GENERAL CHEMISTRY Principles and Modern Applications PETRUCCI HERRING


- Slides: 16

GENERAL CHEMISTRY Principles and Modern Applications PETRUCCI HERRING MADURA Gases TENTH EDITION BISSONNETTE 6 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 1 of 41 General Chemistry: Chapter 1 Copyright © 2011 Pearson Canada Inc.

Gases CONTENTS Slide 2 of 41 6 -1 Properties of Gases: Gas Pressure 6 -2 The Simple Gas Laws 6 -3 Combining the Gas Laws: The Ideal Gas Equation and The General Gas Equation 6 -4 Applications of the Ideal Gas Equation 6 -5 Gases in Chemical Reactions 6 -6 Mixtures of Gases 6 -7 Kinetic—Molecular Theory of Gases 6 -8 Gas Properties Relating to the Kinetic—Molecular Theory 6 -9 Nonideal (Real) Gases General Chemistry: Chapter 1 Copyright © 2011 Pearson Canada Inc.

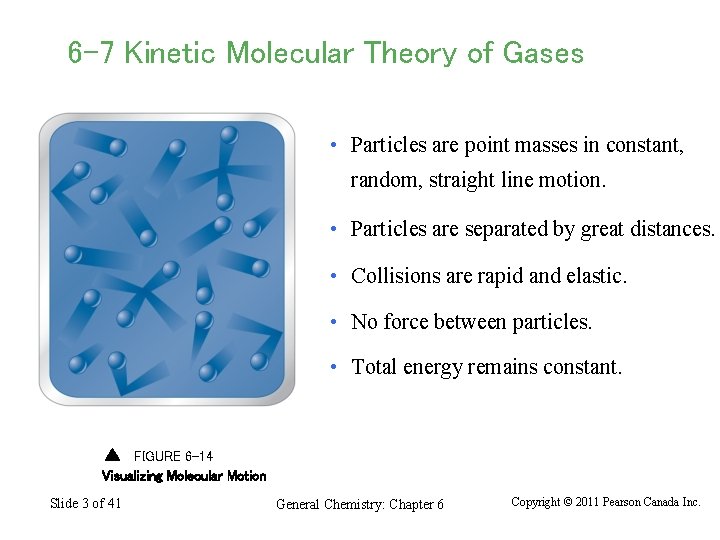
6 -7 Kinetic Molecular Theory of Gases • Particles are point masses in constant, random, straight line motion. • Particles are separated by great distances. • Collisions are rapid and elastic. • No force between particles. • Total energy remains constant. FIGURE 6 -14 Visualizing Molecular Motion Slide 3 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

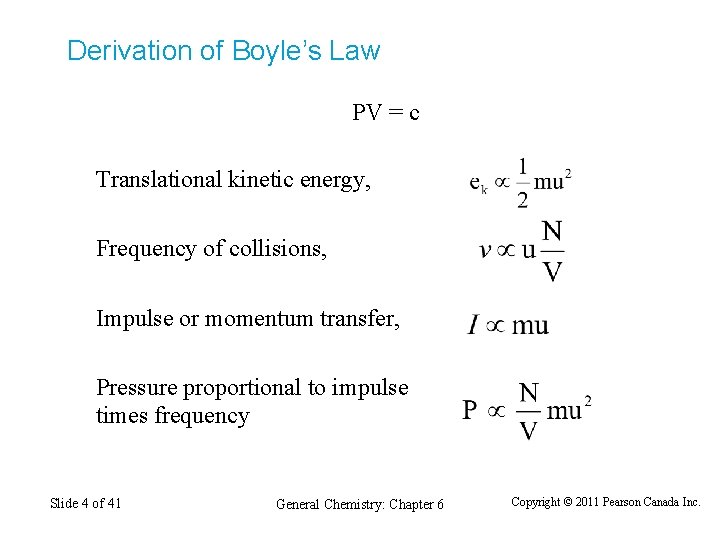
Derivation of Boyle’s Law PV = c Translational kinetic energy, Frequency of collisions, Impulse or momentum transfer, Pressure proportional to impulse times frequency Slide 4 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

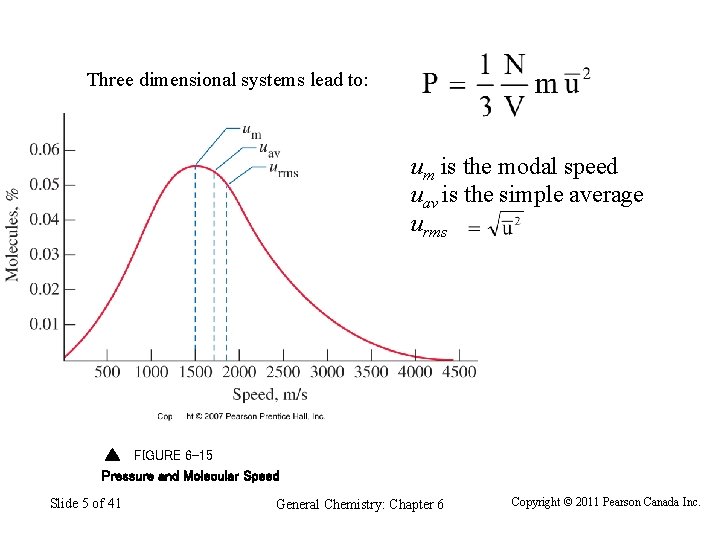
Three dimensional systems lead to: um is the modal speed uav is the simple average urms FIGURE 6 -15 Pressure and Molecular Speed Slide 5 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

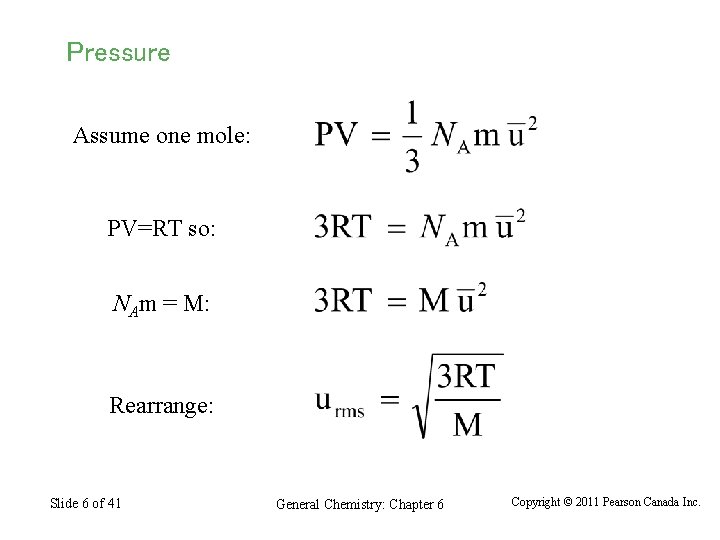
Pressure Assume one mole: PV=RT so: NAm = M: Rearrange: Slide 6 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

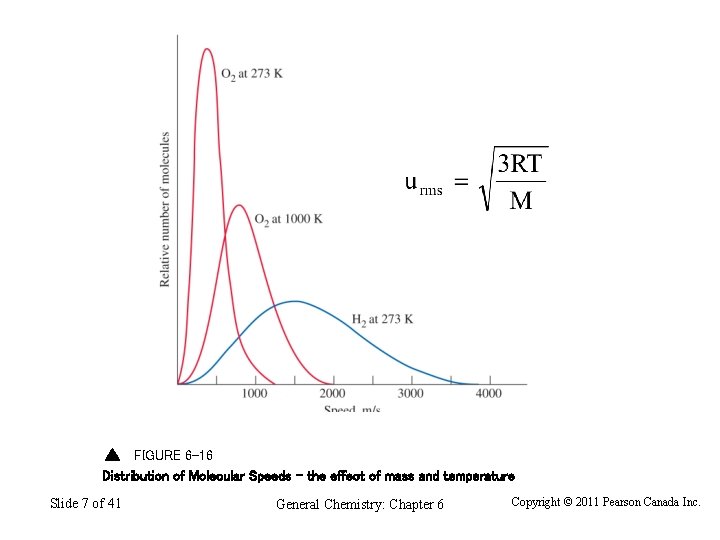
FIGURE 6 -16 Distribution of Molecular Speeds – the effect of mass and temperature Slide 7 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

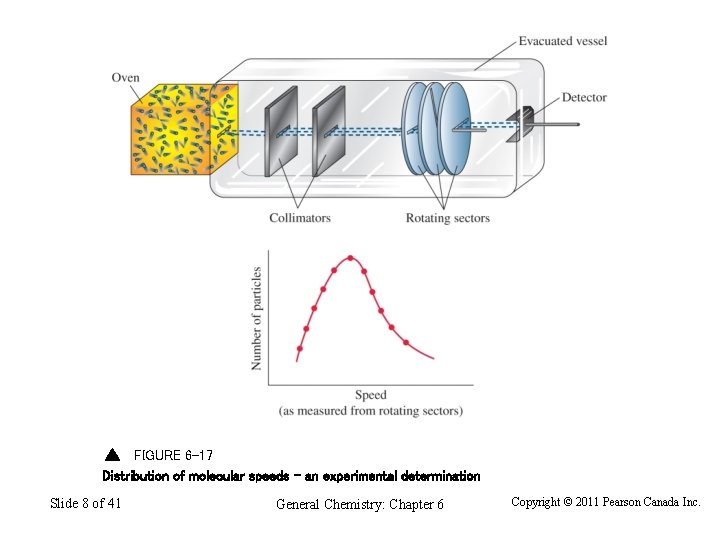
FIGURE 6 -17 Distribution of molecular speeds – an experimental determination Slide 8 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

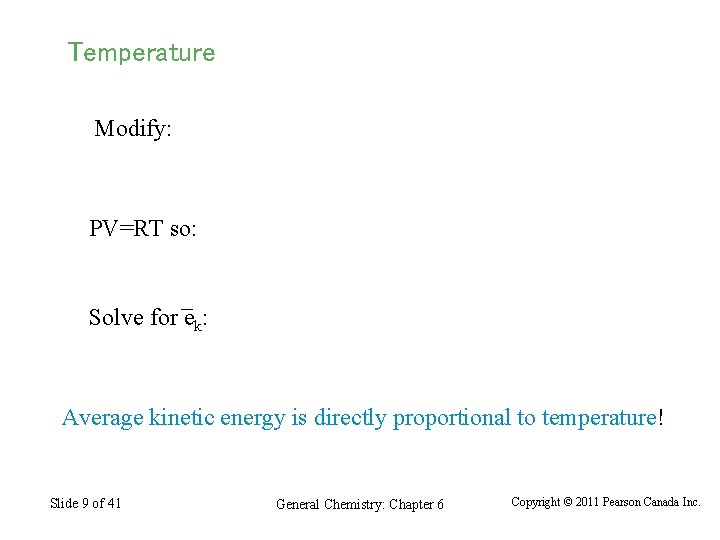
Temperature Modify: PV=RT so: Solve for ek: Average kinetic energy is directly proportional to temperature! Slide 9 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

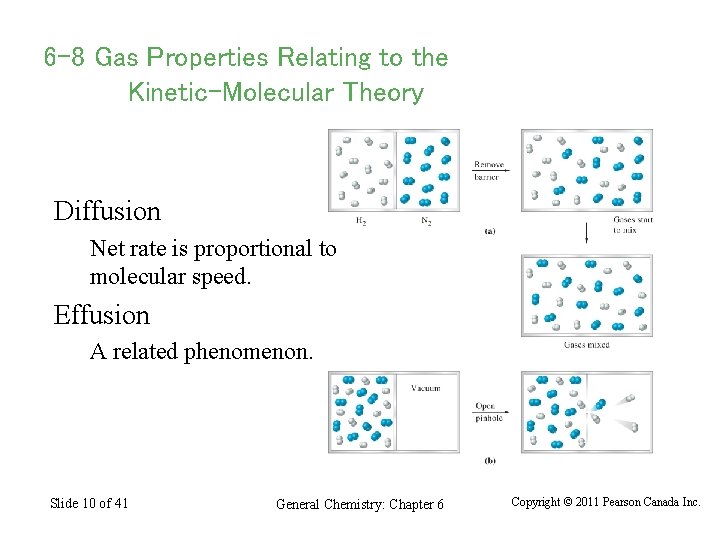
6 -8 Gas Properties Relating to the Kinetic-Molecular Theory Diffusion Net rate is proportional to molecular speed. Effusion A related phenomenon. Slide 10 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

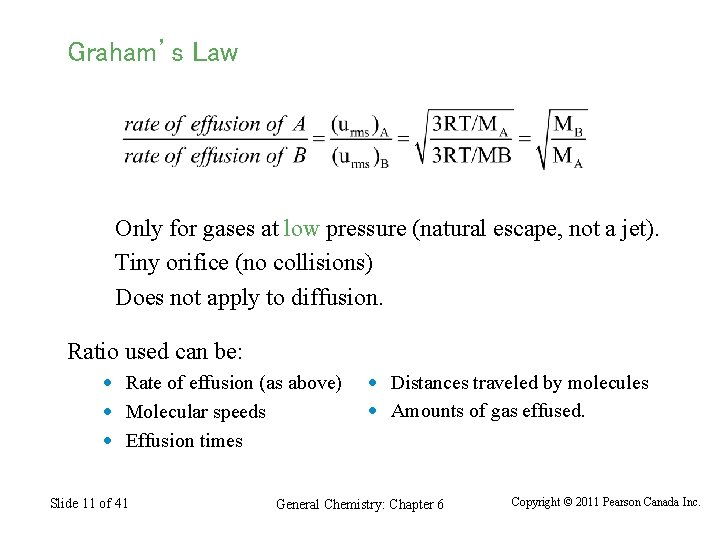
Graham’s Law Only for gases at low pressure (natural escape, not a jet). Tiny orifice (no collisions) Does not apply to diffusion. Ratio used can be: · Rate of effusion (as above) · Molecular speeds · Effusion times Slide 11 of 41 · Distances traveled by molecules · Amounts of gas effused. General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

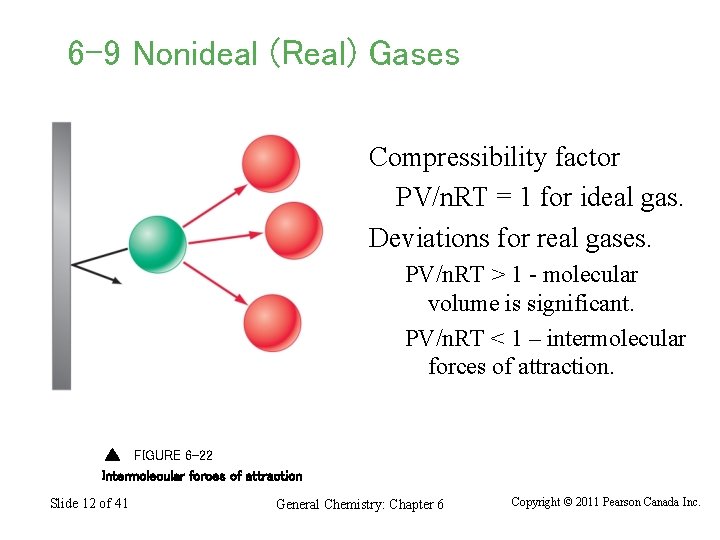
6 -9 Nonideal (Real) Gases Compressibility factor PV/n. RT = 1 for ideal gas. Deviations for real gases. PV/n. RT > 1 - molecular volume is significant. PV/n. RT < 1 – intermolecular forces of attraction. FIGURE 6 -22 Intermolecular forces of attraction Slide 12 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

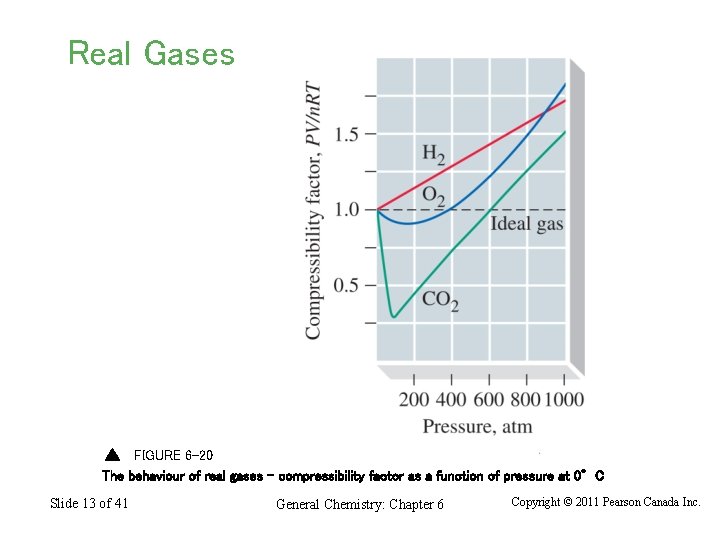
Real Gases FIGURE 6 -20 The behaviour of real gases – compressibility factor as a function of pressure at 0°C Slide 13 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

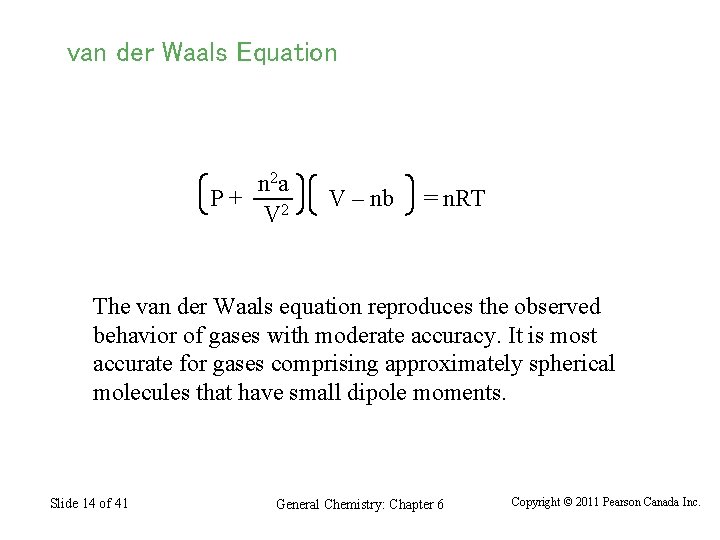
van der Waals Equation n 2 a P+ V 2 V – nb = n. RT The van der Waals equation reproduces the observed behavior of gases with moderate accuracy. It is most accurate for gases comprising approximately spherical molecules that have small dipole moments. Slide 14 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.

Slide 15 of 41 General Chemistry: Chapter 1 Copyright © 2011 Pearson Canada Inc.

End of Chapter Questions A problem is like a knot in a ball of wool: If you pull hard on any loop: The knot will only tighten. The solution (undoing the knot) will not be achieved. If you pull lightly on one loop and then another: You gradually loosen the knot. As more loops are loosened it becomes easier to undo the subsequent ones. Don’t pull too hard on any one piece of information in your problem, it tightens. Slide 16 of 41 General Chemistry: Chapter 6 Copyright © 2011 Pearson Canada Inc.
 Sodyum metalinin su ile tepkimesi
Sodyum metalinin su ile tepkimesi General chemistry ders notları
General chemistry ders notları Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Red herring examples in politics
Red herring examples in politics Red herring fallacy
Red herring fallacy Red herring fallacy
Red herring fallacy False analogy definition
False analogy definition Red herring logical fallacy definition
Red herring logical fallacy definition No true scotsman
No true scotsman Contoh false dilemma
Contoh false dilemma Cod vs herring
Cod vs herring Red herring fallacy in the crucible
Red herring fallacy in the crucible Herring bodies pars nervosa
Herring bodies pars nervosa