General Chemistry An Integrated Approach Hill Petrucci 4

- Slides: 21

General Chemistry: An Integrated Approach Hill, Petrucci, 4 th Edition Chapter 1 Chemistry: Matter and Measurement Mark P. Heitz State University of New York at Brockport © 2005, Prentice Hall, Inc.

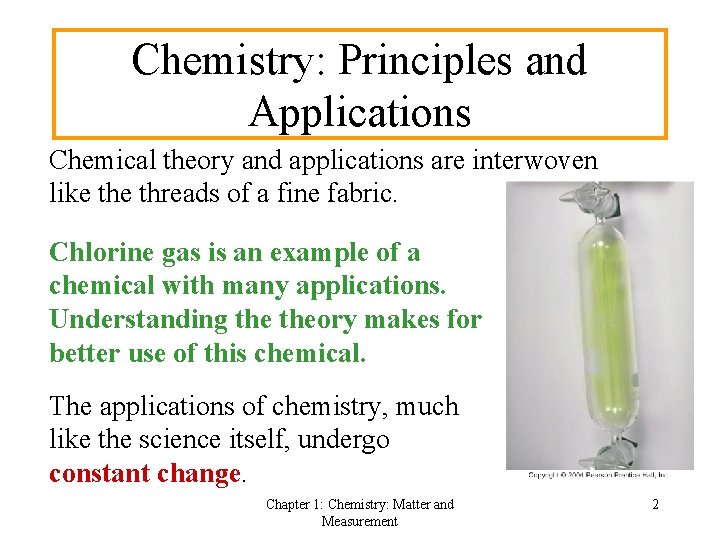
Chemistry: Principles and Applications Chemical theory and applications are interwoven like threads of a fine fabric. Chlorine gas is an example of a chemical with many applications. Understanding theory makes for better use of this chemical. The applications of chemistry, much like the science itself, undergo constant change. Chapter 1: Chemistry: Matter and Measurement 2

Getting Started: Some Key Ideas CHEMISTRY The study of … Composition – what’s in it? E. g. , water is 2 parts Hydrogen and 1 part Oxygen Structure – how is it assembled? E. g. , crystals Chapter 1: Chemistry: Matter and Measurement Properties: E. g. , boiling point, density, flammability EOS 3

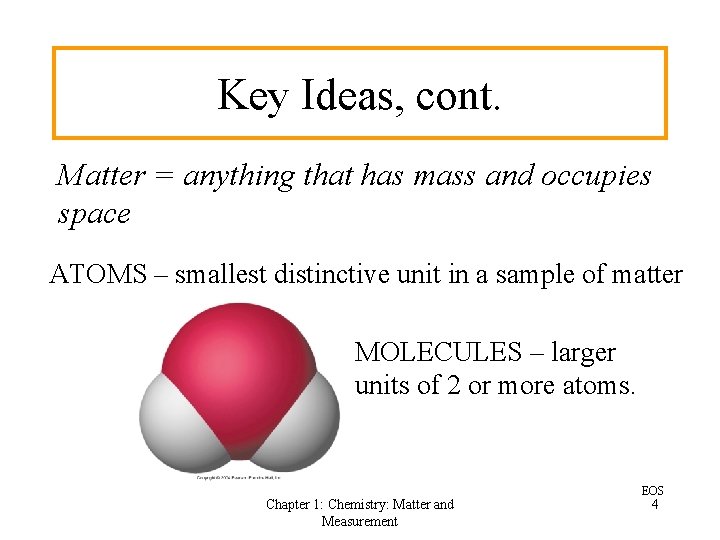
Key Ideas, cont. Matter = anything that has mass and occupies space ATOMS – smallest distinctive unit in a sample of matter MOLECULES – larger units of 2 or more atoms. Chapter 1: Chemistry: Matter and Measurement EOS 4

Properties of Matter Physical property: characteristic displayed by a sample of matter without undergoing any change in its composition e. g. , color Chemical property: characteristics displayed as a result of change in composition e. g. , flammability Chapter 1: Chemistry: Matter and Measurement EOS 5

Physical and Chemical Changes Physical Change: changes in appearance but not in composition e. g. , sublimation of ice in the winter Chemical Change: changes resulting in altered composition and/or molecular structure e. g. , spoilage of foods Chapter 1: Chemistry: Matter and Measurement EOS 6

Classifying Matter Chapter 1: Chemistry: Matter and Measurement 7

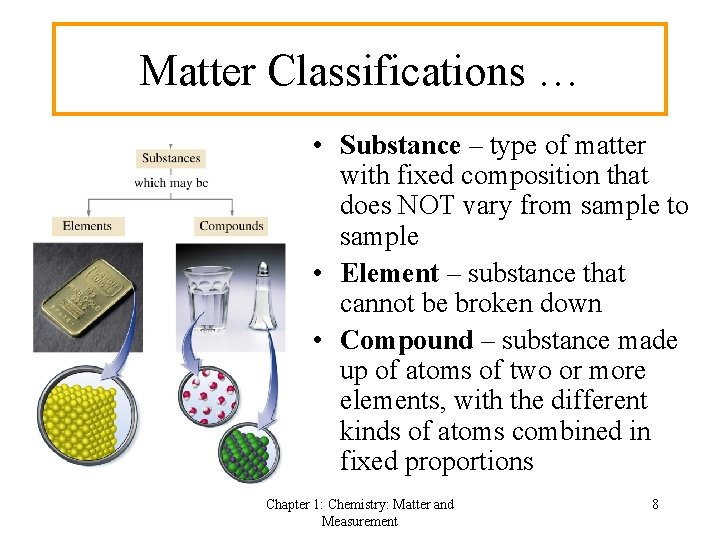
Matter Classifications … • Substance – type of matter with fixed composition that does NOT vary from sample to sample • Element – substance that cannot be broken down • Compound – substance made up of atoms of two or more elements, with the different kinds of atoms combined in fixed proportions Chapter 1: Chemistry: Matter and Measurement 8

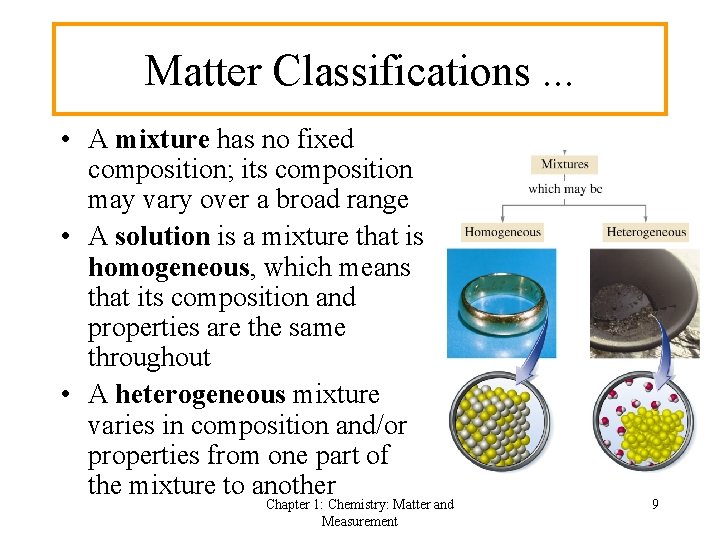
Matter Classifications. . . • A mixture has no fixed composition; its composition may vary over a broad range • A solution is a mixture that is homogeneous, which means that its composition and properties are the same throughout • A heterogeneous mixture varies in composition and/or properties from one part of the mixture to another Chapter 1: Chemistry: Matter and Measurement 9

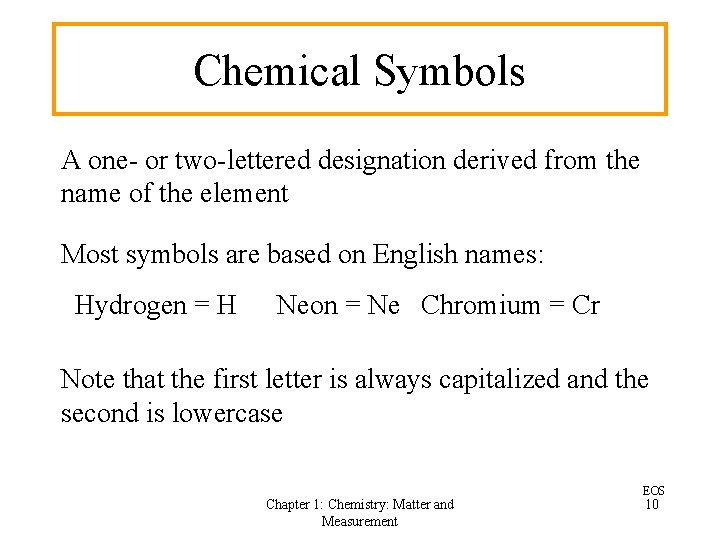
Chemical Symbols A one- or two-lettered designation derived from the name of the element Most symbols are based on English names: Hydrogen = H Neon = Ne Chromium = Cr Note that the first letter is always capitalized and the second is lowercase Chapter 1: Chemistry: Matter and Measurement EOS 10

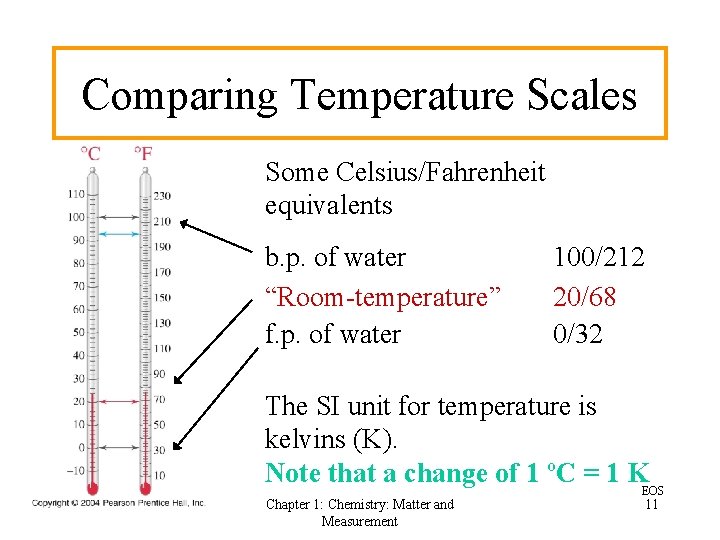
Comparing Temperature Scales Some Celsius/Fahrenheit equivalents b. p. of water “Room-temperature” f. p. of water 100/212 20/68 0/32 The SI unit for temperature is kelvins (K). Note that a change of 1 ºC = 1 KEOS Chapter 1: Chemistry: Matter and Measurement 11

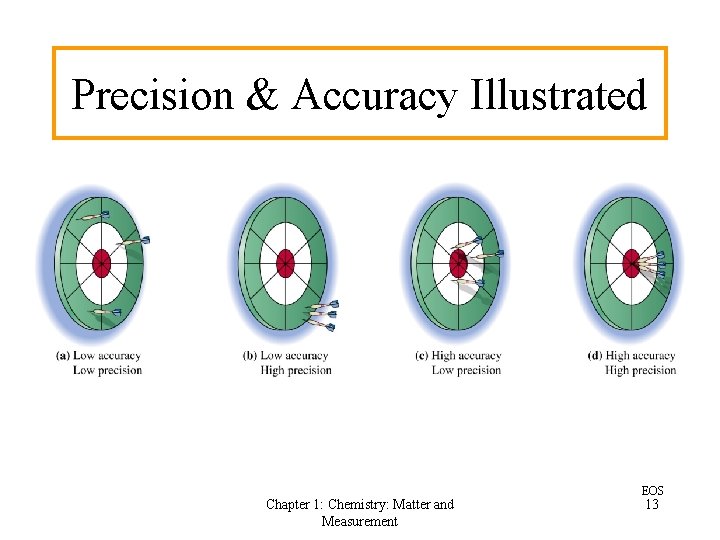
Precision and Accuracy in Measurements • Precision refers to how closely individual scientific measurements agree with one another. • Accuracy refers to the closeness of the average of a set of scientific measurements to the “correct” or “most probable” value. • Sampling errors occur when a group of scientific measurements do not represent the entire population of the variable being studied. Chapter 1: Chemistry: Matter and Measurement EOS 12

Precision & Accuracy Illustrated Chapter 1: Chemistry: Matter and Measurement EOS 13

Significant Figures • All digits in a number that are known with certainty plus the first uncertain digit • The more significant digits obtained, the better the precision of a measurement • The concept of significant figures applies only to measurements • Exact values have an unlimited number of significant figures Chapter 1: Chemistry: Matter and Measurement EOS 14

Rules for Zeros in Significant Figures Zeros between two other significant digits ARE significant e. g. , 10023 A zero preceding a decimal point is not significant e. g. , 0. 10023 Zeros between the decimal point and the first nonzero digit are not significant e. g. , 0. 0010023 Chapter 1: Chemistry: Matter and Measurement EOS 15

Rules for Zeros in Significant Figures Zeros at the end of a number are significant if they are to the right of the decimal point e. g. , 0. 1002300 1023. 00 Zeros at the end of a number may or may not be significant if the number is written without a decimal point e. g. , 1000. compared to 1000 Chapter 1: Chemistry: Matter and Measurement EOS 16

Rules for Significant Figures in Calculations KEY POINT: A calculated quantity can be no more precise than the least precise data used in the calculation … and the reported result should reflect this fact Analogy: a chain is only as strong as its weakest link Chapter 1: Chemistry: Matter and Measurement EOS 17

Significant Figures in Calculations Multiplication and Division: the reported results should have no more significant figures than the factor with the fewest significant figures 1. 827 m × 0. 762 m = ? 0. 762 has 3 sigfigs so the reported answer is 1. 39 m 2 Chapter 1: Chemistry: Matter and Measurement EOS 18

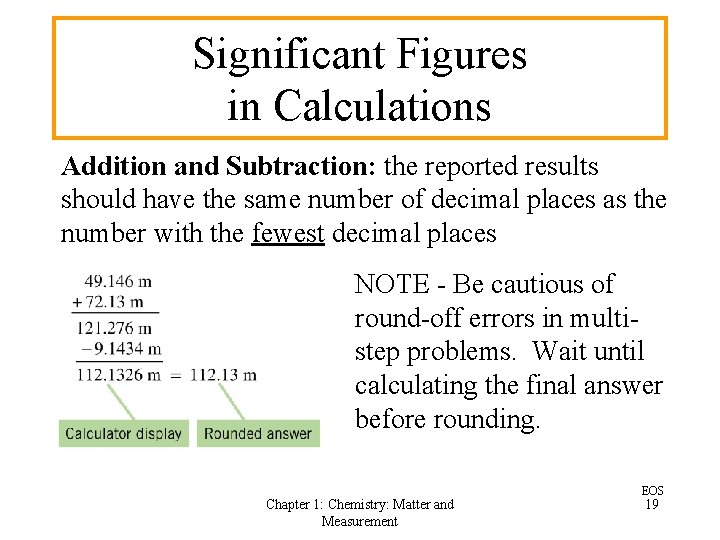
Significant Figures in Calculations Addition and Subtraction: the reported results should have the same number of decimal places as the number with the fewest decimal places NOTE - Be cautious of round-off errors in multistep problems. Wait until calculating the final answer before rounding. Chapter 1: Chemistry: Matter and Measurement EOS 19

Density is the ratio of mass per unit volume of a substance Chapter 1: Chemistry: Matter and Measurement EOS 20

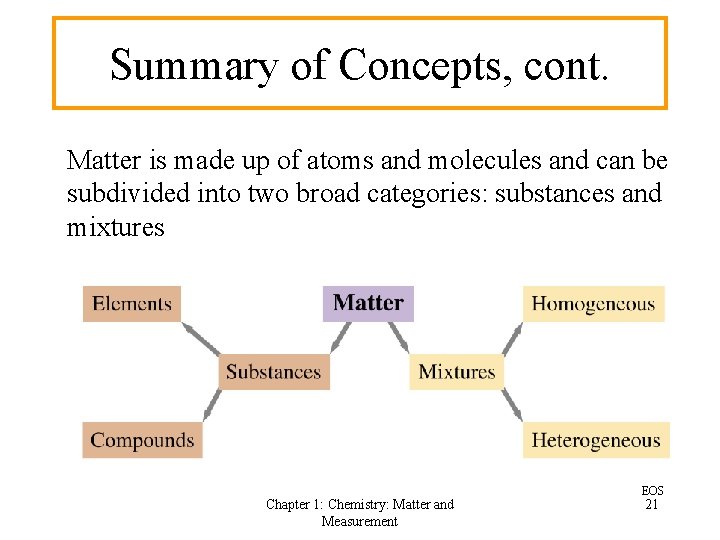
Summary of Concepts, cont. Matter is made up of atoms and molecules and can be subdivided into two broad categories: substances and mixtures Chapter 1: Chemistry: Matter and Measurement EOS 21
 Klorpentan
Klorpentan Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Genel kimya 1 gazlar
Genel kimya 1 gazlar Petrucci
Petrucci Petrucci
Petrucci Mole hill chemistry
Mole hill chemistry An integrated approach to business studies
An integrated approach to business studies Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Is chalk natural or manmade
Is chalk natural or manmade Democritus atomic model diagram
Democritus atomic model diagram Datagram vs virtual circuit
Datagram vs virtual circuit Theoretical models of counseling
Theoretical models of counseling Waterfall and sprinkler strategy
Waterfall and sprinkler strategy Multiple approach avoidance
Multiple approach avoidance Bandura's reciprocal determinism
Bandura's reciprocal determinism Research approach means
Research approach means Traditional development approach
Traditional development approach Tony wagner's seven survival skills
Tony wagner's seven survival skills General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry