Enzymes Regulatory enzymes are usually the enzymes that




- Slides: 28

Enzymes �Regulatory enzymes are usually the enzymes that are the rate-limiting, or committed step, in a pathway, meaning that after this step a particular reaction pathway will go to completion �There are five primary forms of enzyme regulation: substrate availability, allosteric, post-translational modification, interaction with control proteins

Properties of Enzymes �In general, chemical reactions that release energy can occur without input of energy �The oxidation of glucose releases energy, but the reaction does not occur without an input of energy �Activation energy: the energy required to start such a reaction �Enzymes lower the activation energy so reactions can occur at mild temperatures in living cells

Enzymes �Provide a surface on which reactions take place �Active site: the area on the enzyme surface where the enzyme forms a loose association with the substrate �Substrate: the substance on which the enzyme acts �Enzyme-substrate complex: formed when the substrate molecule collides with the active site of its enzyme �Enzymes generally have a high degree of specificity �Endoenzymes (intracellular)/exoenzymes (extracellular)

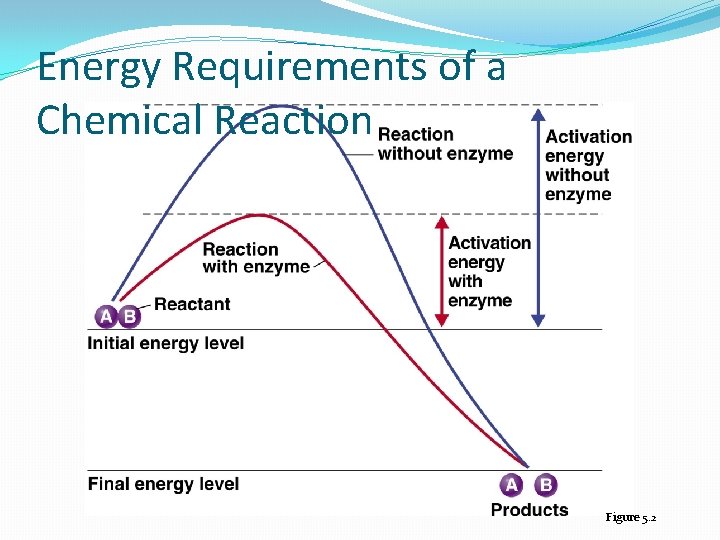
Energy Requirements of a Chemical Reaction Figure 5. 2

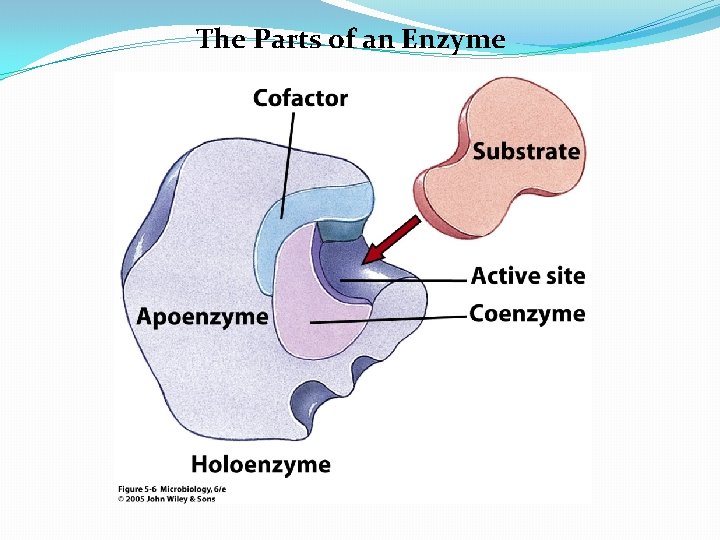
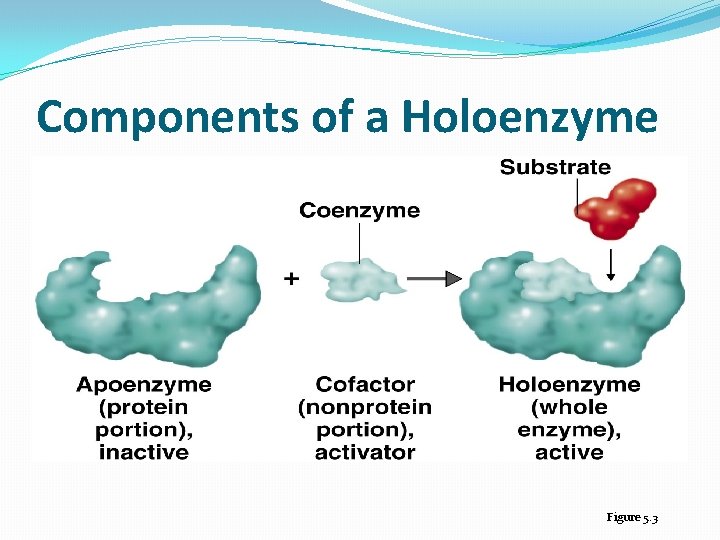
Enzyme Components �Biological catalysts �Specific for a chemical reaction; not used up in that reaction �Apoenzyme: Protein �Cofactor: Nonprotein component �Coenzyme: Organic cofactor �Holoenzyme: Apoenzyme plus cofactor

The Parts of an Enzyme

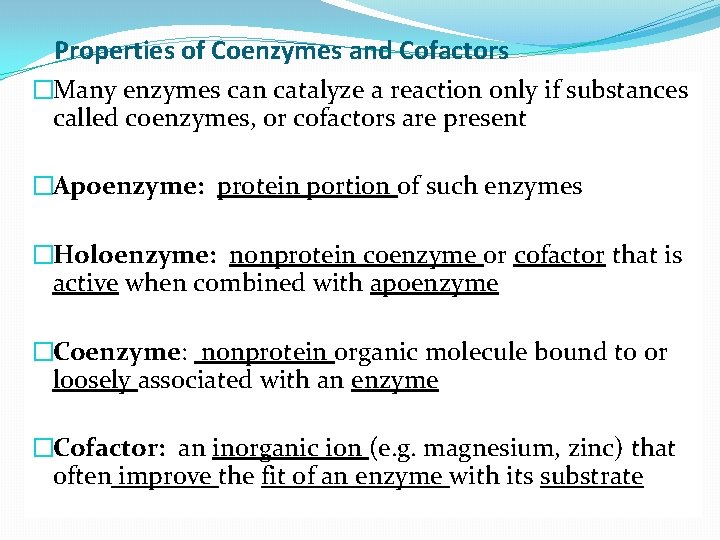
Properties of Coenzymes and Cofactors �Many enzymes can catalyze a reaction only if substances called coenzymes, or cofactors are present �Apoenzyme: protein portion of such enzymes �Holoenzyme: nonprotein coenzyme or cofactor that is active when combined with apoenzyme �Coenzyme: nonprotein organic molecule bound to or loosely associated with an enzyme �Cofactor: an inorganic ion (e. g. magnesium, zinc) that often improve the fit of an enzyme with its substrate

Components of a Holoenzyme Figure 5. 3

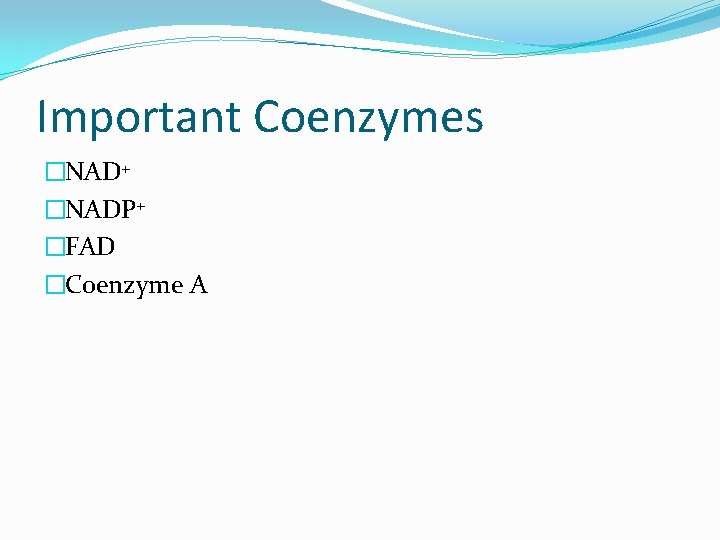
Important Coenzymes �NAD+ �NADP+ �FAD �Coenzyme A

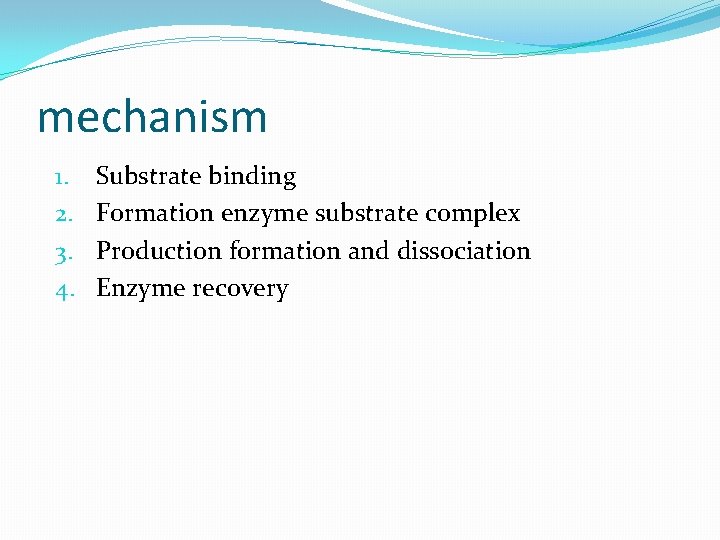
mechanism 1. 2. 3. 4. Substrate binding Formation enzyme substrate complex Production formation and dissociation Enzyme recovery

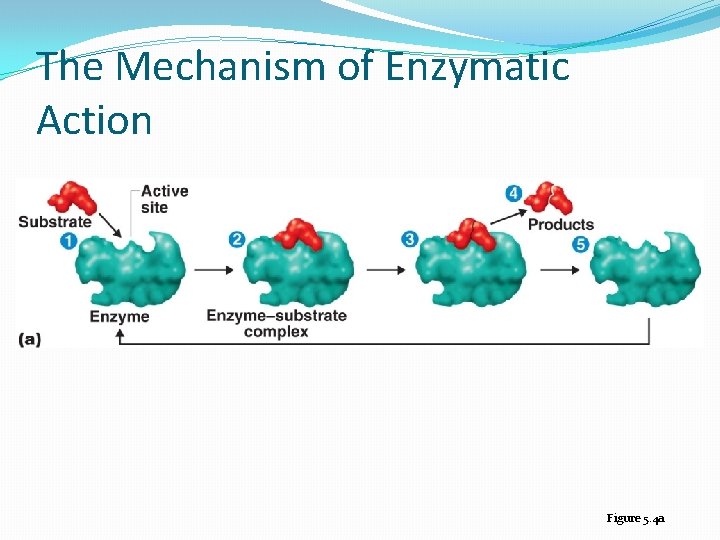
The Mechanism of Enzymatic Action Figure 5. 4 a

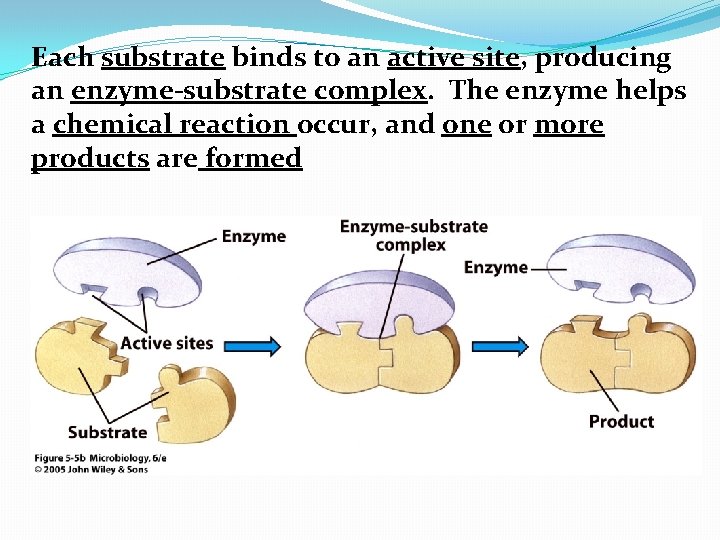
Each substrate binds to an active site, producing an enzyme-substrate complex. The enzyme helps a chemical reaction occur, and one or more products are formed

Enzyme Classification �Oxidoreductase: Oxidation-reduction reactions �Transferase: Transfer functional groups �Hydrolase: Hydrolysis �Lyase: Removal of atoms without hydrolysis �Isomerase: Rearrangement of atoms �Ligase: Joining of molecules, uses ATP

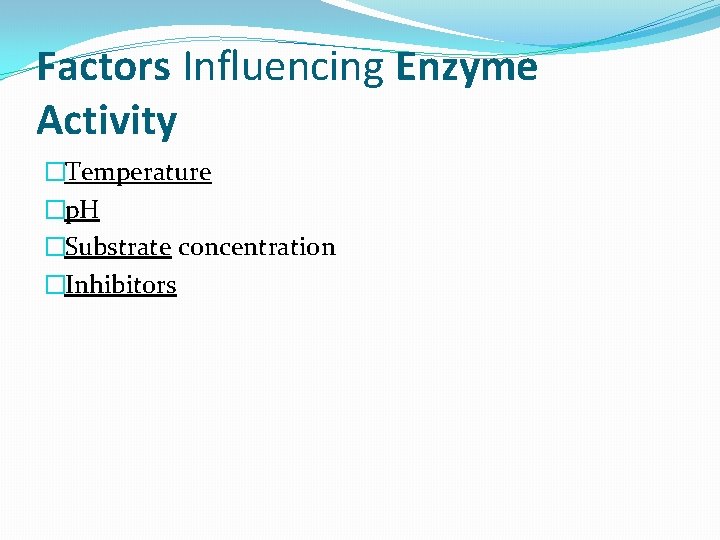
Factors Influencing Enzyme Activity �Temperature �p. H �Substrate concentration �Inhibitors

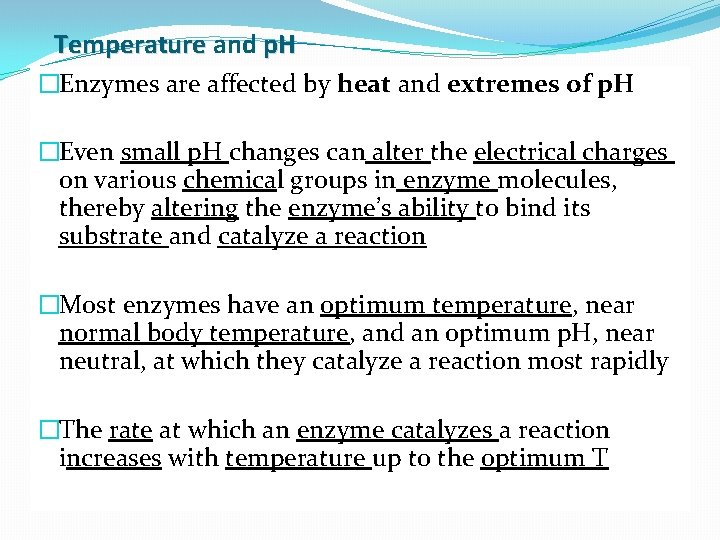
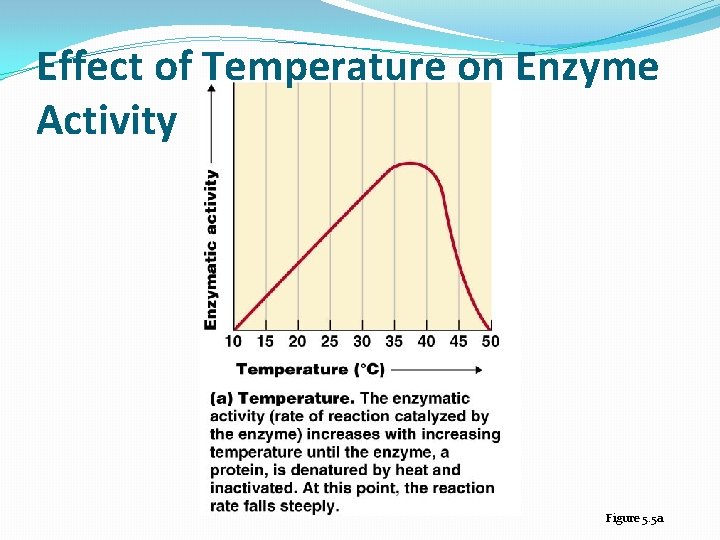
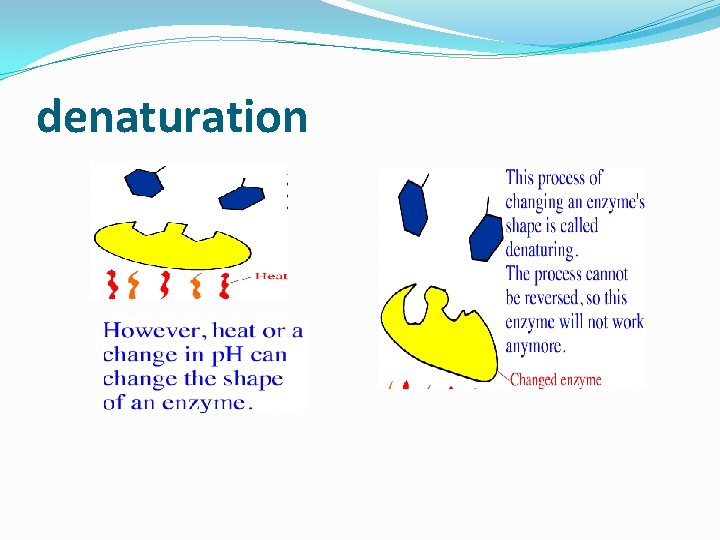
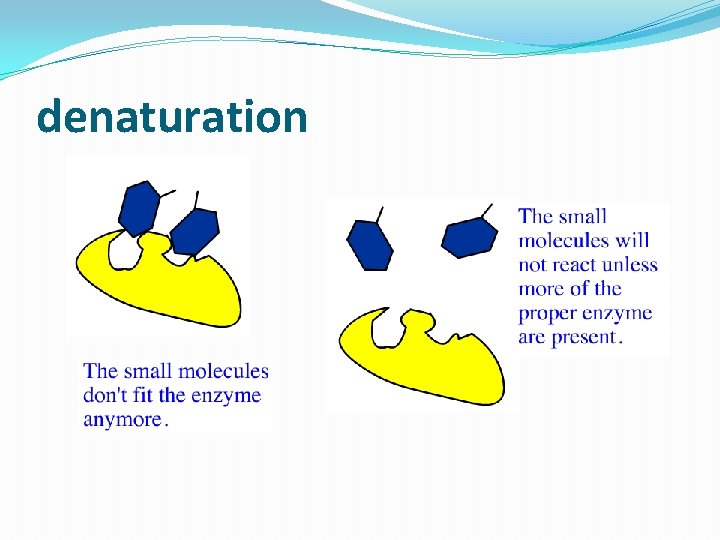
Temperature and p. H �Enzymes are affected by heat and extremes of p. H �Even small p. H changes can alter the electrical charges on various chemical groups in enzyme molecules, thereby altering the enzyme’s ability to bind its substrate and catalyze a reaction �Most enzymes have an optimum temperature, near normal body temperature, and an optimum p. H, near neutral, at which they catalyze a reaction most rapidly �The rate at which an enzyme catalyzes a reaction increases with temperature up to the optimum T

Effect of Temperature on Enzyme Activity Figure 5. 5 a

Effect of p. H on Enzyme Activity Figure 5. 5 b

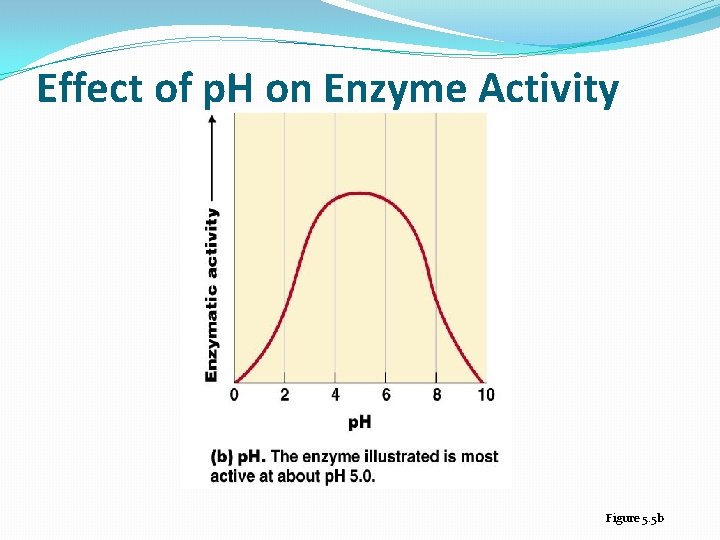
Effect of Substrate Concentration on Enzyme Activity Figure 5. 5 c

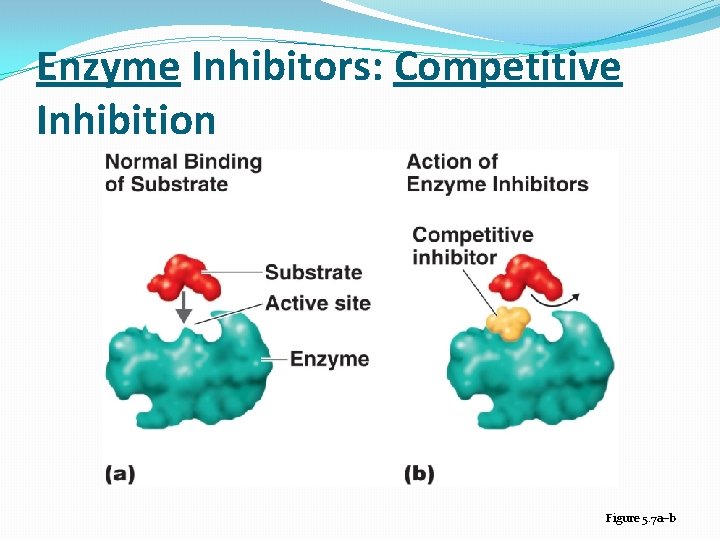
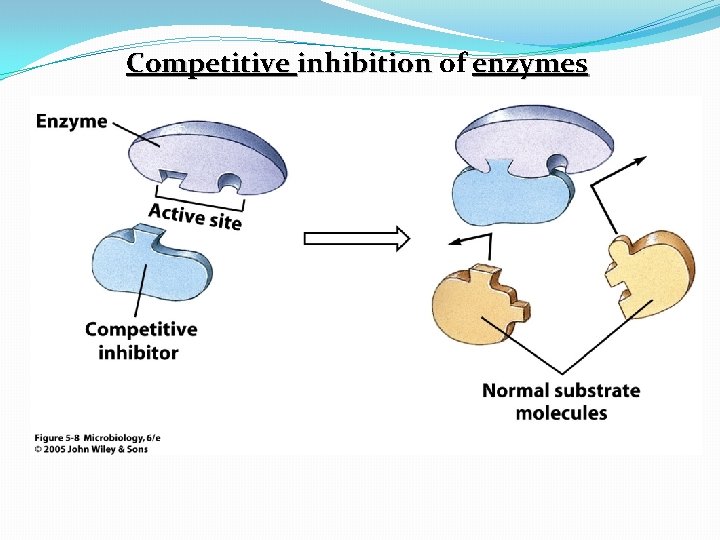
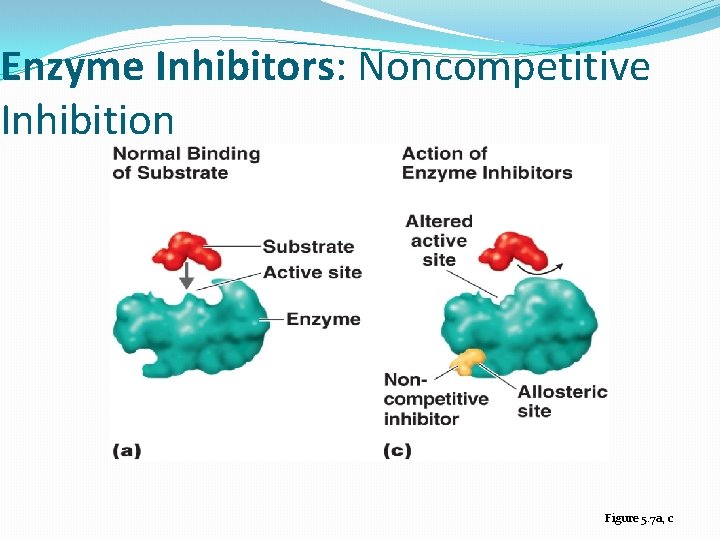
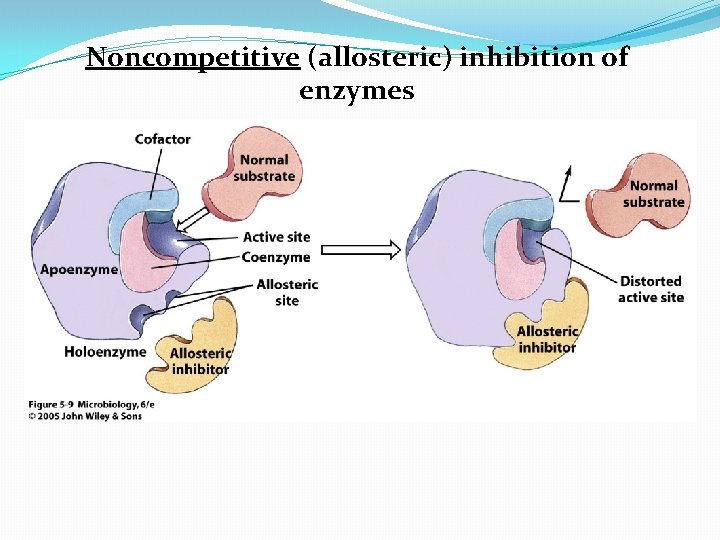
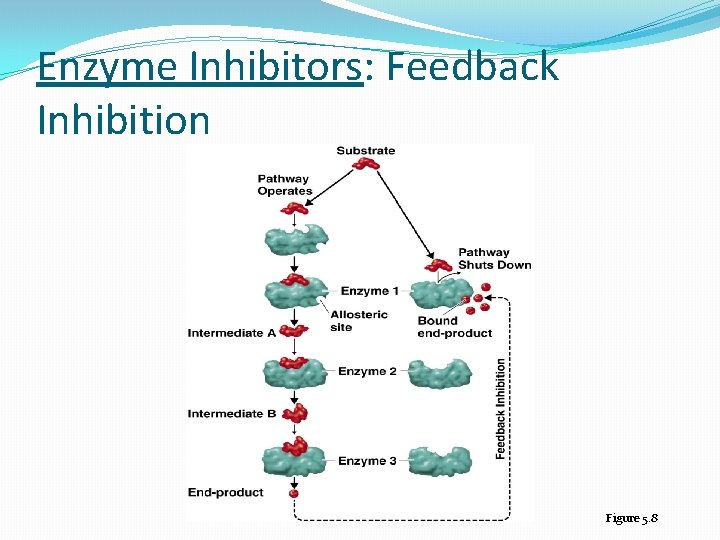
Enzyme Inhibition �Competitive inhibitor: A molecule similar in structure to a substrate can bind to an enzyme’s active site and compete with substrate �Noncompetitive inhibitors: attach to the enzyme at an allosteric site, which is a site other than the active site � noncompetitive inhibitors: distort the tertiary protein structure and alter the shape of the active site �Feedback inhibition: regulates the rate of many metabolic pathways when an end product of a pathway accumulates and binds to and inactivates the first enzyme in the metabolic pathway

Enzyme Inhibitors: Competitive Inhibition Figure 5. 7 a–b

Competitive inhibition of enzymes

Allosteric regulation of enzyme activity � Allosteric regulation = the activation or inhibition of an enzyme’s activity due to binding of an effectors molecule at a regulatory site that is distinct from the active site of the enzyme � Allosteric regulators generally act by increasing or decreasing the enzyme’s affinity for the substrate

Enzyme Inhibitors: Noncompetitive Inhibition Figure 5. 7 a, c

Noncompetitive (allosteric) inhibition of enzymes

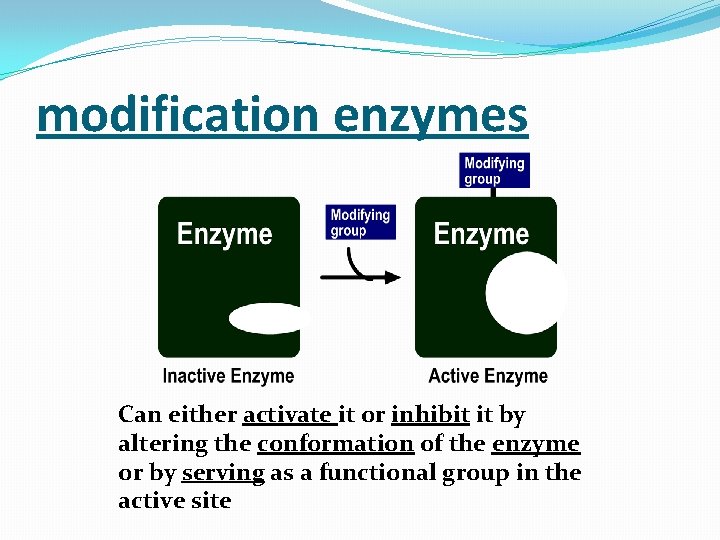
modification enzymes Can either activate it or inhibit it by altering the conformation of the enzyme or by serving as a functional group in the active site

denaturation

denaturation

Enzyme Inhibitors: Feedback Inhibition Figure 5. 8
 Insidan region jh
Insidan region jh Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown
Phân độ lown Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất độ 3
Block nhĩ thất độ 3 Thẻ vin
Thẻ vin Cái miệng nó xinh thế
Cái miệng nó xinh thế Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là V cc
V cc Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Lời thề hippocrates
Lời thề hippocrates Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em đại từ thay thế
đại từ thay thế Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thang điểm glasgow
Thang điểm glasgow Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dot
Dot