Petrucci Harwood Herring Madura GENERAL CHEMISTRY Ninth Edition



- Slides: 23

Petrucci • Harwood • Herring • Madura GENERAL CHEMISTRY Ninth Edition Principles and Modern Applications Chapter 7: Thermochemistry 1

Contents 7 -1 7 -2 7 -3 7 -4 7 -5 7 -6 7 -7 7 -8 2 Getting Started: Some Terminology Heats of Reaction and Calorimetry Work The First Law of Thermodynamics Heats of Reaction: U and H The Indirect Determination of H: Hess’s Law Standard Enthalpies of Formation

6 -1 Getting Started: Some Terminology ¨ System ¨ Surroundings 3

Terminology ¨ Energy, U · The capacity to do work. ¨ Work · Force acting through a distance. ¨ Kinetic Energy · The energy of motion. 4

Energy ¨ Potential Energy · Energy due to condition, position, or composition. · Associated with forces of attraction or repulsion between objects. ¨ Energy can change from potential to kinetic. 5

Energy and Temperature ¨ Thermal Energy · Kinetic energy associated with random molecular motion. · In general proportional to temperature. · An intensive property. ¨ Heat and Work · q and w. · Energy changes. 6

Heat Energy transferred between a system and its surroundings as a result of a temperature difference. ¨ Heat flows from hotter to colder. · Temperature may change. · Phase may change (an isothermal process). 7

Units of Heat ¨ Calorie (cal) · The quantity of heat required to change the temperature of one gram of water by one degree Celsius. ¨ Joule (J) · SI unit for heat 1 cal = 4. 184 J 8

Heat Capacity ¨ The quantity of heat required to change the temperature of a system by one degree. · Molar heat capacity. ◦ System is one mole of substance. · Specific heat capacity, c. q = mc T ◦ System is one gram of substance · Heat capacity ◦ Mass specific heat. 9 q = C T

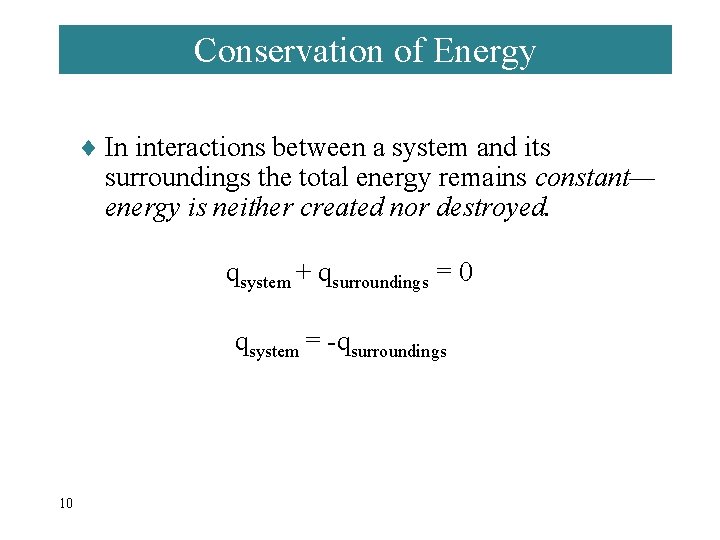
Conservation of Energy ¨ In interactions between a system and its surroundings the total energy remains constant— energy is neither created nor destroyed. qsystem + qsurroundings = 0 qsystem = -qsurroundings 10

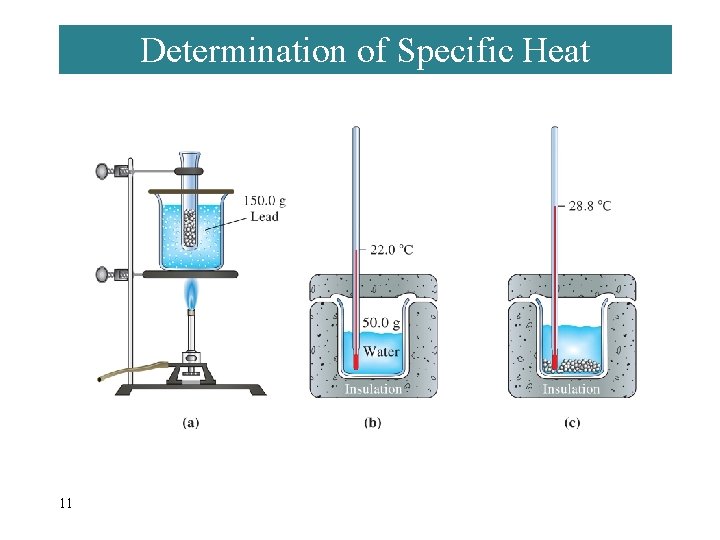
Determination of Specific Heat 11

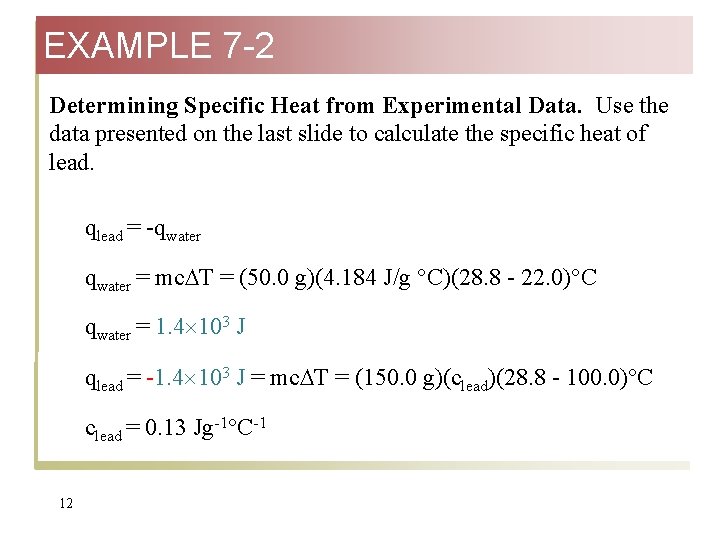
EXAMPLE 7 -2 Determining Specific Heat from Experimental Data. Use the data presented on the last slide to calculate the specific heat of lead. qlead = -qwater = mc T = (50. 0 g)(4. 184 J/g °C)(28. 8 - 22. 0)°C qwater = 1. 4 103 J qlead = -1. 4 103 J = mc T = (150. 0 g)(clead)(28. 8 - 100. 0)°C clead = 0. 13 Jg-1°C-1 12

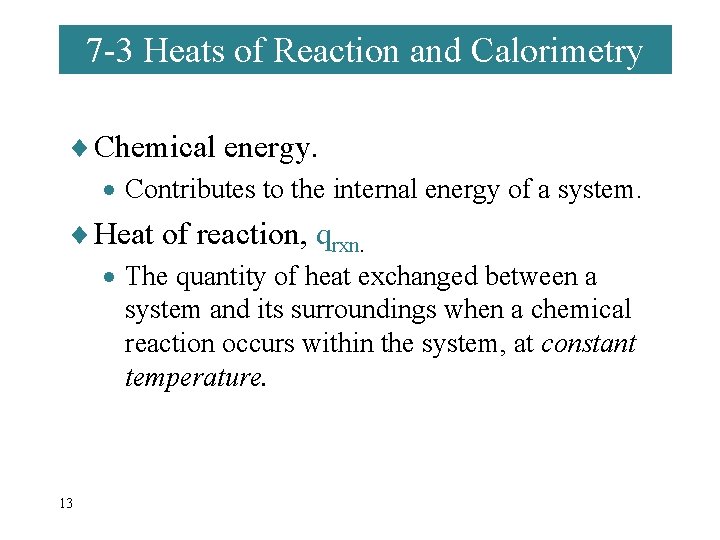
7 -3 Heats of Reaction and Calorimetry ¨ Chemical energy. · Contributes to the internal energy of a system. ¨ Heat of reaction, qrxn. · The quantity of heat exchanged between a system and its surroundings when a chemical reaction occurs within the system, at constant temperature. 13

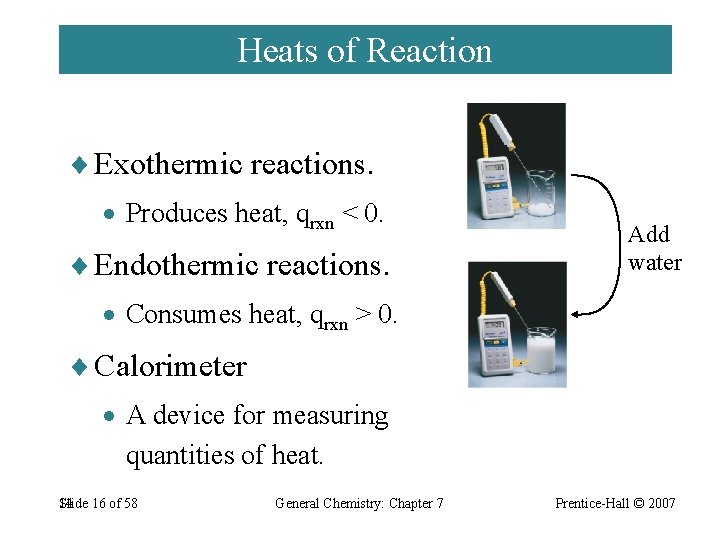
Heats of Reaction ¨ Exothermic reactions. · Produces heat, qrxn < 0. ¨ Endothermic reactions. Add water · Consumes heat, qrxn > 0. ¨ Calorimeter · A device for measuring quantities of heat. 14 Slide 16 of 58 General Chemistry: Chapter 7 Prentice-Hall © 2007

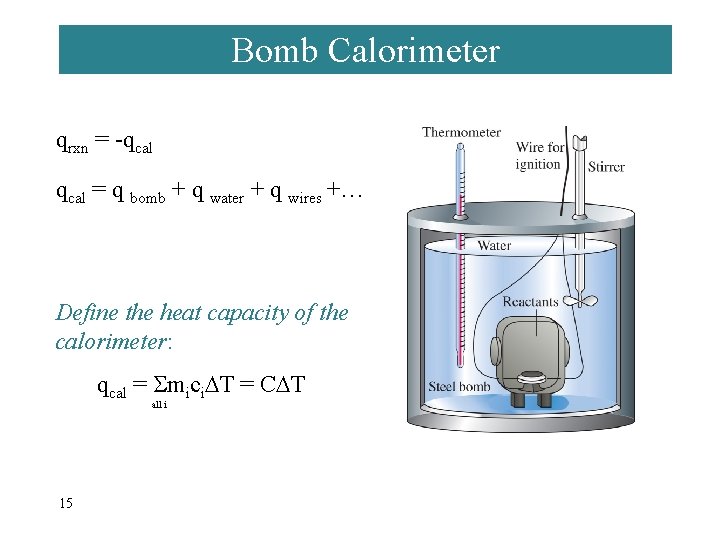
Bomb Calorimeter qrxn = -qcal = q bomb + q water + q wires +… Define the heat capacity of the calorimeter: qcal = mici T = C T all i 15 heat

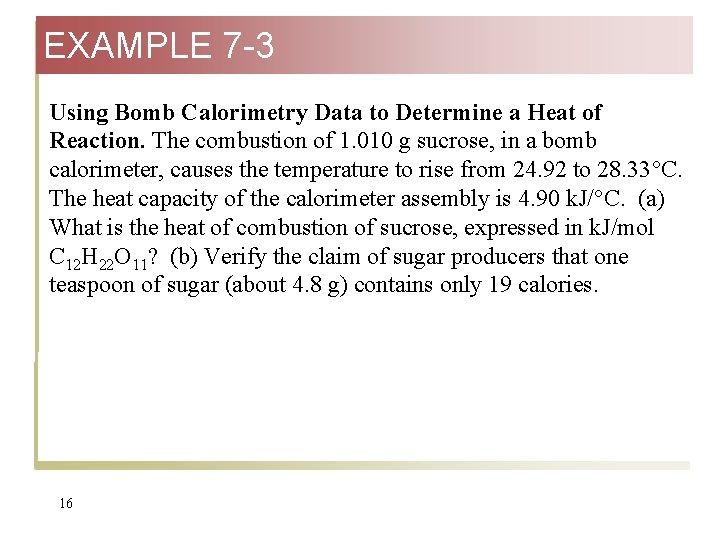
EXAMPLE 7 -3 Using Bomb Calorimetry Data to Determine a Heat of Reaction. The combustion of 1. 010 g sucrose, in a bomb calorimeter, causes the temperature to rise from 24. 92 to 28. 33°C. The heat capacity of the calorimeter assembly is 4. 90 k. J/°C. (a) What is the heat of combustion of sucrose, expressed in k. J/mol C 12 H 22 O 11? (b) Verify the claim of sugar producers that one teaspoon of sugar (about 4. 8 g) contains only 19 calories. 16

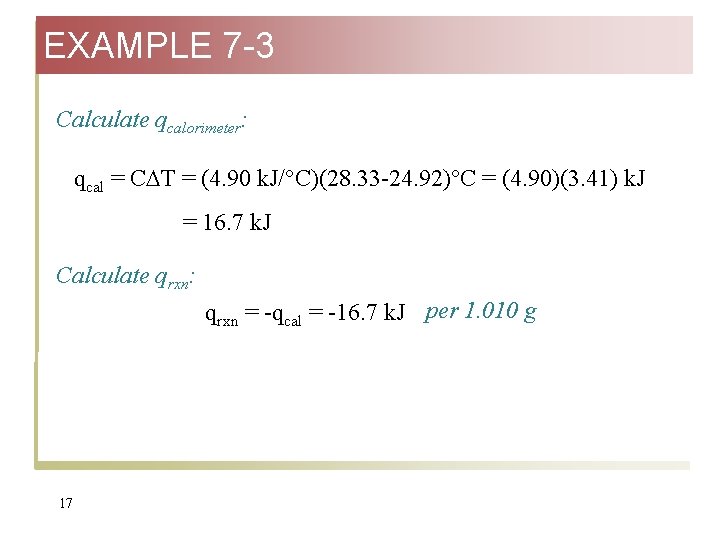
EXAMPLE 7 -3 Calculate qcalorimeter: qcal = C T = (4. 90 k. J/°C)(28. 33 -24. 92)°C = (4. 90)(3. 41) k. J = 16. 7 k. J Calculate qrxn: qrxn = -qcal = -16. 7 k. J per 1. 010 g 17

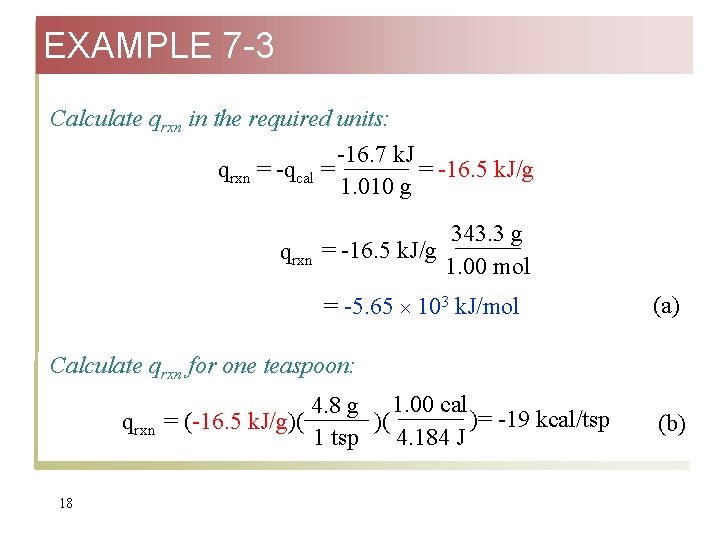
EXAMPLE 7 -3 Calculate qrxn in the required units: -16. 7 k. J qrxn = -qcal = = -16. 5 k. J/g 1. 010 g qrxn 343. 3 g = -16. 5 k. J/g 1. 00 mol = -5. 65 103 k. J/mol (a) Calculate qrxn for one teaspoon: qrxn 18 4. 8 g 1. 00 cal )= -19 kcal/tsp = (-16. 5 k. J/g)( )( 4. 184 J 1 tsp (b)

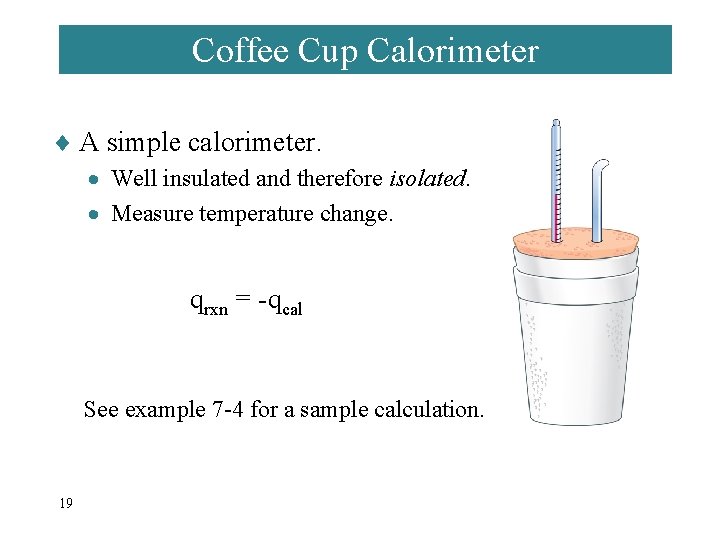
Coffee Cup Calorimeter ¨ A simple calorimeter. · Well insulated and therefore isolated. · Measure temperature change. qrxn = -qcal See example 7 -4 for a sample calculation. 19

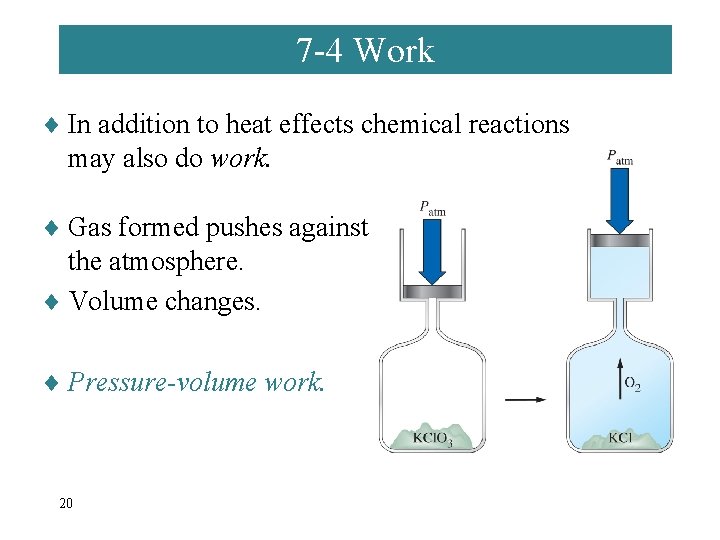
7 -4 Work ¨ In addition to heat effects chemical reactions may also do work. ¨ Gas formed pushes against the atmosphere. ¨ Volume changes. ¨ Pressure-volume work. 20

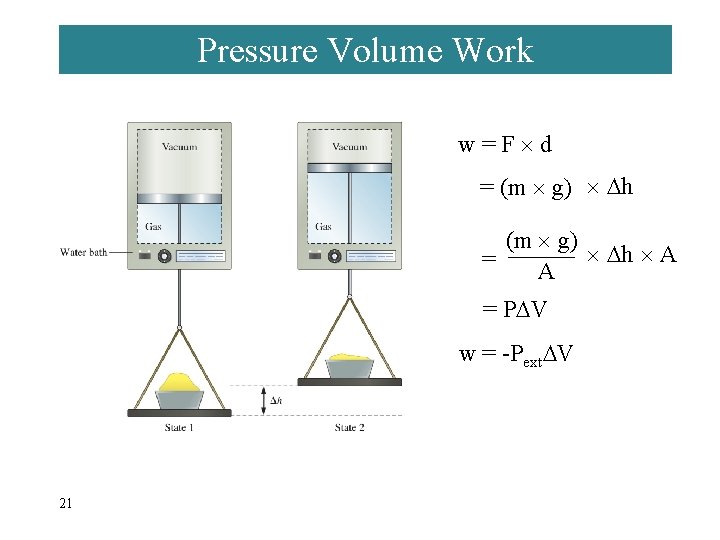
Pressure Volume Work w=F d = (m g) h A = P V w = -Pext V 21

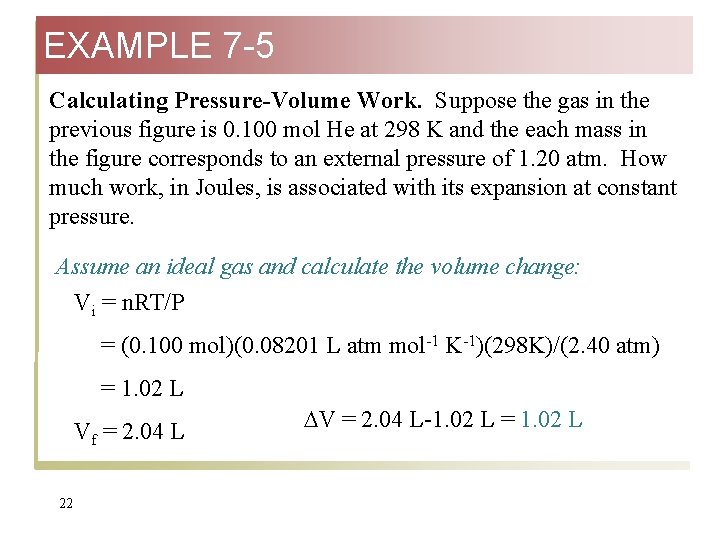
EXAMPLE 7 -5 Calculating Pressure-Volume Work. Suppose the gas in the previous figure is 0. 100 mol He at 298 K and the each mass in the figure corresponds to an external pressure of 1. 20 atm. How much work, in Joules, is associated with its expansion at constant pressure. Assume an ideal gas and calculate the volume change: Vi = n. RT/P = (0. 100 mol)(0. 08201 L atm mol-1 K-1)(298 K)/(2. 40 atm) = 1. 02 L Vf = 2. 04 L 22 V = 2. 04 L-1. 02 L = 1. 02 L

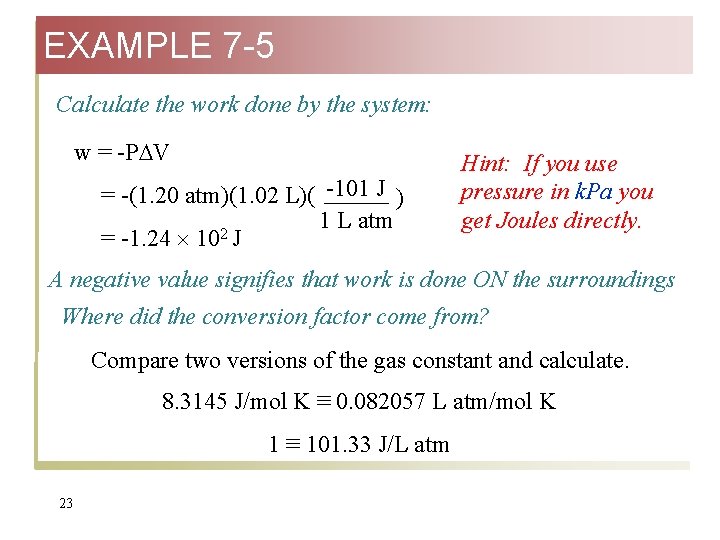
EXAMPLE 7 -5 Calculate the work done by the system: w = -P V = -(1. 20 atm)(1. 02 L)( -101 J ) 1 L atm 2 = -1. 24 10 J Hint: If you use pressure in k. Pa you get Joules directly. A negative value signifies that work is done ON the surroundings Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8. 3145 J/mol K ≡ 0. 082057 L atm/mol K 1 ≡ 101. 33 J/L atm 23
 Genel kimya petrucci harwood herring
Genel kimya petrucci harwood herring Klorpentan
Klorpentan Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Psychology ninth edition in modules
Psychology ninth edition in modules Macroeconomics mankiw 9th edition
Macroeconomics mankiw 9th edition Human anatomy & physiology edition 9
Human anatomy & physiology edition 9 General adaptation syndrome
General adaptation syndrome Social psychology ninth edition
Social psychology ninth edition Biology ninth edition
Biology ninth edition Child development laura berk 9th edition
Child development laura berk 9th edition Child development ninth edition
Child development ninth edition Abnormal psychology comer 9th edition
Abnormal psychology comer 9th edition Psychology ninth edition in modules
Psychology ninth edition in modules Psychology ninth edition david g myers
Psychology ninth edition david g myers Biology ninth edition
Biology ninth edition Campbell ninth edition
Campbell ninth edition International financial management 13th edition
International financial management 13th edition Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci General chemistry
General chemistry Doug harwood
Doug harwood Harwood imalat işletmesi araştırması
Harwood imalat işletmesi araştırması Crusher hire harwood
Crusher hire harwood












































