General Chemistry An Integrated Approach Hill Petrucci 4



- Slides: 40

General Chemistry: An Integrated Approach Hill, Petrucci, 4 th Edition Chapter 22 The d-Block Elements and Coordination Chemistry Mark P. Heitz State University of New York at Brockport © 2005, Prentice Hall, Inc. Chapter 22: d-Block Elements

On the Periodic Table … d-block elements contain the transition metals Chapter 22: d-Block Elements EOS 2

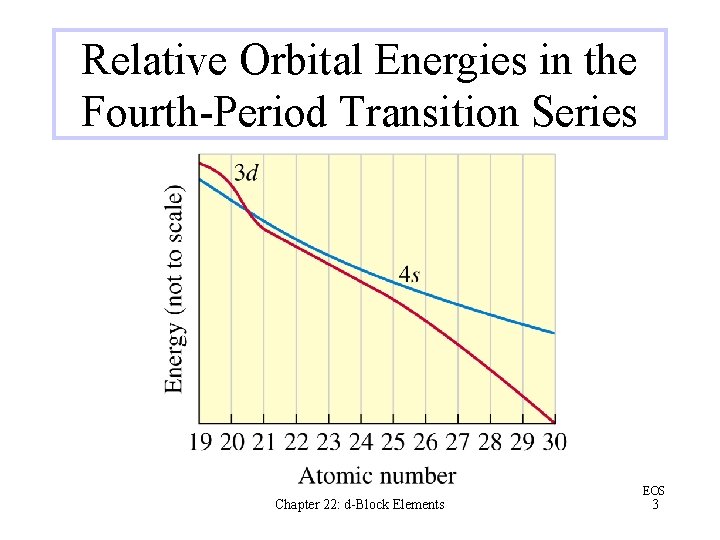
Relative Orbital Energies in the Fourth-Period Transition Series Chapter 22: d-Block Elements EOS 3

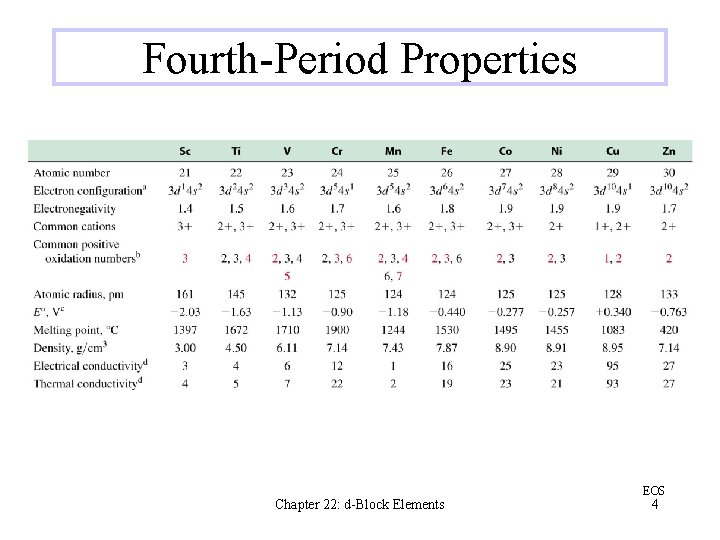
Fourth-Period Properties Chapter 22: d-Block Elements EOS 4

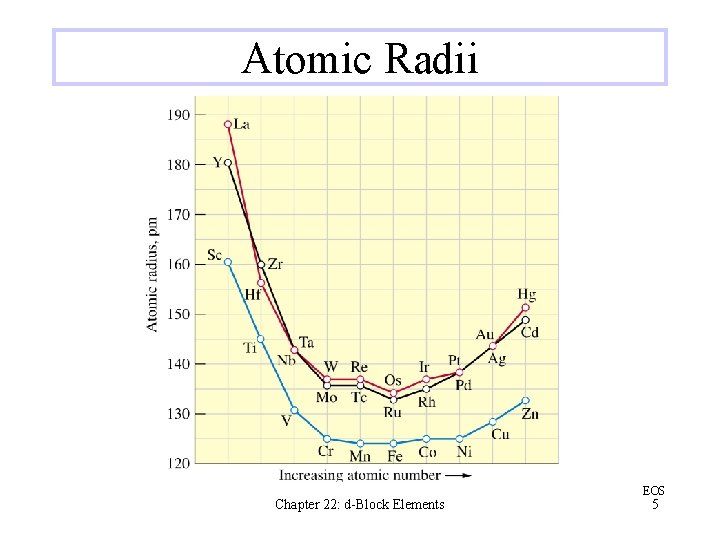
Atomic Radii Chapter 22: d-Block Elements EOS 5

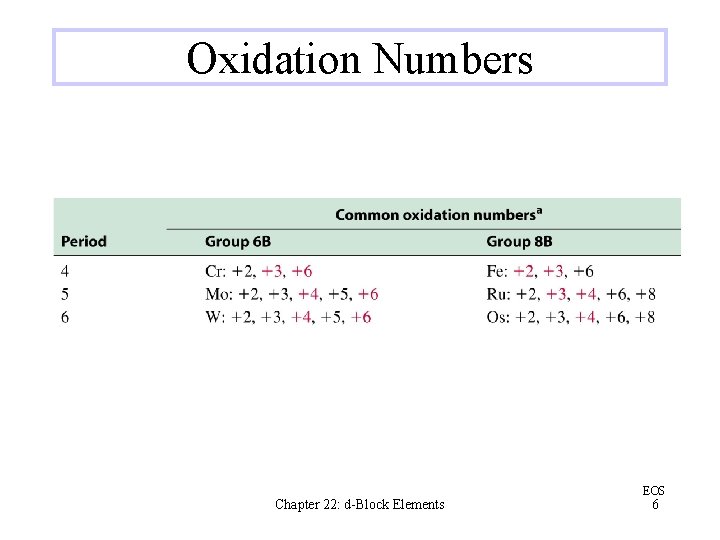
Oxidation Numbers Chapter 22: d-Block Elements EOS 6

Scandium is rather widely distributed in Earth’s crust, but there is only one mineral from which it is extracted, thortveitite, Sc 2 Si 2 O 7 Despite its relative rarity, the physical and chemical properties of scandium have been well characterized. Its chemistry is based mostly on the Sc 3+ ion Scandium properties resemble main group metals, particularly aluminum Chapter 22: d-Block Elements EOS 7

Titanium is the ninth most abundant element in Earth’s crust and the second most abundant transition metal (after iron) Three desirable properties make titanium a highly useful metal: (1) low density; (2) high structural strength, even at high temperatures; and (3) corrosion resistance The most important compound of titanium is the oxide, Ti. O 2, and its most important use is as a white pigment in paints, papers, and plastics Chapter 22: d-Block Elements EOS 8

Vanadium is reasonably abundant in Earth’s solid crust, ranking nineteenth among the elements It is mostly obtained as a by-product of the production of uranium from carnotite, K 2(UO 2)2(VO 4)2· 3 H 2 O Vanadium is of interest because of (1) its use as an alloying element in steel; (2) the catalytic activity of some of its compounds, principally V 2 O 5; and (3) the range of oxidation number in its ions The different oxidation states of vanadium gives a variety of distinctive colors Chapter 22: d-Block Elements EOS 9

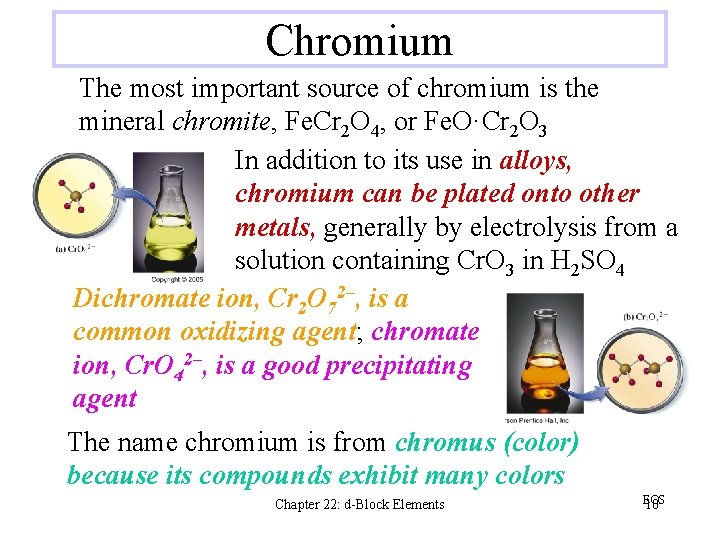
Chromium The most important source of chromium is the mineral chromite, Fe. Cr 2 O 4, or Fe. O·Cr 2 O 3 In addition to its use in alloys, chromium can be plated onto other metals, generally by electrolysis from a solution containing Cr. O 3 in H 2 SO 4 Dichromate ion, Cr 2 O 72–, is a common oxidizing agent; chromate ion, Cr. O 42–, is a good precipitating agent The name chromium is from chromus (color) because its compounds exhibit many colors Chapter 22: d-Block Elements EOS 10

Manganese is obtained mainly from the mineral pyrolusite, Mn. O 2 Ferromanganese alloys are wear resistant and shock resistant and are used for railroad tracks, bulldozers, and road scrapers Manganese dioxide is the starting point for making most other manganese compounds Potassium permanganate, KMn. O 4, is an important oxidizing agent that is used in both analytical and organic chemistry laboratories Chapter 22: d-Block Elements EOS 11

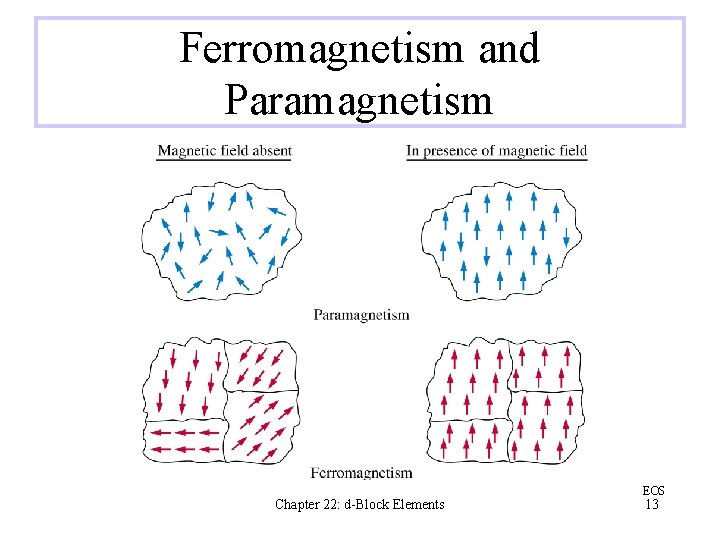
The Iron Triad: Fe, Co, and Ni Iron is the fourth most abundant element in Earth’s crust. Cobalt and nickel are not nearly as common, but they are still sufficiently abundant that their annual production is thousands of tons All three iron triad elements form 2+ ions Ferromagnetism is a much stronger magnetic effect than paramagnetism Chapter 22: d-Block Elements EOS 12

Ferromagnetism and Paramagnetism Chapter 22: d-Block Elements EOS 13

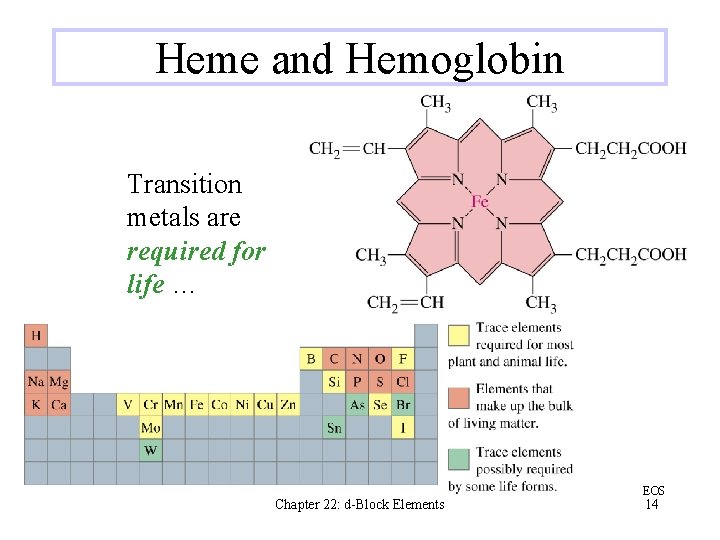
Heme and Hemoglobin Transition metals are required for life … Chapter 22: d-Block Elements EOS 14

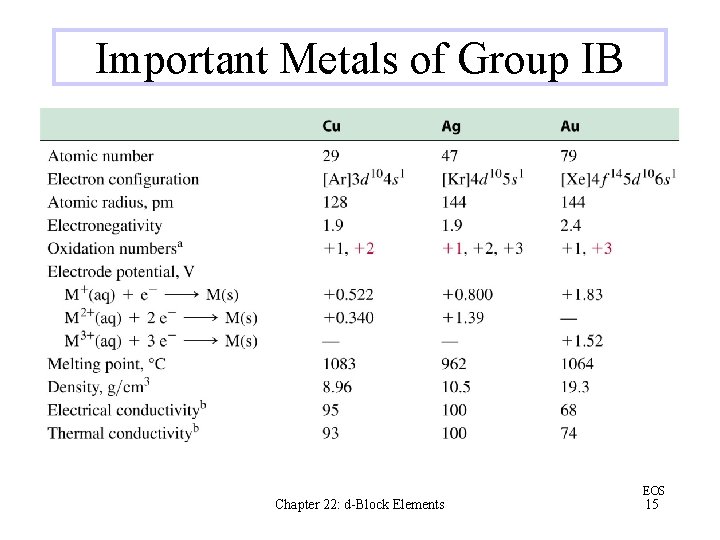
Important Metals of Group IB Chapter 22: d-Block Elements EOS 15

Zinc has many uses, such as in alloys, and is a good electrical conductor Brass is a copper alloy having from 20 to 45% zinc and small quantities of tin, lead, and iron Zinc oxidizes in air to form a thin, adherent oxide coating that protects the underlying metal from further corrosion Because zinc is more easily oxidized than iron, zinc is used in making galvanized iron Chapter 22: d-Block Elements EOS 16

Cadmium can substitute for zinc in coating metals for certain applications Its primary uses are in alloys and as electrodes in batteries Because of its capacity to absorb neutrons, cadmium is used in control rods in nuclear reactors Zinc in trace amounts is an essential element for humans; cadmium is quite toxic Its effect may be to substitute for zinc in certain enzymes Chapter 22: d-Block Elements EOS 17

Mercury differs from zinc and cadmium in at least six significant ways Mercury forms few water-soluble compounds and most of its compounds are not hydrated The physical properties of mercury, especially its metallic and liquid properties and its high density, determine many of its uses Long-term exposure can present a serious health hazard Mercury poisons the body’s systems, in part by interfering with sulfur-containing enzymes Chapter 22: d-Block Elements EOS 18

The Lanthanide Elements The elements cerium through lutetium comprise the first series of the f-block and are called the inner transition elements These elements are frequently called “rare earths, ” a name of historical origin but a clear misnomer because several are not rare at all For many uses, the lanthanides do not have to be separated from one another. A mixture of the lanthanide metals with about 25% La is used in certain steel and magnesium-based alloys. Some of the oxides are used to color glass EOS Chapter 22: d-Block Elements 19

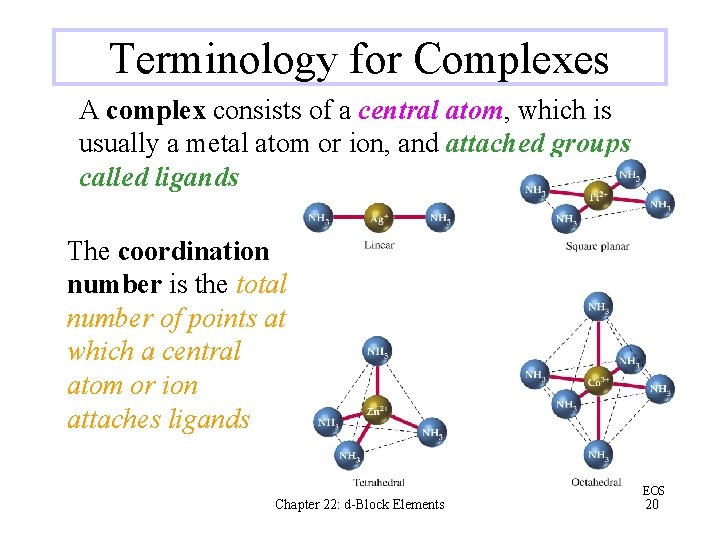
Terminology for Complexes A complex consists of a central atom, which is usually a metal atom or ion, and attached groups called ligands The coordination number is the total number of points at which a central atom or ion attaches ligands Chapter 22: d-Block Elements EOS 20

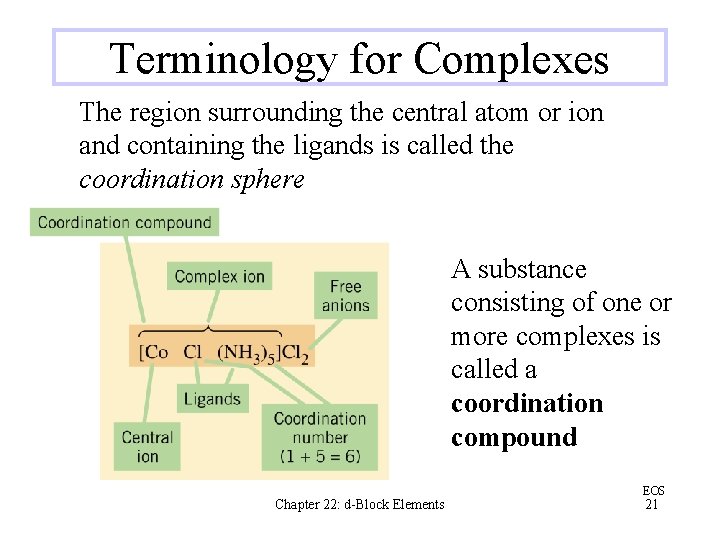
Terminology for Complexes The region surrounding the central atom or ion and containing the ligands is called the coordination sphere A substance consisting of one or more complexes is called a coordination compound Chapter 22: d-Block Elements EOS 21

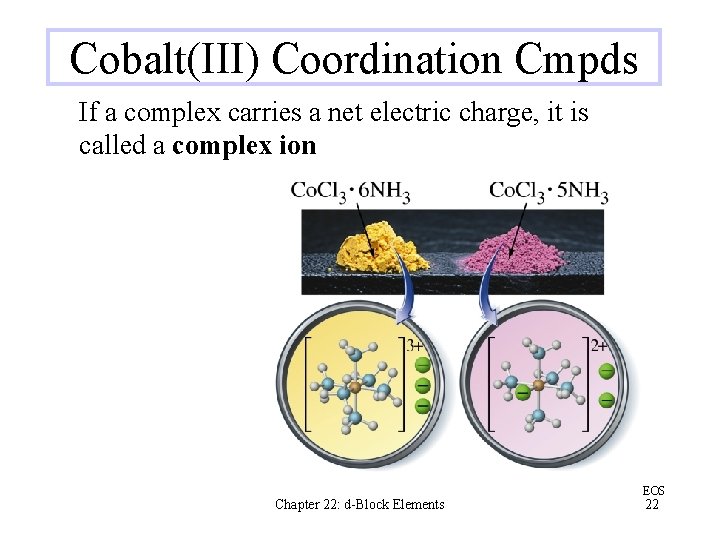
Cobalt(III) Coordination Cmpds If a complex carries a net electric charge, it is called a complex ion Chapter 22: d-Block Elements EOS 22

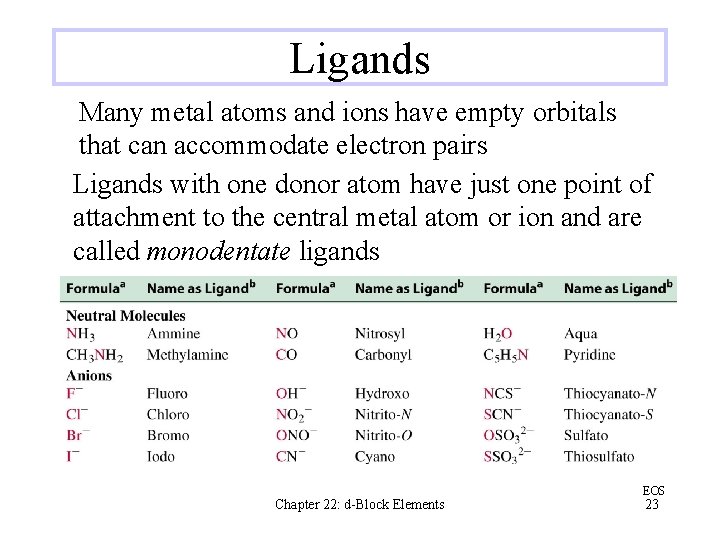
Ligands Many metal atoms and ions have empty orbitals that can accommodate electron pairs Ligands with one donor atom have just one point of attachment to the central metal atom or ion and are called monodentate ligands Chapter 22: d-Block Elements EOS 23

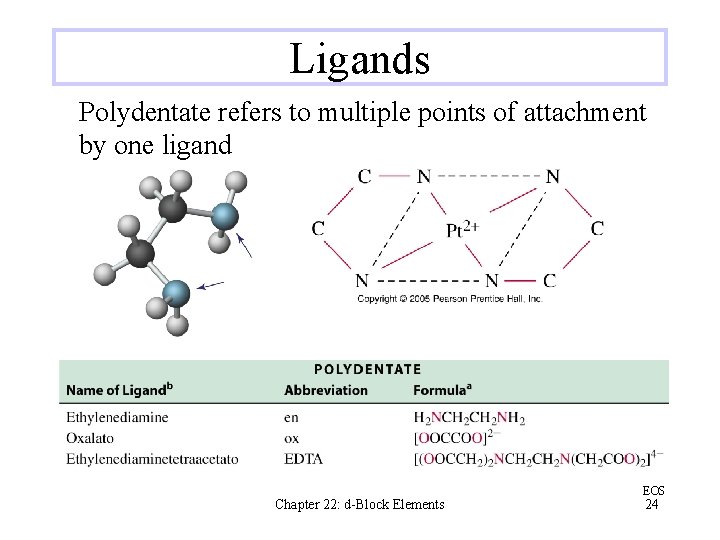
Ligands Polydentate refers to multiple points of attachment by one ligand Chapter 22: d-Block Elements EOS 24

Naming Complex Ions In naming a complex, first name the ligands and then the central metal atom or ion as a single compound word Name the ligands in alphabetical order based on the first letters of their names and ignoring prefixes Designate the number of ligands with prefixes Use the unmodified name of the central metal in a complex cation. In a complex anion, add the ending -ate to the name of the central metal Write the names in the order of cation followed by anion Chapter 22: d-Block Elements EOS 25

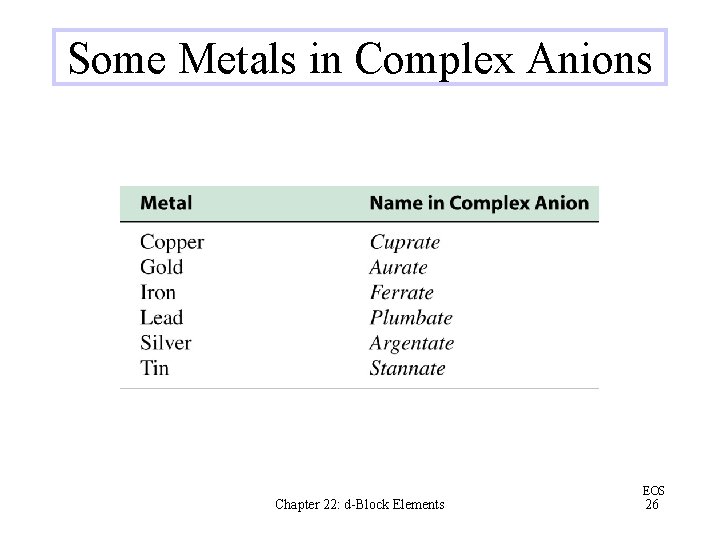
Some Metals in Complex Anions Chapter 22: d-Block Elements EOS 26

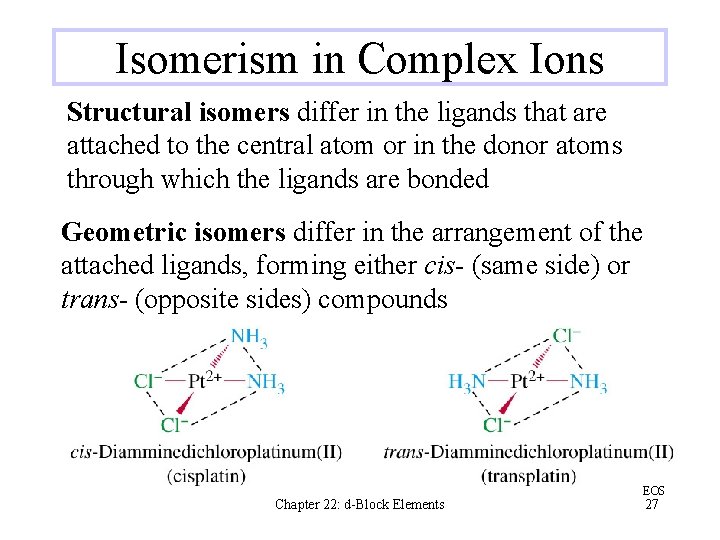
Isomerism in Complex Ions Structural isomers differ in the ligands that are attached to the central atom or in the donor atoms through which the ligands are bonded Geometric isomers differ in the arrangement of the attached ligands, forming either cis- (same side) or trans- (opposite sides) compounds Chapter 22: d-Block Elements EOS 27

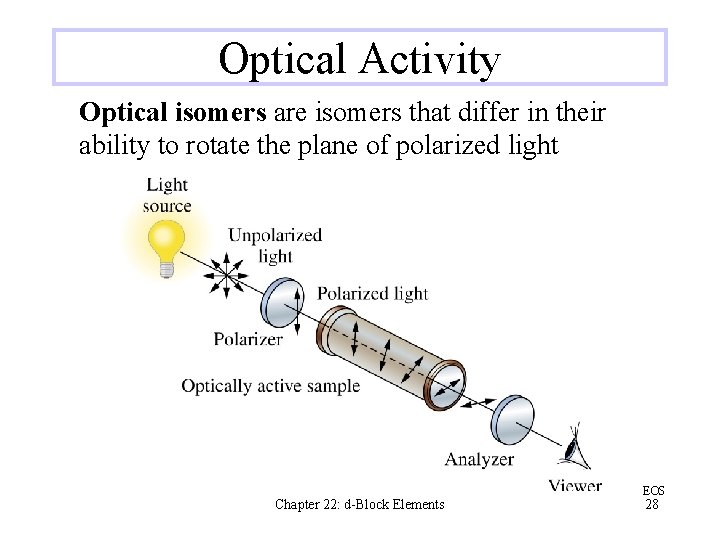
Optical Activity Optical isomers are isomers that differ in their ability to rotate the plane of polarized light Chapter 22: d-Block Elements EOS 28

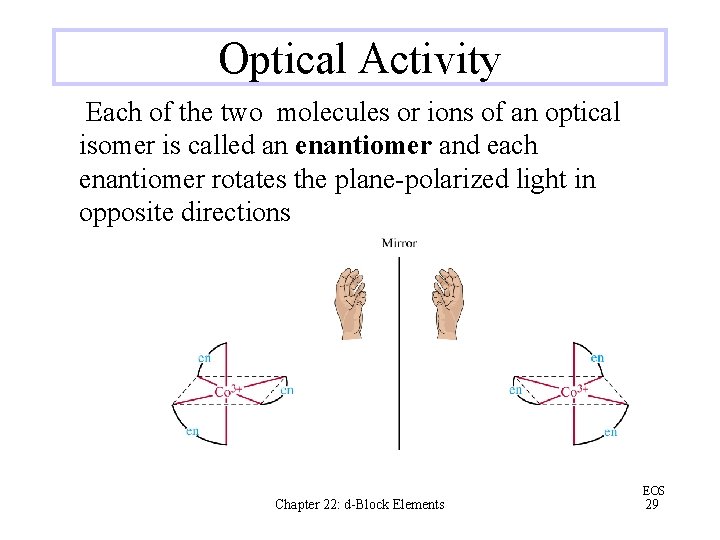
Optical Activity Each of the two molecules or ions of an optical isomer is called an enantiomer and each enantiomer rotates the plane-polarized light in opposite directions Chapter 22: d-Block Elements EOS 29

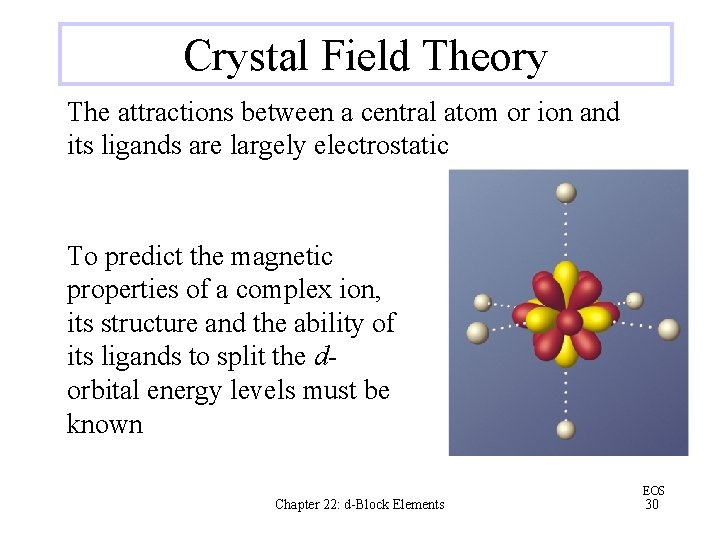
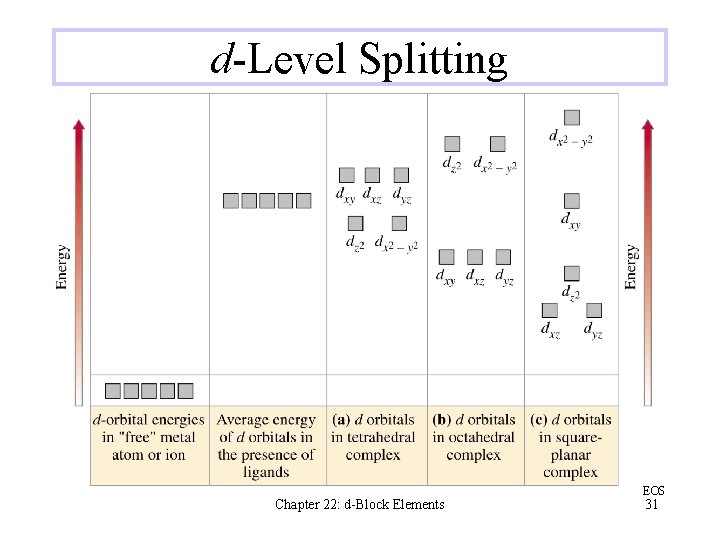
Crystal Field Theory The attractions between a central atom or ion and its ligands are largely electrostatic To predict the magnetic properties of a complex ion, its structure and the ability of its ligands to split the dorbital energy levels must be known Chapter 22: d-Block Elements EOS 30

d-Level Splitting Chapter 22: d-Block Elements EOS 31

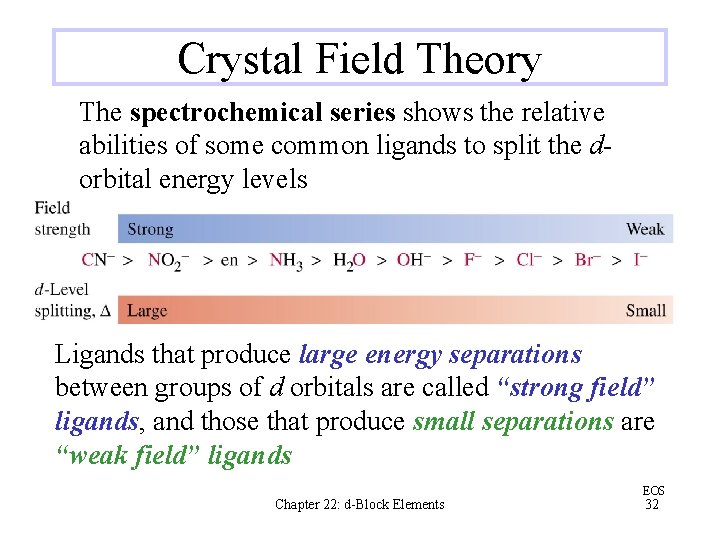
Crystal Field Theory The spectrochemical series shows the relative abilities of some common ligands to split the dorbital energy levels Ligands that produce large energy separations between groups of d orbitals are called “strong field” ligands, and those that produce small separations are “weak field” ligands Chapter 22: d-Block Elements EOS 32

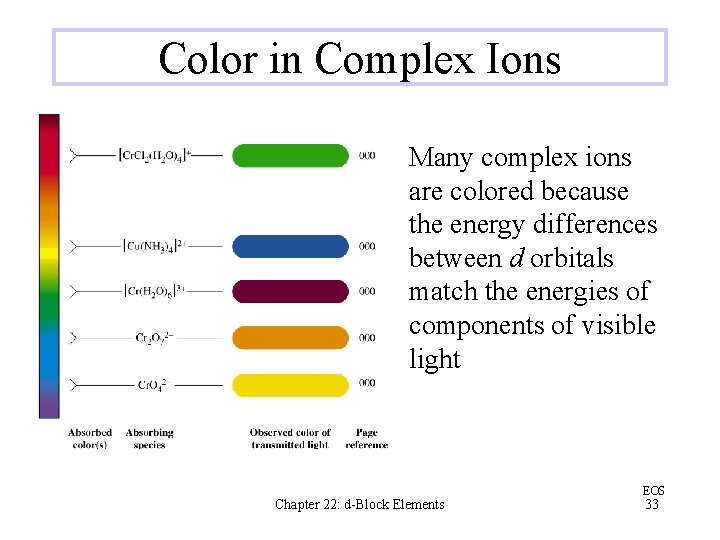
Color in Complex Ions Many complex ions are colored because the energy differences between d orbitals match the energies of components of visible light Chapter 22: d-Block Elements EOS 33

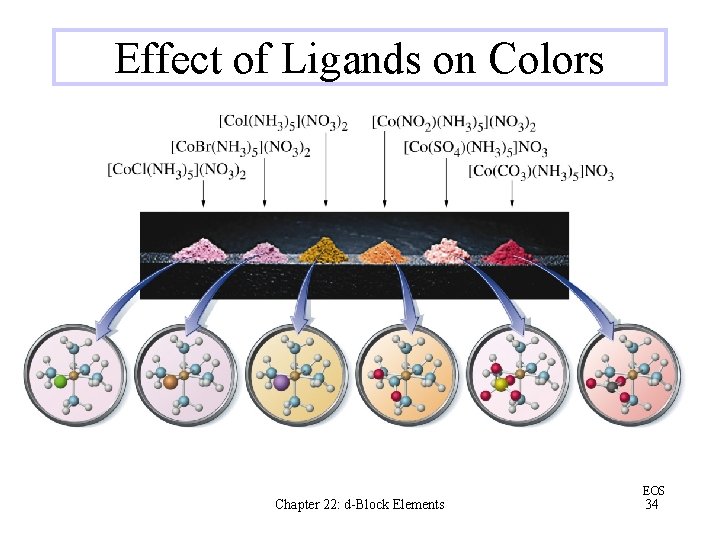
Effect of Ligands on Colors Chapter 22: d-Block Elements EOS 34

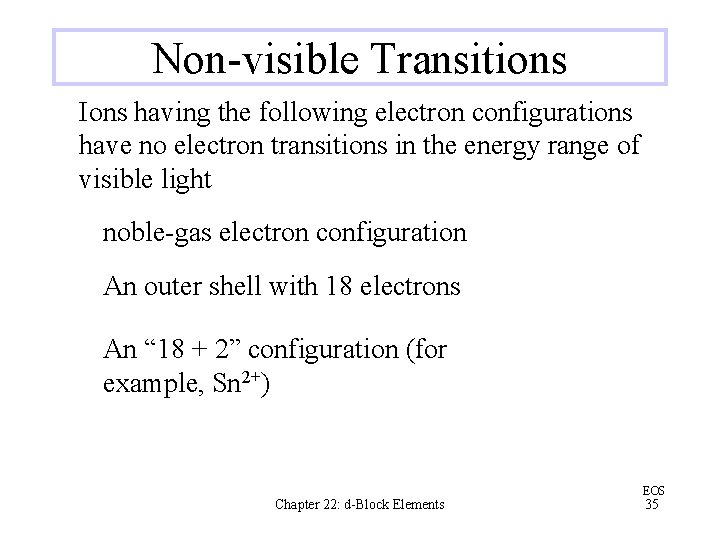
Non-visible Transitions Ions having the following electron configurations have no electron transitions in the energy range of visible light noble-gas electron configuration An outer shell with 18 electrons An “ 18 + 2” configuration (for example, Sn 2+) Chapter 22: d-Block Elements EOS 35

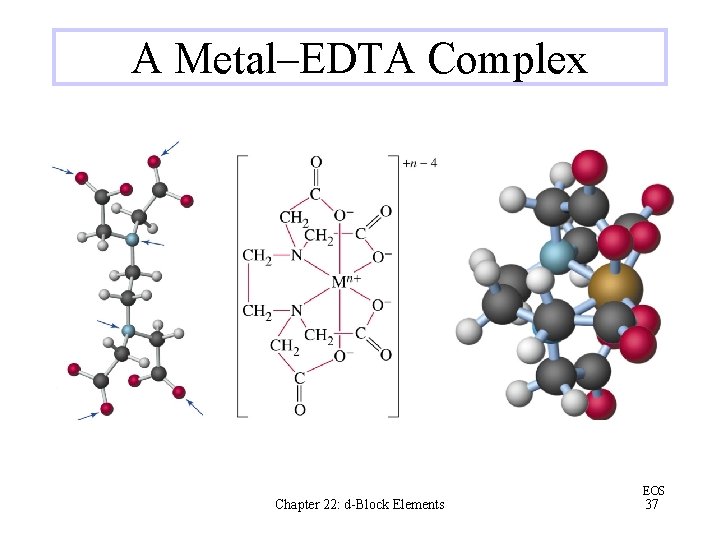
Chelates generally are much more stable than complexes with monodentate ligands The increase in entropy during chelation is an important factor in the stabilities of chelates EDTA (ethylenediaminetetraacetic acid) is commonly used to sequester metal ions in solution Chelation therapy is sometimes used to treat heavy metal ion poisoning Chapter 22: d-Block Elements EOS 36

A Metal–EDTA Complex Chapter 22: d-Block Elements EOS 37

Summary of Concepts • All the elements of the d block are metals • Most metals exist in several oxidation states and form many complex ions and colored compounds • The early members in a period in the d block are active metals but later members are less active • Scandium resembles aluminum but is not widely used • The uses of titanium depend on its high strength, low density, and corrosion resistance • Dichromate and permanganate ions are widely used oxidizing agents Chapter 22: d-Block Elements EOS 38

Summary (cont’d) • Copper, silver, and gold are much less active than earlier members of their periods • Zinc, cadmium, and mercury are not transition elements; their atoms and ions all have filled d subshells • The central metal atom or ion of a metal complex is a Lewis acid; it forms coordinate covalent bonds by accepting lone-pair electrons from ligands, which are Lewis bases • Isomerism among complexes is of two general types: structural and optical Chapter 22: d-Block Elements EOS 39

Summary (cont’d) • Interactions between lone-pair electrons on ligands and electrons in the d orbitals of the central metal atom or ion produce a splitting of the d-orbital energy level • Electron transitions between d orbitals of different energy provide a way for a complex to absorb some wavelength components of visible light and transmit others, giving rise to color • Explanations of the colors and magnetic properties of complexes are facilitated by a special listing of common ligands called the spectrochemical series Chapter 22: d-Block Elements EOS 40
 General chemistry ders notları
General chemistry ders notları Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Genel kimya 1 gazlar
Genel kimya 1 gazlar Petrucci
Petrucci Petrucci
Petrucci Mole hill chemistry
Mole hill chemistry An integrated approach to business studies
An integrated approach to business studies Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter Democritus atomic model diagram
Democritus atomic model diagram Datagram approach
Datagram approach Theoretical models of counseling
Theoretical models of counseling Waterfall market entry strategy
Waterfall market entry strategy Multiple approach avoidance
Multiple approach avoidance Bandura's reciprocal determinism
Bandura's reciprocal determinism Research approach meaning
Research approach meaning Traditional development approach
Traditional development approach Deep learning approach and surface learning approach
Deep learning approach and surface learning approach General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry The hand approach general paper
The hand approach general paper Aice general paper hand approach
Aice general paper hand approach Back face detection
Back face detection Aice general paper essay format
Aice general paper essay format Aice general paper exam
Aice general paper exam Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Whrhs.org
Whrhs.org Temporal island of vision
Temporal island of vision Esteri del colesterolo
Esteri del colesterolo