disorder at the microscopic level (however, entropy is not “disorder”). • There are two driving forces behind spontaneous processes: the tendency to achieve a lower energy state (enthalpy change) and the tendency for energy to be distributed among states (entropy). • In many cases, however, the two factors work in opposition. One may increase and the other decrease or vice versa. In these cases, we must determine which factor predominates. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

14 Assessing Entropy Change • The difference in entropy (S) between two states is the entropy change (DS). • The greater the number of configurations of the microscopic particles (atoms, ions, molecules) among the energy levels in a particular state of a system, the greater is the entropy of the system. • Entropy generally increases when: – Solids melt to form liquids. – Solids or liquids vaporize to form gases. – Solids or liquids dissolve in a solvent to form nonelectrolyte solutions. – A chemical reaction produces an increase in the number of molecules of gases. – A substance is heated. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

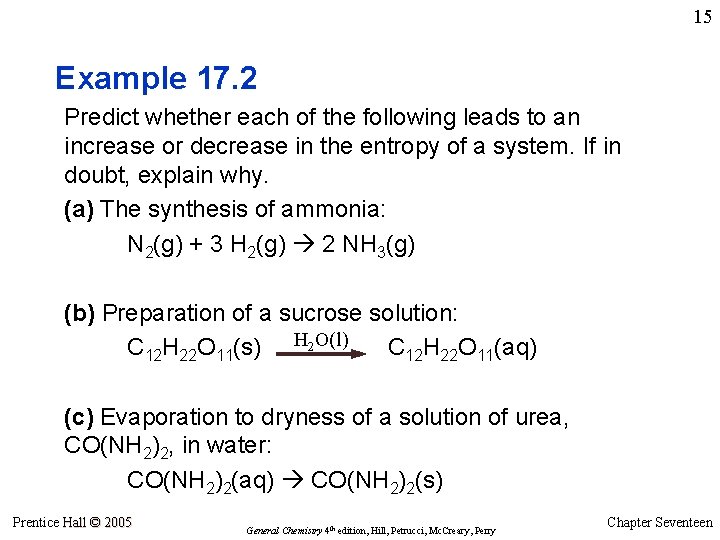
15 Example 17. 2 Predict whether each of the following leads to an increase or decrease in the entropy of a system. If in doubt, explain why. (a) The synthesis of ammonia: N 2(g) + 3 H 2(g) 2 NH 3(g) (b) Preparation of a sucrose solution: C 12 H 22 O 11(s) H 2 O(l) C 12 H 22 O 11(aq) (c) Evaporation to dryness of a solution of urea, CO(NH 2)2, in water: CO(NH 2)2(aq) CO(NH 2)2(s) Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

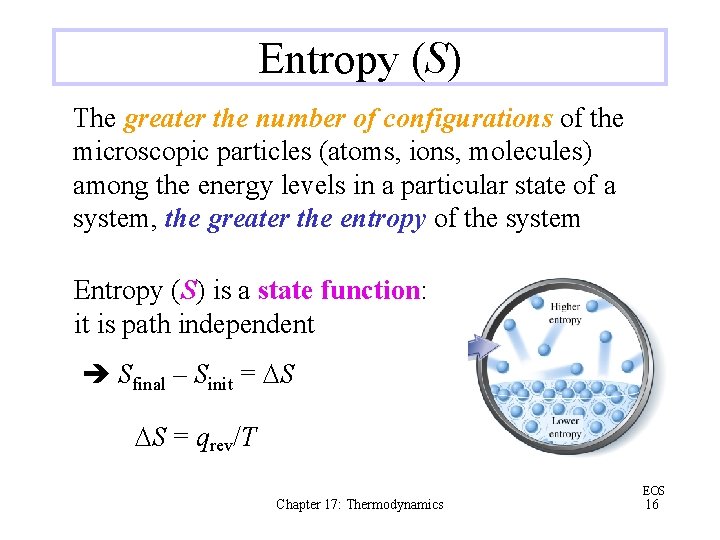
Entropy (S) The greater the number of configurations of the microscopic particles (atoms, ions, molecules) among the energy levels in a particular state of a system, the greater the entropy of the system Entropy (S) is a state function: it is path independent Sfinal – Sinit = DS DS = qrev/T Chapter 17: Thermodynamics EOS 16

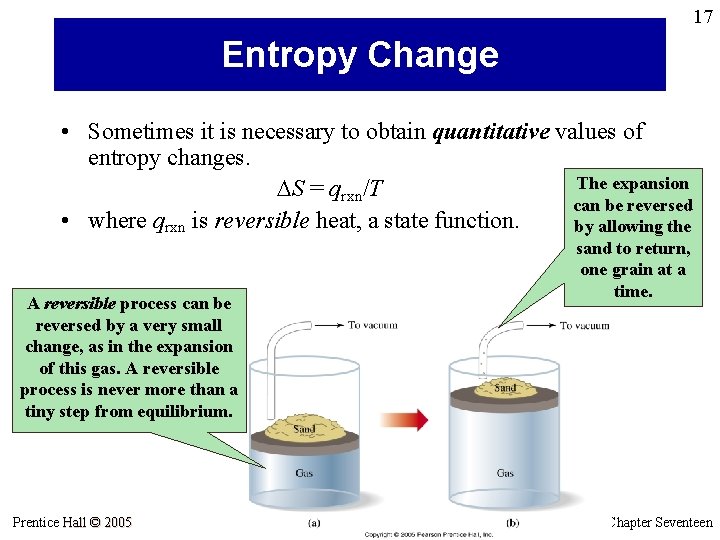
17 Entropy Change • Sometimes it is necessary to obtain quantitative values of entropy changes. The expansion DS = qrxn/T can be reversed • where qrxn is reversible heat, a state function. by allowing the sand to return, one grain at a time. A reversible process can be reversed by a very small change, as in the expansion of this gas. A reversible process is never more than a tiny step from equilibrium. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

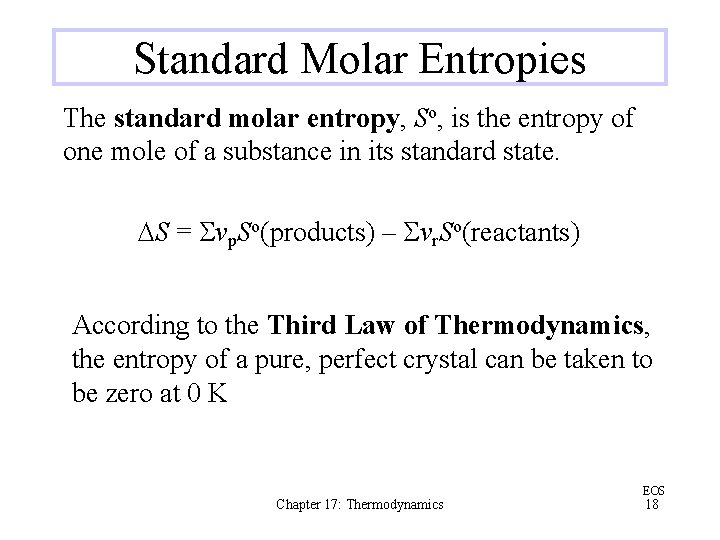
Standard Molar Entropies The standard molar entropy, So, is the entropy of one mole of a substance in its standard state. DS = Svp. So(products) – Svr. So(reactants) According to the Third Law of Thermodynamics, the entropy of a pure, perfect crystal can be taken to be zero at 0 K Chapter 17: Thermodynamics EOS 18

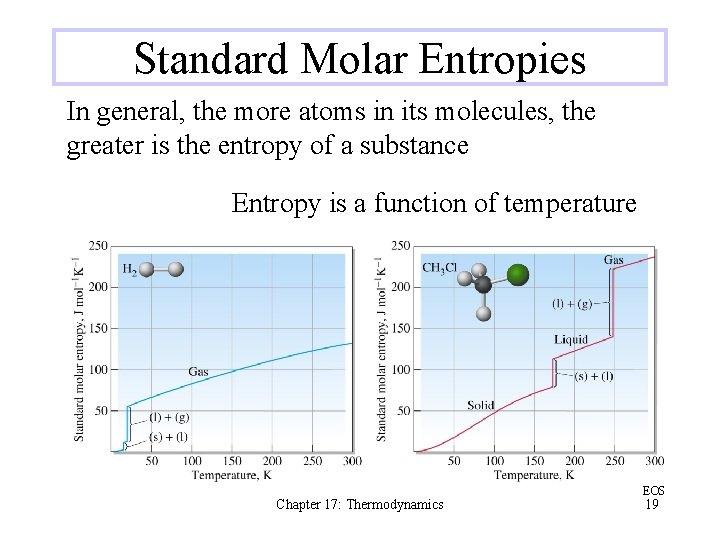
Standard Molar Entropies In general, the more atoms in its molecules, the greater is the entropy of a substance Entropy is a function of temperature Chapter 17: Thermodynamics EOS 19

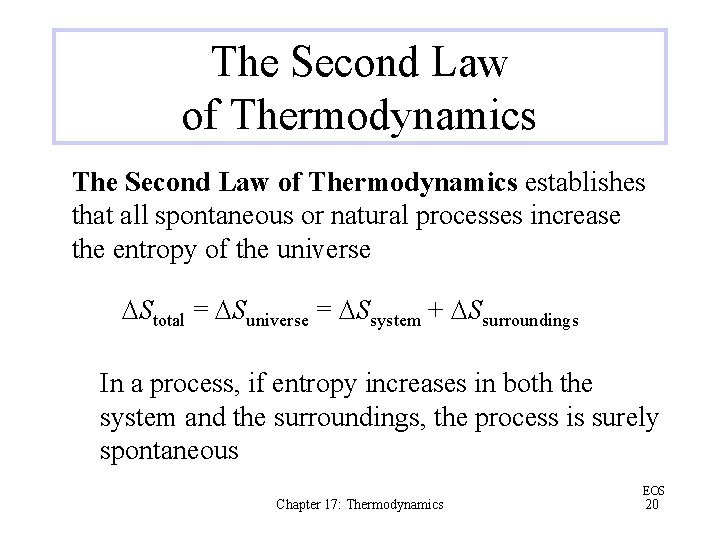
The Second Law of Thermodynamics establishes that all spontaneous or natural processes increase the entropy of the universe DStotal = DSuniverse = DSsystem + DSsurroundings In a process, if entropy increases in both the system and the surroundings, the process is surely spontaneous Chapter 17: Thermodynamics EOS 20

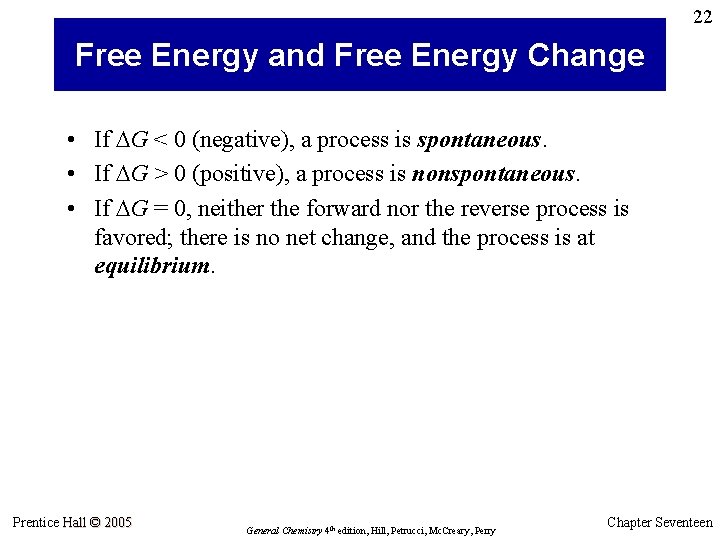
21 Free Energy and Free Energy Change • What is the significance of: –TΔSuniv = ΔHsys – TΔSsys ? • The entropy change of the universe—our criterion for spontaneity—has now been defined entirely in terms of the system. • The quantity –TΔSuniv is called the free energy change (DG). • For a process at constant temperature and pressure: DGsys = DHsys – TDSsys Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

22 Free Energy and Free Energy Change • If DG < 0 (negative), a process is spontaneous. • If DG > 0 (positive), a process is nonspontaneous. • If DG = 0, neither the forward nor the reverse process is favored; there is no net change, and the process is at equilibrium. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

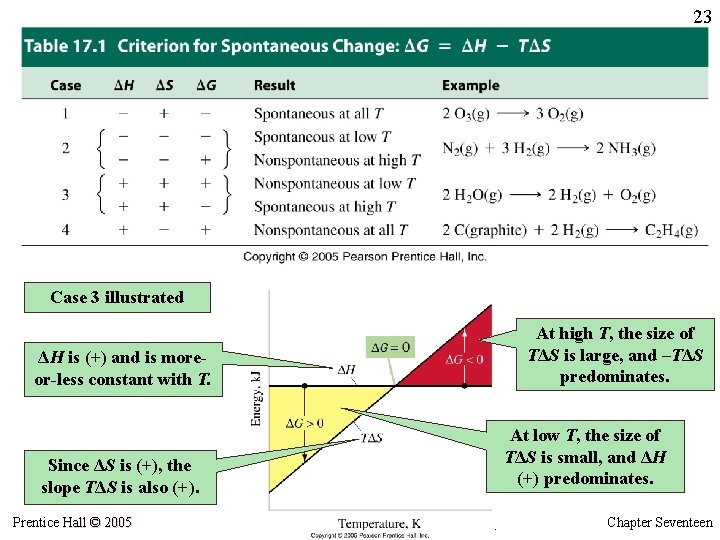
23 Case 3 illustrated At high T, the size of TΔS is large, and –TΔS predominates. ΔH is (+) and is moreor-less constant with T. At low T, the size of TΔS is small, and ΔH (+) predominates. Since ΔS is (+), the slope TΔS is also (+). Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

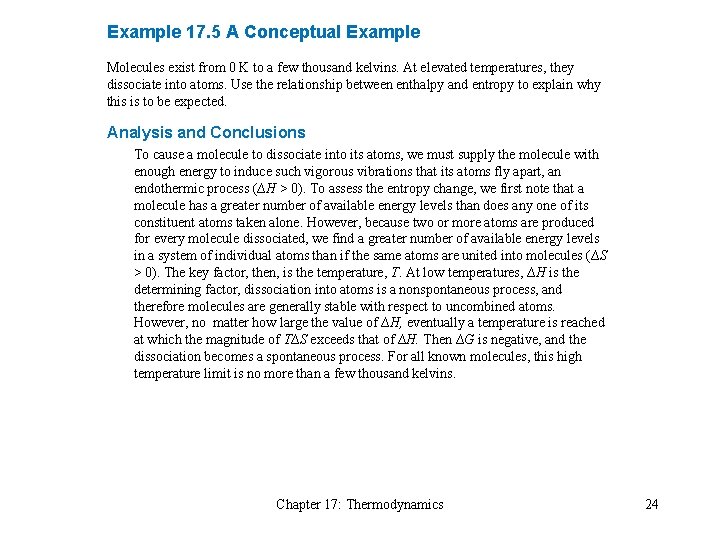
Example 17. 5 A Conceptual Example Molecules exist from 0 K to a few thousand kelvins. At elevated temperatures, they dissociate into atoms. Use the relationship between enthalpy and entropy to explain why this is to be expected. Analysis and Conclusions To cause a molecule to dissociate into its atoms, we must supply the molecule with enough energy to induce such vigorous vibrations that its atoms fly apart, an endothermic process (∆H > 0). To assess the entropy change, we first note that a molecule has a greater number of available energy levels than does any one of its constituent atoms taken alone. However, because two or more atoms are produced for every molecule dissociated, we find a greater number of available energy levels in a system of individual atoms than if the same atoms are united into molecules (∆S > 0). The key factor, then, is the temperature, T. At low temperatures, ∆H is the determining factor, dissociation into atoms is a nonspontaneous process, and therefore molecules are generally stable with respect to uncombined atoms. However, no matter how large the value of ∆H, eventually a temperature is reached at which the magnitude of T∆S exceeds that of ∆H. Then ∆G is negative, and the dissociation becomes a spontaneous process. For all known molecules, this high temperature limit is no more than a few thousand kelvins. Chapter 17: Thermodynamics 24

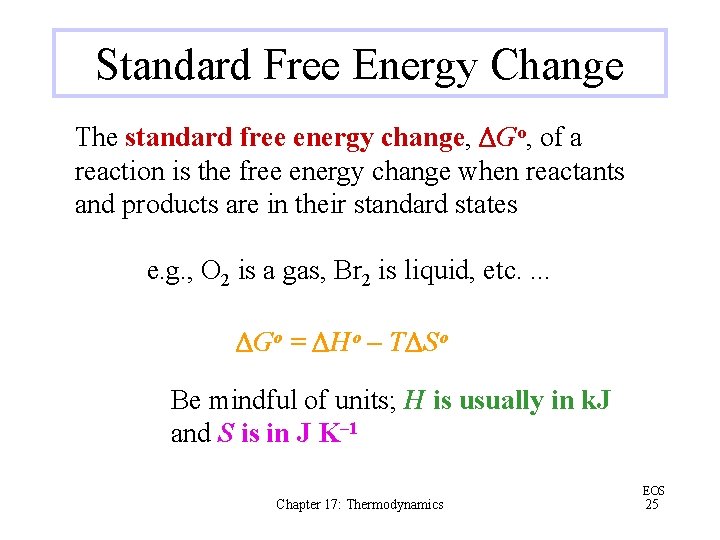
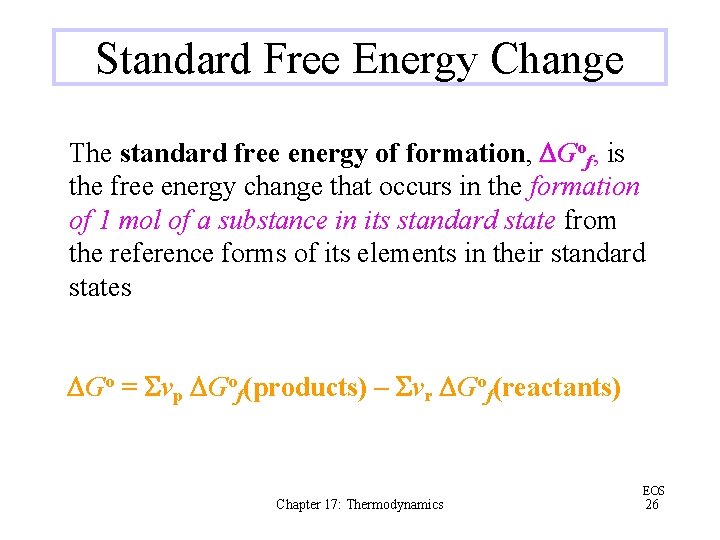
Standard Free Energy Change The standard free energy change, DGo, of a reaction is the free energy change when reactants and products are in their standard states e. g. , O 2 is a gas, Br 2 is liquid, etc. . DGo = DHo – TDSo Be mindful of units; H is usually in k. J and S is in J K– 1 Chapter 17: Thermodynamics EOS 25

Standard Free Energy Change The standard free energy of formation, DGof, is the free energy change that occurs in the formation of 1 mol of a substance in its standard state from the reference forms of its elements in their standard states DGo = Svp DGof(products) – Svr DGof(reactants) Chapter 17: Thermodynamics EOS 26

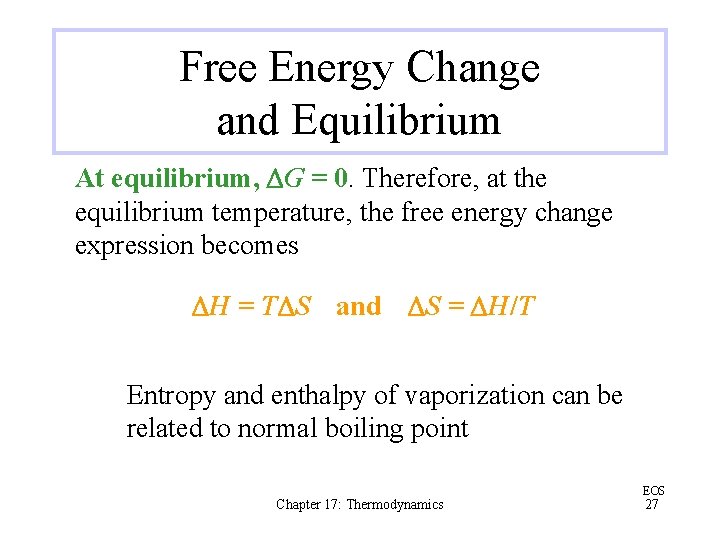
Free Energy Change and Equilibrium At equilibrium, DG = 0. Therefore, at the equilibrium temperature, the free energy change expression becomes DH = TDS and DS = DH/T Entropy and enthalpy of vaporization can be related to normal boiling point Chapter 17: Thermodynamics EOS 27

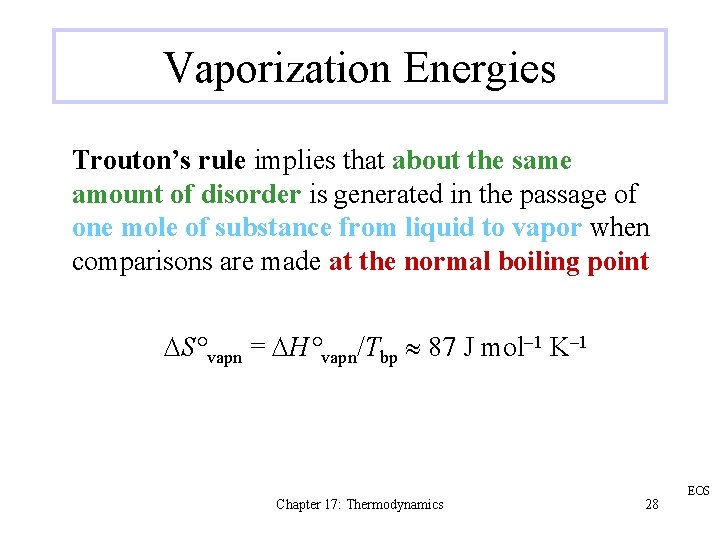
Vaporization Energies Trouton’s rule implies that about the same amount of disorder is generated in the passage of one mole of substance from liquid to vapor when comparisons are made at the normal boiling point DS°vapn = DH°vapn/Tbp 87 J mol– 1 K– 1 Chapter 17: Thermodynamics 28 EOS

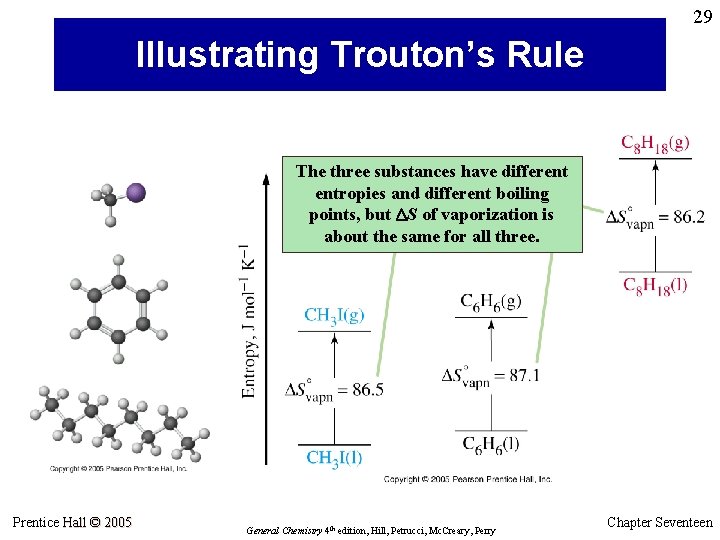
29 Illustrating Trouton’s Rule The three substances have different entropies and different boiling points, but DS of vaporization is about the same for all three. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

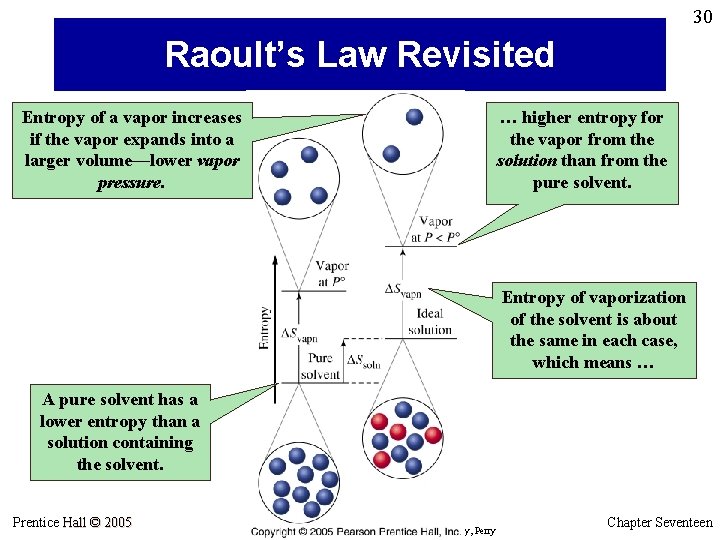
30 Raoult’s Law Revisited Entropy of a vapor increases if the vapor expands into a larger volume—lower vapor pressure. … higher entropy for the vapor from the solution than from the pure solvent. Entropy of vaporization of the solvent is about the same in each case, which means … A pure solvent has a lower entropy than a solution containing the solvent. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Seventeen

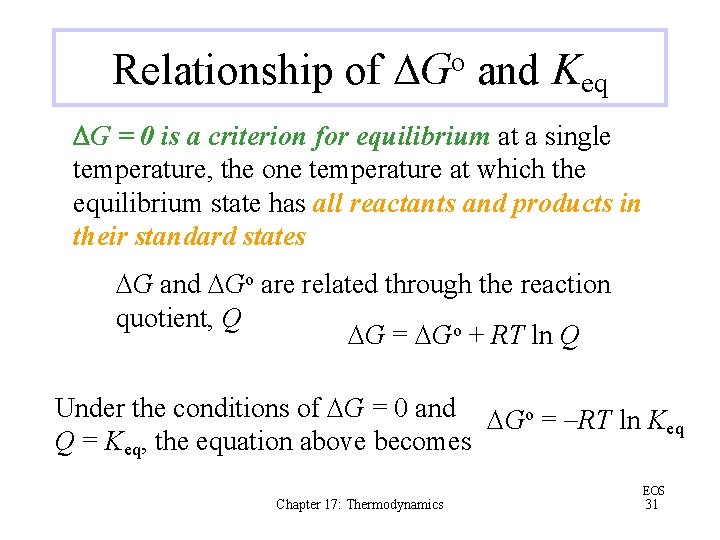
Relationship of DGo and Keq DG = 0 is a criterion for equilibrium at a single temperature, the one temperature at which the equilibrium state has all reactants and products in their standard states DG and DGo are related through the reaction quotient, Q DG = DGo + RT ln Q Under the conditions of DG = 0 and DGo = -RT ln K eq Q = Keq, the equation above becomes Chapter 17: Thermodynamics EOS 31

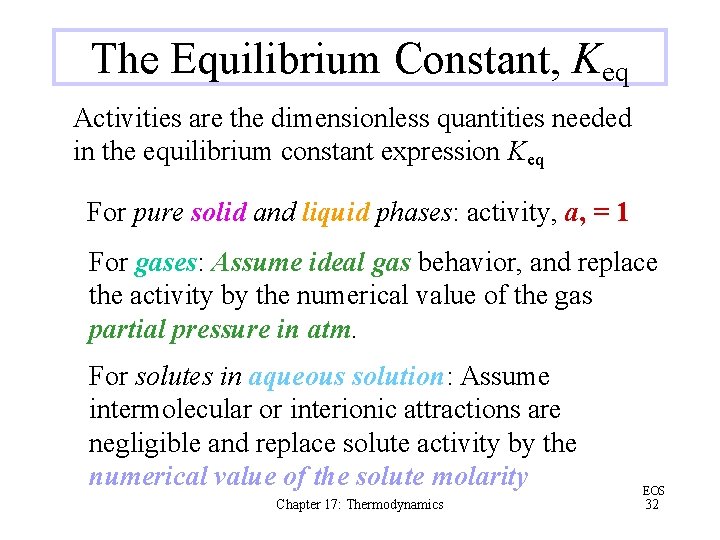
The Equilibrium Constant, Keq Activities are the dimensionless quantities needed in the equilibrium constant expression Keq For pure solid and liquid phases: activity, a, = 1 For gases: Assume ideal gas behavior, and replace the activity by the numerical value of the gas partial pressure in atm. For solutes in aqueous solution: Assume intermolecular or interionic attractions are negligible and replace solute activity by the numerical value of the solute molarity Chapter 17: Thermodynamics EOS 32

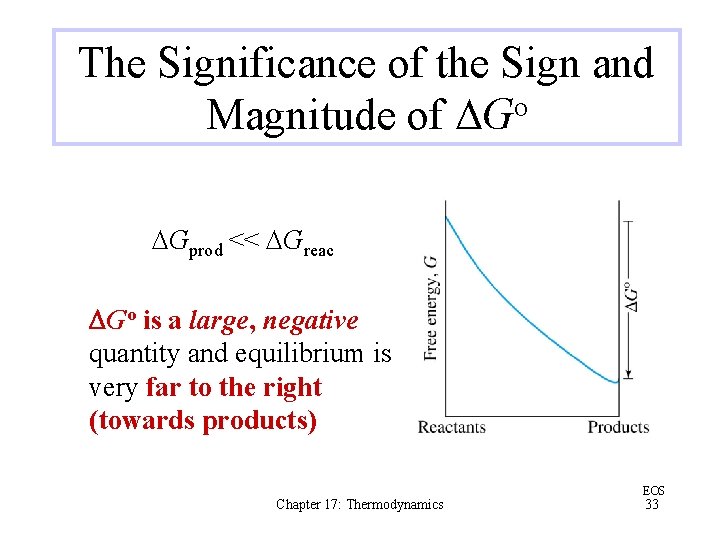
The Significance of the Sign and Magnitude of DGo DGprod << DGreac DGo is a large, negative quantity and equilibrium is very far to the right (towards products) Chapter 17: Thermodynamics EOS 33

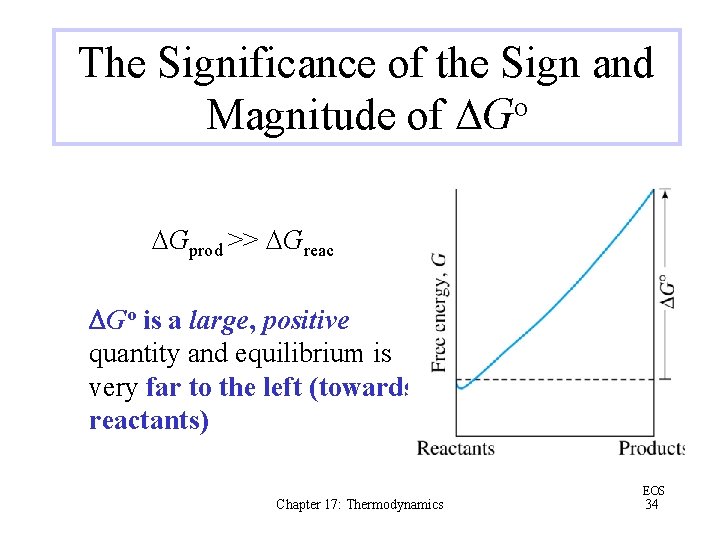
The Significance of the Sign and Magnitude of DGo DGprod >> DGreac DGo is a large, positive quantity and equilibrium is very far to the left (towards reactants) Chapter 17: Thermodynamics EOS 34

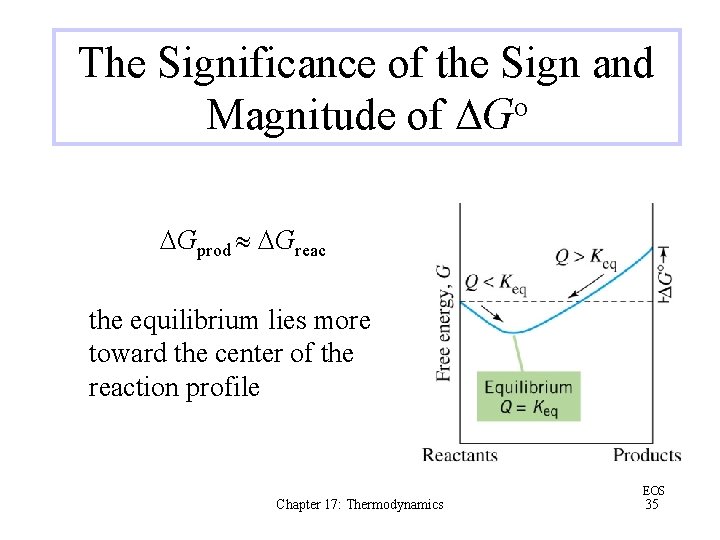
The Significance of the Sign and Magnitude of DGo DGprod DGreac the equilibrium lies more toward the center of the reaction profile Chapter 17: Thermodynamics EOS 35

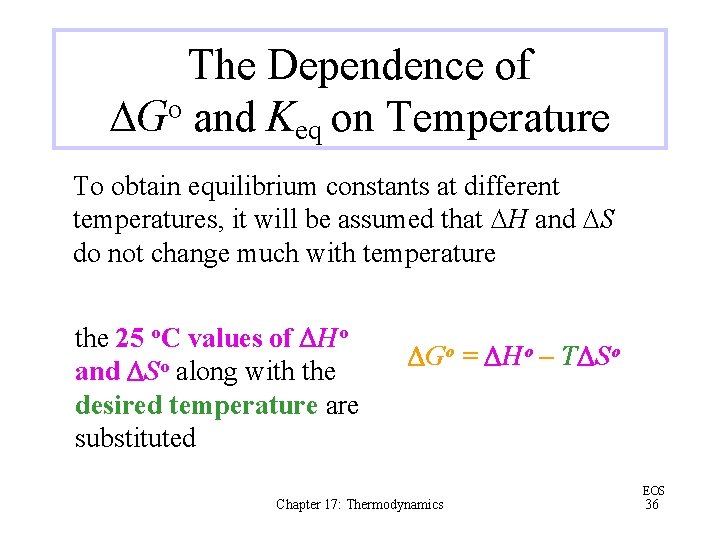
The Dependence of DGo and Keq on Temperature To obtain equilibrium constants at different temperatures, it will be assumed that DH and DS do not change much with temperature the 25 o. C values of DHo and DSo along with the desired temperature are substituted DGo = DHo – TDSo Chapter 17: Thermodynamics EOS 36

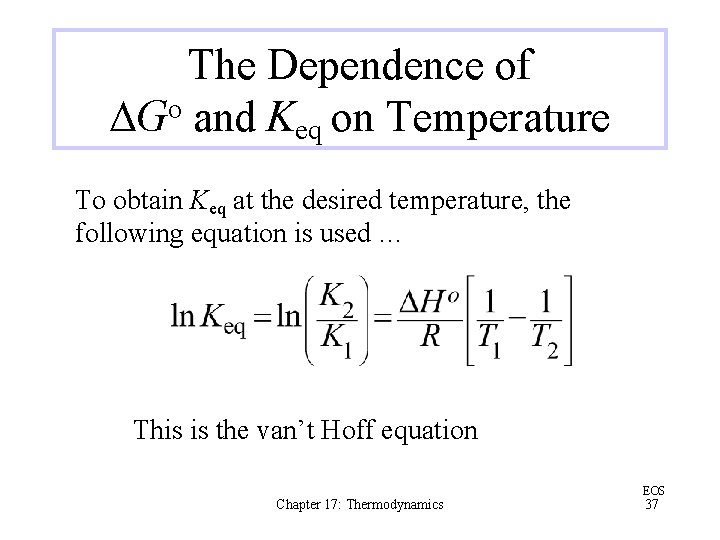
The Dependence of DGo and Keq on Temperature To obtain Keq at the desired temperature, the following equation is used … This is the van’t Hoff equation Chapter 17: Thermodynamics EOS 37

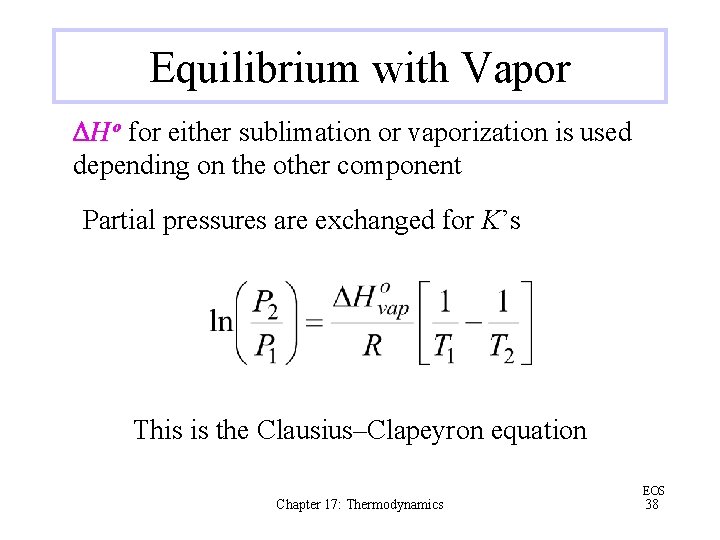
Equilibrium with Vapor DHo for either sublimation or vaporization is used depending on the other component Partial pressures are exchanged for K’s This is the Clausius–Clapeyron equation Chapter 17: Thermodynamics EOS 38

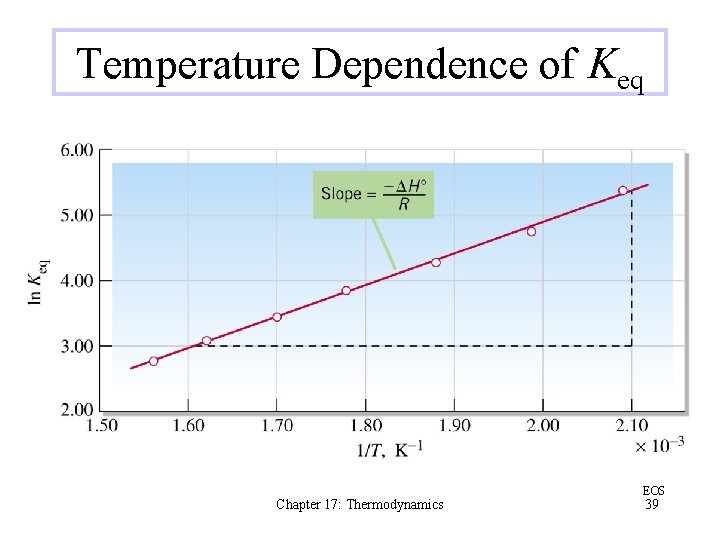
Temperature Dependence of Keq Chapter 17: Thermodynamics EOS 39

Summary of Concepts • A spontaneous change is one that occurs by itself without outside intervention • The third law of thermodynamics states that the entropy of a pure, perfect crystal at 0 K can be taken to be zero • The direction of spontaneous change is that in which total entropy increases • The free energy change, DG, is equal to –TDS, and it applies just to the system itself Chapter 17: Thermodynamics EOS 40

Summary (cont’d) • The standard free energy change, DGo, can be calculated by substituting standard enthalpies and entropies of reaction and a Kelvin temperature into the Gibbs equation, or, by combining standard free energies of formation • The condition of equilibrium is one for which DG =0 Chapter 17: Thermodynamics EOS 41

Summary (cont’d) • The value of DGo is in itself often sufficient to determine how a reaction will proceed • Values of DGof, DHof, and DS are generally tabulated for 25 o. C. To obtain values of Keq at other temperatures, the van’t Hoff equation must be used • The Clausius–Clapeyron equation connects solid/vapor or liquid/vapor equilibria to varying temperature Chapter 17: Thermodynamics EOS 42
 General chemistry ders notları
General chemistry ders notları Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Kısmi basınç
Kısmi basınç Petrucci
Petrucci Petrucci
Petrucci Mole hill chemistry
Mole hill chemistry An integrated approach to business studies
An integrated approach to business studies Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Is chalk natural or manmade
Is chalk natural or manmade Democritus atomic model diagram
Democritus atomic model diagram Datagram switching vs virtual circuit switching
Datagram switching vs virtual circuit switching Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Waterfall and sprinkler strategy
Waterfall and sprinkler strategy Multiple approach avoidance conflict
Multiple approach avoidance conflict Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Research approach means
Research approach means Approach to system development
Approach to system development Tony wagner's seven survival skills
Tony wagner's seven survival skills General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Discursive prompt examples
Discursive prompt examples Aice general paper hand approach
Aice general paper hand approach What is the general approach for back face detection
What is the general approach for back face detection Aice general paper exam
Aice general paper exam Aice general paper exam
Aice general paper exam Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Whrhs.org
Whrhs.org Baring of blind spot in glaucoma
Baring of blind spot in glaucoma Coefficiente di hill
Coefficiente di hill Lydia darragh
Lydia darragh Robert frost marriage
Robert frost marriage