Energetics Year 11 Chemistry R Slider Exothermic and































- Slides: 58

Energetics Year 11 Chemistry R Slider

Exothermic and Endothermic

Chemical Energy. Enthalpy Kinetic energy is the energy of motion • Particles that make up matter are constantly in motion Potential energy is stored energy (no motion) • Every bond between atoms and ions has stored energy within the bonds • This stored energy is known as chemical potential energy Total energy = Kinetic energy +Potential Energy (known as Enthalpy or heat content– symbol H)

Change in Enthalpy (ΔH) Because it is difficult to measure the amount of energy in individual reactants or products, Chemists measure the change in enthalpy (ΔH) that occurs when a reaction takes place. ΔH is the difference in the enthalpy of the products and the enthalpy of the reactants. Enthalpy changes result from bonds breaking and new bonds reforming. This breaking and reforming of bonds results in energy changes within the system which are measured as temperature changes Note: Standard enthalpy changes are measured under standard temperature (298 K) and pressure (101. 3 k. Pa)

Endothermic or Exothermic? Endothermic Some chemical reactions absorb heat from the surroundings so the products of the reaction contain more heat. The surroundings feel colder, ΔH = + Examples: photosynthesis, cold packs Exothermic Some chemical reactions release heat to the surroundings so the products have less heat than the reactants The surroundings feel hotter, ΔH = Examples: combustion, neutralisation reactions

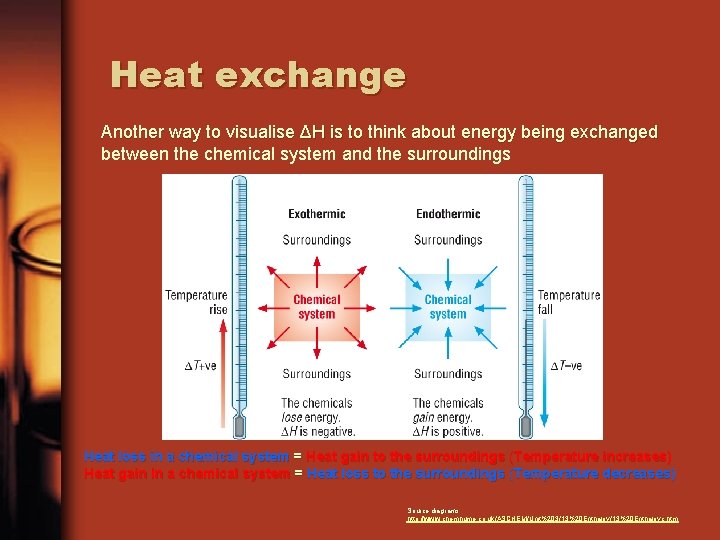
Heat exchange Another way to visualise ΔH is to think about energy being exchanged between the chemical system and the surroundings Heat loss in a chemical system = Heat gain to the surroundings (Temperature increases) Heat gain in a chemical system = Heat loss to the surroundings (Temperature decreases) Source diagrams: http: //www. chemhume. co. uk/ASCHEM/Unit%203/13%20 Enthalpyc. htm

Combustion

Combustion is exothermic Combustion is the process of burning Most often, the combustion of a material involves its combination with oxygen gas. Because combustion reactions release energy in the form of heat and light, they are exothermic. Example: The combustion of methane (natural gas) in oxygen: CH 4 + 2 O 2 CO 2 + 2 H 2 O ∆ Hrxn = -832 k. J/mol (exothermic) Fire in the Penola Forest (SA), Ash Wednesday 1983 (Source: www. austehc. unimelb. edu. au/fam/1611_image. html)

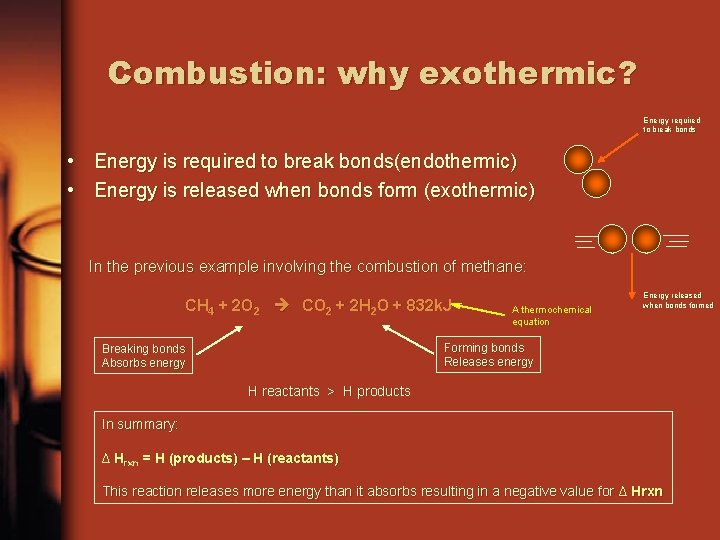
Combustion: why exothermic? Energy required to break bonds • Energy is required to break bonds(endothermic) • Energy is released when bonds form (exothermic) In the previous example involving the combustion of methane: CH 4 + 2 O 2 CO 2 + 2 H 2 O + 832 k. J A thermochemical equation Energy released when bonds formed Forming bonds Releases energy Breaking bonds Absorbs energy H reactants > H products In summary: ∆ Hrxn = H (products) – H (reactants) This reaction releases more energy than it absorbs resulting in a negative value for ∆ Hrxn

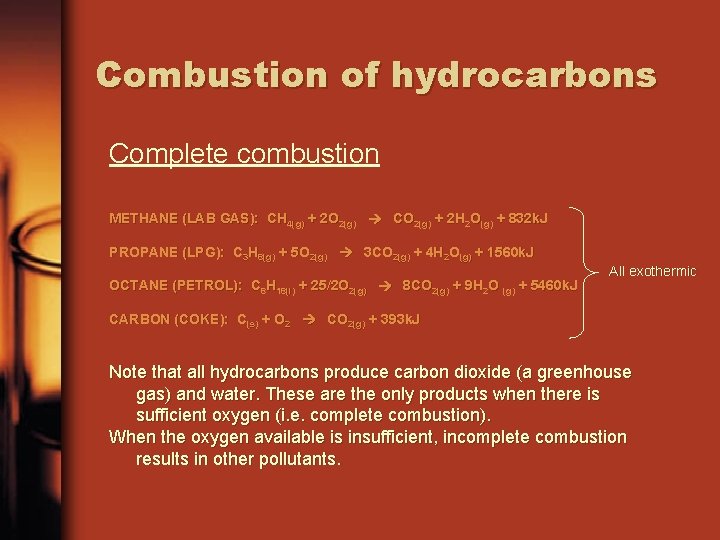
Combustion of hydrocarbons Complete combustion METHANE (LAB GAS): CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) + 832 k. J PROPANE (LPG): C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) + 1560 k. J OCTANE (PETROL): C 8 H 18(l) + 25/2 O 2(g) 8 CO 2(g) + 9 H 2 O (g) + 5460 k. J All exothermic CARBON (COKE): C(s) + O 2 CO 2(g) + 393 k. J Note that all hydrocarbons produce carbon dioxide (a greenhouse gas) and water. These are the only products when there is sufficient oxygen (i. e. complete combustion). When the oxygen available is insufficient, incomplete combustion results in other pollutants.

Combustion of hydrocarbons Incomplete combustion As the quantity of oxygen decreases, combustion of hydrocarbons becomes more and more incomplete, leading to the production of the pollutants carbon monoxide and carbon (soot). Consider the hydrocarbon pentane: Complete: C 5 H 12(l) + 8 O 2(g) 5 CO 2(g) + 6 H 2 O (g) Incomplete (less O 2): C 5 H 12(l) + 6 O 2(g) 4 CO(g) + CO 2(g) + 6 H 2 O (g) Incomplete (even less O 2): C 5 H 12(l) + 4 O 2(g) 2 CO(g) + 3 C(s) + 6 H 2 O (g) Incomplete combustion products Incomplete combustion of hydrocarbons can produce CO and C.

Other Combustion Pollutants Sulfur Dioxide/Trioxide Sulfur is an impurity in fossil fuels, mainly coal (up to 5%). When sulfur burns in air sulfur dioxide is produced: S(s) + O 2(g) SO 2(g) Sulfur trioxide can be produced by further oxidation of SO 2 2 SO 2(g) + O 2(g) 2 SO 3(g) These two gases can combine with water in the atmosphere to produce acid rain: SO 2(g) + H 2 O(l) H 2 SO 3(aq) (sulfurous acid) SO 3(g) + H 2 O(l) H 2 SO 4(aq) (sulfuric acid) www. robl. w 1. com Acid rain effects

Other Combustion Pollutants Oxides of Nitrogen (NOx) Nitrogen and oxygen in the air can react at high temperatures that are present inside car engines: N 2(g) + O 2(g) 2 NO (g) 2 NO(g) + O 2(g) 2 NO 2(g) Nitrogen dioxide produces the brown haze known as photochemical smog. This causes respiratory problems in many people. In addition, NO 2 can react with UV light to produce toxic ozone O 3. NO 2(g) + UV 2 NO 2(g) + O(g) + O 2(g) O 3(g) Photochemical smog www. birmingham. gov. uk


Controlling Pollution • Keep car engines tuned properly so that they are at maximum efficiency and incomplete combustion is minimised. • Catalytic converters mounted to motor vehicle exhaust systems remove unburned hydrocarbons and oxides of nitrogen. 2 NO(g) + 2 CO(g) N 2(g) + 2 CO 2(g) (more detailed chemistry on next slide) • Alternative fuels and fuel systems

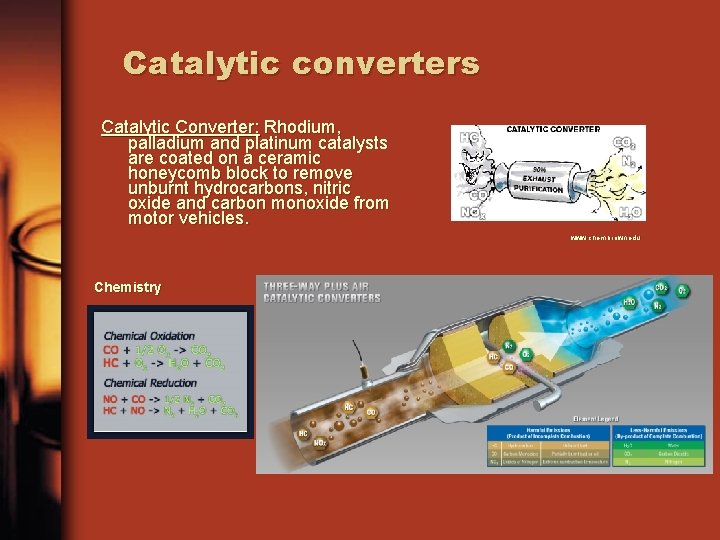
Catalytic converters Catalytic Converter: Rhodium, palladium and platinum catalysts are coated on a ceramic honeycomb block to remove unburnt hydrocarbons, nitric oxide and carbon monoxide from motor vehicles. www. chem. brown. edu Chemistry

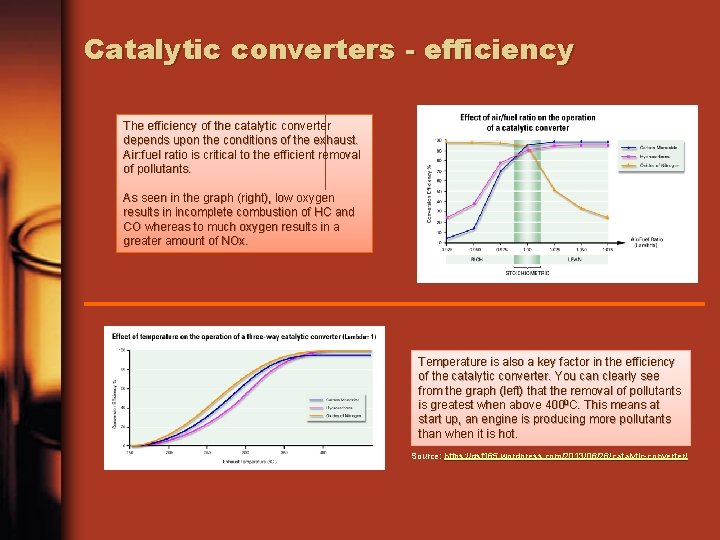
Catalytic converters - efficiency The efficiency of the catalytic converter depends upon the conditions of the exhaust. Air: fuel ratio is critical to the efficient removal of pollutants. As seen in the graph (right), low oxygen results in incomplete combustion of HC and CO whereas to much oxygen results in a greater amount of NOx. Temperature is also a key factor in the efficiency of the catalytic converter. You can clearly see from the graph (left) that the removal of pollutants is greatest when above 4000 C. This means at start up, an engine is producing more pollutants than when it is hot. Source: https: //gsf 165. wordpress. com/2013/06/26/catalytic-converter/

Energy profile diagrams

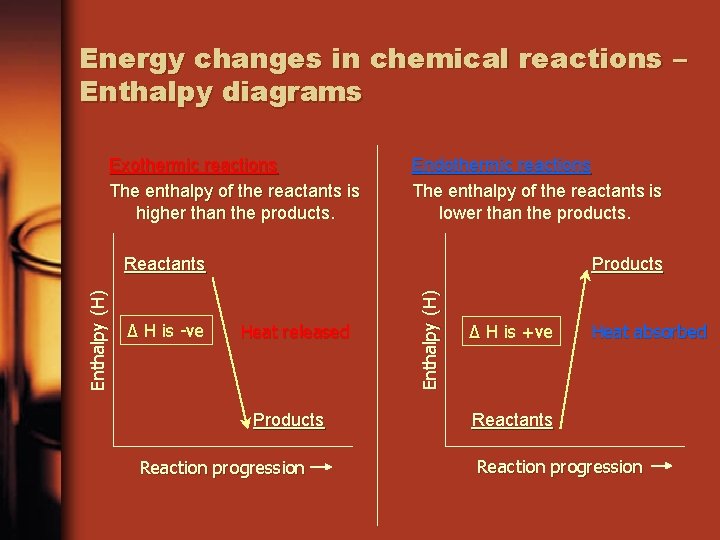
Energy changes in chemical reactions – Enthalpy diagrams Exothermic reactions The enthalpy of the reactants is higher than the products. Endothermic reactions The enthalpy of the reactants is lower than the products. ∆ H is -ve Products Heat released Products Reaction progression Enthalpy (H) Reactants ∆ H is +ve Heat absorbed Reactants Reaction progression

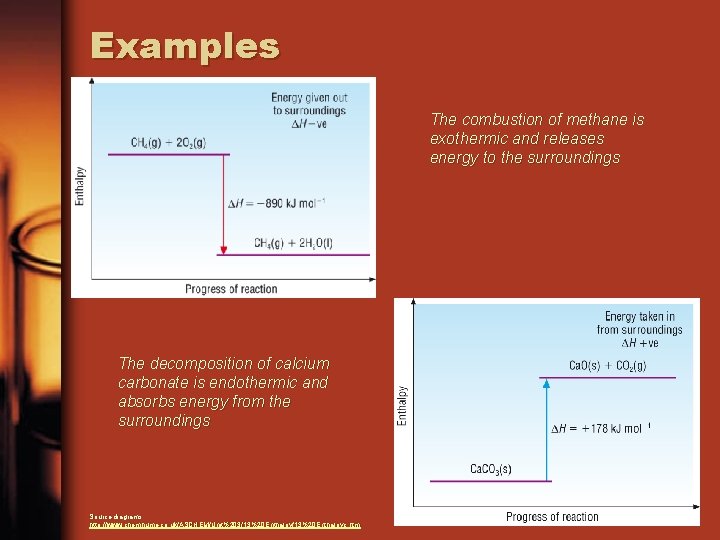
Examples The combustion of methane is exothermic and releases energy to the surroundings The decomposition of calcium carbonate is endothermic and absorbs energy from the surroundings Source diagrams: http: //www. chemhume. co. uk/ASCHEM/Unit%203/13%20 Enthalpyc. htm

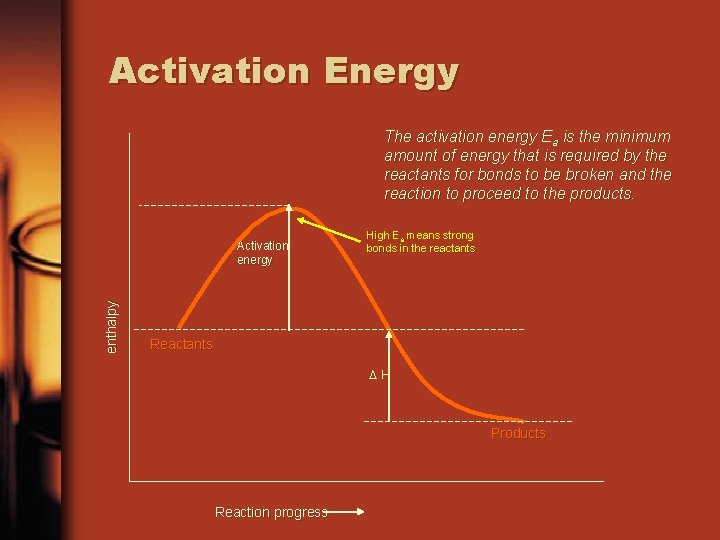
Activation Energy The activation energy Ea is the minimum amount of energy that is required by the reactants for bonds to be broken and the reaction to proceed to the products. enthalpy Activation energy High Ea means strong bonds in the reactants Reactants ∆H Products Reaction progress

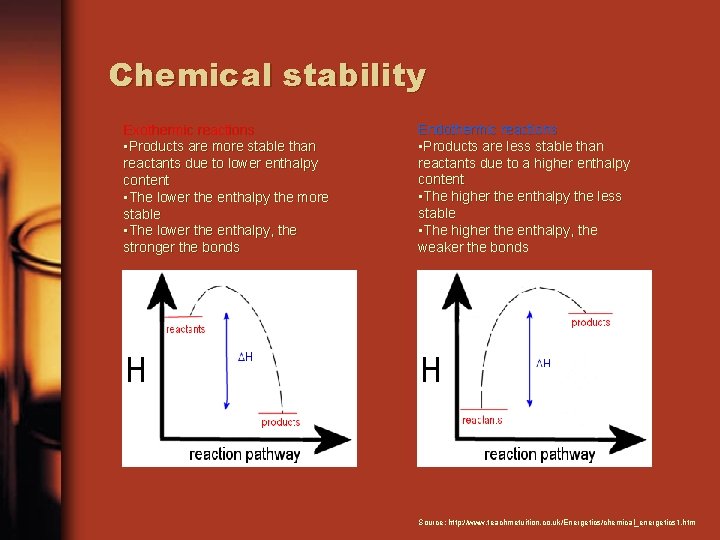
Chemical stability Exothermic reactions • Products are more stable than reactants due to lower enthalpy content • The lower the enthalpy the more stable • The lower the enthalpy, the stronger the bonds Endothermic reactions • Products are less stable than reactants due to a higher enthalpy content • The higher the enthalpy the less stable • The higher the enthalpy, the weaker the bonds Source: http: //www. teachmetuition. co. uk/Energetics/chemical_energetics 1. htm

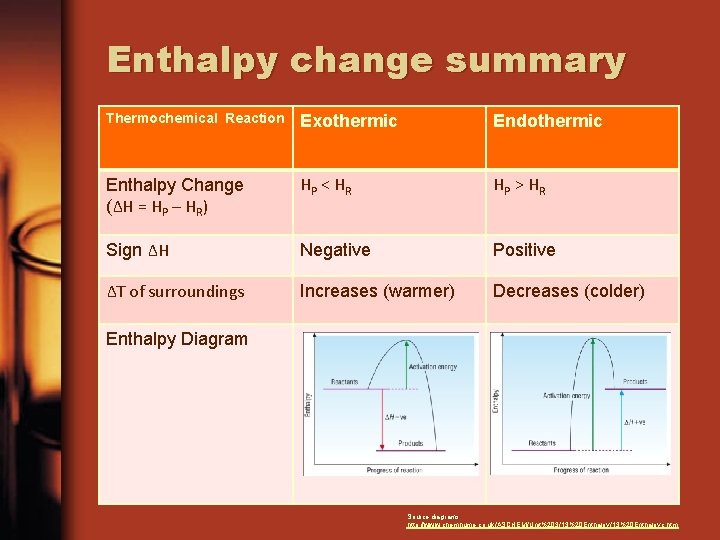
Enthalpy change summary Thermochemical Reaction Exothermic Endothermic Enthalpy Change (ΔH = HP – HR) HP < H R HP > H R Sign ΔH Negative Positive ΔT of surroundings Increases (warmer) Decreases (colder) Enthalpy Diagram Source diagrams: http: //www. chemhume. co. uk/ASCHEM/Unit%203/13%20 Enthalpyc. htm

Exercises 1. Classify each of the reactions below as endothermic or exothermic a) C 6 H 12 O 6(aq) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l) ΔH = -2801 k. J mol-1 b) 6 CO 2(g) + 6 H 2 O(l) C 6 H 12 O 6(aq) + 6 O 2(g) ΔH = +2801 k. J mol-1 c) H 2(g) + 1/2 O 2(g) H 2 O(l) + 286 k. Jmol-1 2. When a spark is introduced into a vessel containing a mixture of hydrogen and oxygen gases, they react explosively. a) What is the role of the spark in this reaction? b) What bonds are broken when this reaction is initiated? c) What bonds are generated when the products are formed? d) Is the reaction endothermic or exothermic? Justify your answer. 3. 10. 0 g of ammonium nitrate is dissolved in 100 cm 3 of water and the temperature of the solution decreases from 19. 00 C to 10. 500 C. a) Which is greater, the enthalpy of the reactants or the products? b) Is the reaction endothermic or exothermic? c) What chemical bonds were broken when the ammonium nitrate dissolved in water? 4. Consider the reaction represented by the following equation: A + 2 B C In this reaction, the total enthalpy of the reactants is 180 k. Jmol-1, the total enthalpy for the products is 10 k. J. mol-1 and the activation energy for the forward reaction is 120 k. J. mol-1. a) Draw a diagram for the energy profile for this reaction. Label the diagram clearly to show ΔH and Ea. b) State whether the forward reaction is endothermic or exothermic c) Calculate the change in enthalpy d) Calculate the amount of energy released as the products are formed e) Calculate the activation energy of the reverse reaction: C A 2 B

Exercises - Answers 1. a) exothermic b) endothermic c) exothermic 2. a)spark is to overcome the activation energy b) H-H and O=O covalent bonds broken c) O-H bonds are made during the reaction d) exothermic as energy is released 3. a) products>reactants b) endothermic c) ionic bonds between NH 4+ and NO 34. a) diagram should show exothermic forward rxn b) exothermic c) ΔH = 10 – 180 k. J mol-1 = -170 k. J mol-1 d) 290 k. J mol -1 e) 290 k. J mol-1

Calculating Enthalpy

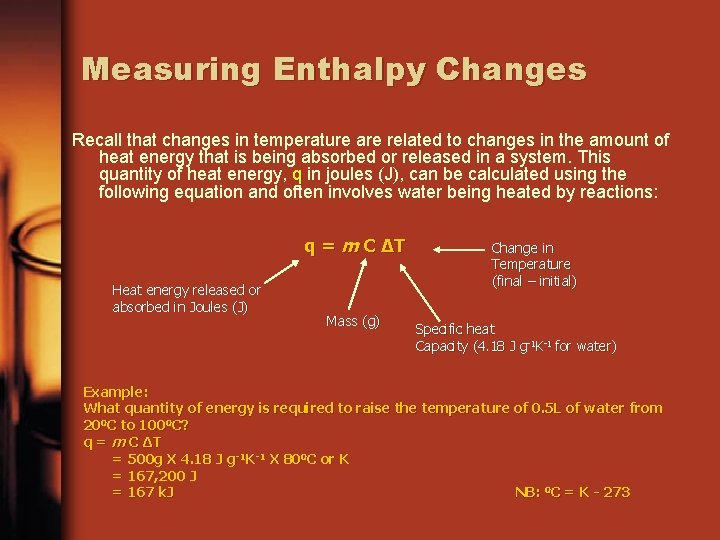
Measuring Enthalpy Changes Recall that changes in temperature are related to changes in the amount of heat energy that is being absorbed or released in a system. This quantity of heat energy, q in joules (J), can be calculated using the following equation and often involves water being heated by reactions: q = m C ∆T Heat energy released or absorbed in Joules (J) Mass (g) Change in Temperature (final – initial) Specific heat Capacity (4. 18 J g-1 K-1 for water) Example: What quantity of energy is required to raise the temperature of 0. 5 L of water from 200 C to 1000 C? q = m C ∆T = 500 g X 4. 18 J g-1 K-1 X 800 C or K = 167, 200 J = 167 k. J NB: 0 C = K - 273

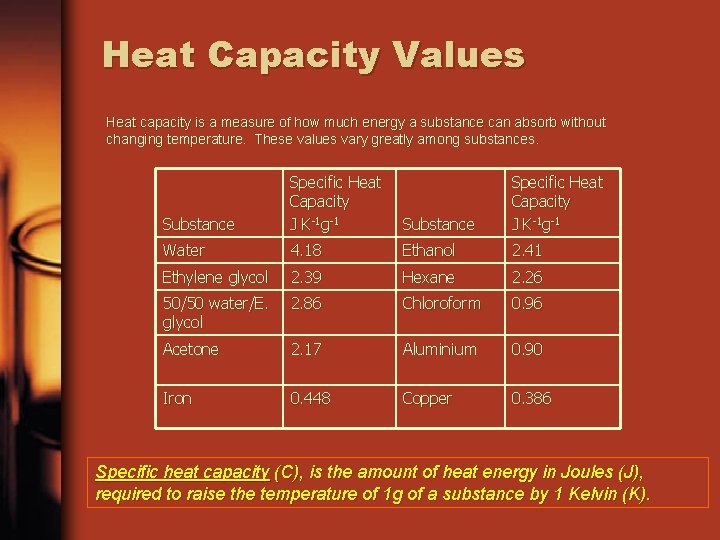
Heat Capacity Values Heat capacity is a measure of how much energy a substance can absorb without changing temperature. These values vary greatly among substances. Substance Specific Heat Capacity J K-1 g-1 Water 4. 18 Ethanol 2. 41 Ethylene glycol 2. 39 Hexane 2. 26 50/50 water/E. glycol 2. 86 Chloroform 0. 96 Acetone 2. 17 Aluminium 0. 90 Iron 0. 448 Copper 0. 386 Specific heat capacity (C), is the amount of heat energy in Joules (J), required to raise the temperature of 1 g of a substance by 1 Kelvin (K).

Practice Questions 1. A Bunsen burner was used to heat 100 cm 3 of water for 10 min. The temperature of the water increased from 15. 00 C to 80. 00 C. Determine the heat energy change of the water. (note: the density of water is 1. 0 g cm-3) 2. The same Bunsen burner was used to heat a 500 g block of copper for 10 minutes. Assuming the same amount of energy is transferred from the Bunsen burner to the block as in question 1, determine the highest temperature that the block could reach if the starting temperature was 15. 00 C. 3. Explain the difference in the temperatures considering the same amount of energy was put into the water and the copper Solutions: 1)27. 2 k. J 2) 1560 C 3) The water has a much higher heat capacity than the copper which means the water can absorb much more heat energy before changing temperature.

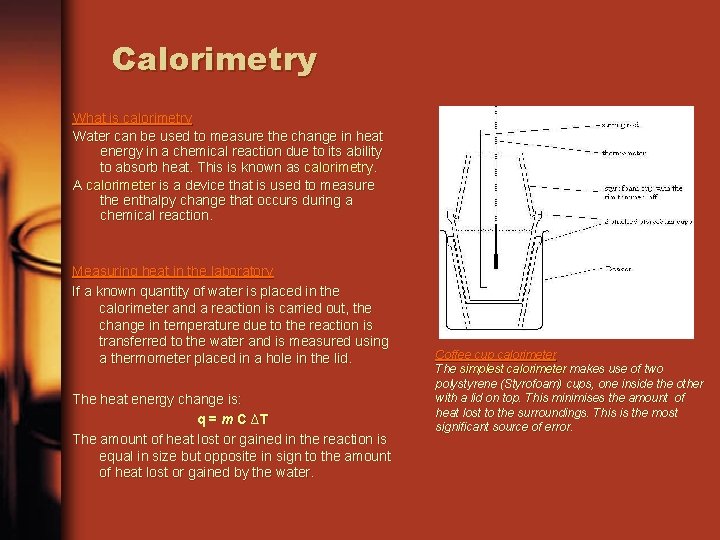
Calorimetry What is calorimetry Water can be used to measure the change in heat energy in a chemical reaction due to its ability to absorb heat. This is known as calorimetry. A calorimeter is a device that is used to measure the enthalpy change that occurs during a chemical reaction. Measuring heat in the laboratory If a known quantity of water is placed in the calorimeter and a reaction is carried out, the change in temperature due to the reaction is transferred to the water and is measured using a thermometer placed in a hole in the lid. The heat energy change is: q = m C ∆T The amount of heat lost or gained in the reaction is equal in size but opposite in sign to the amount of heat lost or gained by the water. Coffee cup calorimeter The simplest calorimeter makes use of two polystyrene (Styrofoam) cups, one inside the other with a lid on top. This minimises the amount of heat lost to the surroundings. This is the most significant source of error.

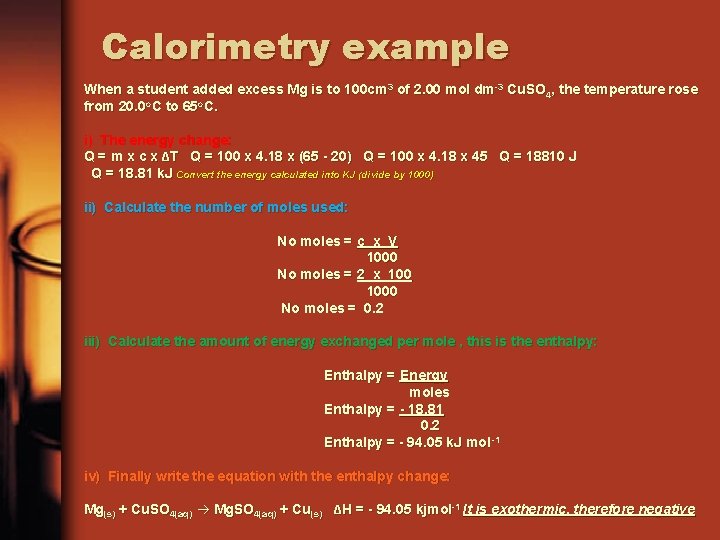
Calorimetry example When a student added excess Mg is to 100 cm 3 of 2. 00 mol dm-3 Cu. SO 4, the temperature rose from 20. 0 o. C to 65 o. C. i) The energy change: Q = m x c x ΔT Q = 100 x 4. 18 x (65 - 20) Q = 100 x 4. 18 x 45 Q = 18810 J Q = 18. 81 k. J Convert the energy calculated into KJ (divide by 1000) ii) Calculate the number of moles used: No moles = c x V 1000 No moles = 2 x 1000 No moles = 0. 2 iii) Calculate the amount of energy exchanged per mole , this is the enthalpy: Enthalpy = Energy moles Enthalpy = - 18. 81 0. 2 Enthalpy = - 94. 05 k. J mol -1 iv) Finally write the equation with the enthalpy change: Mg(s) + Cu. SO 4(aq) Mg. SO 4(aq) + Cu(s) ΔH = - 94. 05 kjmol-1 It is exothermic, therefore negative

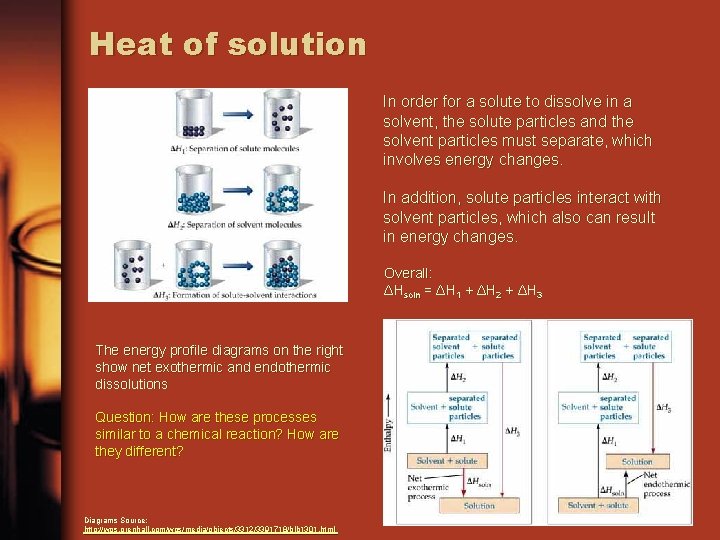
Heat of solution In order for a solute to dissolve in a solvent, the solute particles and the solvent particles must separate, which involves energy changes. In addition, solute particles interact with solvent particles, which also can result in energy changes. Overall: ΔHsoln = ΔH 1 + ΔH 2 + ΔH 3 The energy profile diagrams on the right show net exothermic and endothermic dissolutions Question: How are these processes similar to a chemical reaction? How are they different? Diagrams Source: http: //wps. prenhall. com/wps/media/objects/3312/3391718/blb 1301. html

Heat of Combustion (ΔHc) • Heat of Combustion of a substance is the heat liberated when 1 mole of the substance undergoes complete combustion with oxygen at constant pressure. • Combustion is always exothermic, ΔH is negative. • By definition, the heat of combustion is minus the enthalpy change for the combustion reaction. • By definition, the heat of combustion is a positive value. • Heat of Combustion can be measured experimentally.

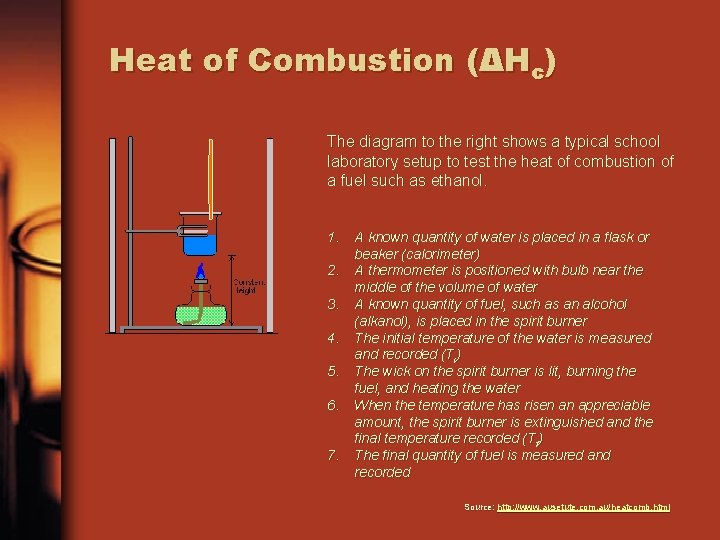
Heat of Combustion (ΔHc) The diagram to the right shows a typical school laboratory setup to test the heat of combustion of a fuel such as ethanol. 1. A known quantity of water is placed in a flask or beaker (calorimeter) 2. A thermometer is positioned with bulb near the middle of the volume of water 3. A known quantity of fuel, such as an alcohol (alkanol), is placed in the spirit burner 4. The initial temperature of the water is measured and recorded (Ti) 5. The wick on the spirit burner is lit, burning the fuel, and heating the water 6. When the temperature has risen an appreciable amount, the spirit burner is extinguished and the final temperature recorded (Tf) 7. The final quantity of fuel is measured and recorded Source: http: //www. ausetute. com. au/heatcomb. html

Heat of Combustion Example A student used the apparatus on the previous slide to determine the heat of combustion of ethanol. Their results for one trial are below: initial water temperature (Ti) = 20 o. C intial mass burner + ethanol = 37. 25 g final water temperature (Tf)= 75 o. C final mass burner + ethanol = 35. 50 g change in temperature = Tf - Ti = 55 o. C mass ethanol used = 1. 75 g Use the results above to determine the heat of combustion for ethanol in k. J/mol assuming 200 cm 3 of water is used in the calorimeter Also, note MM of ethanol is 46 g. mol-1. (Solution on the next slide)

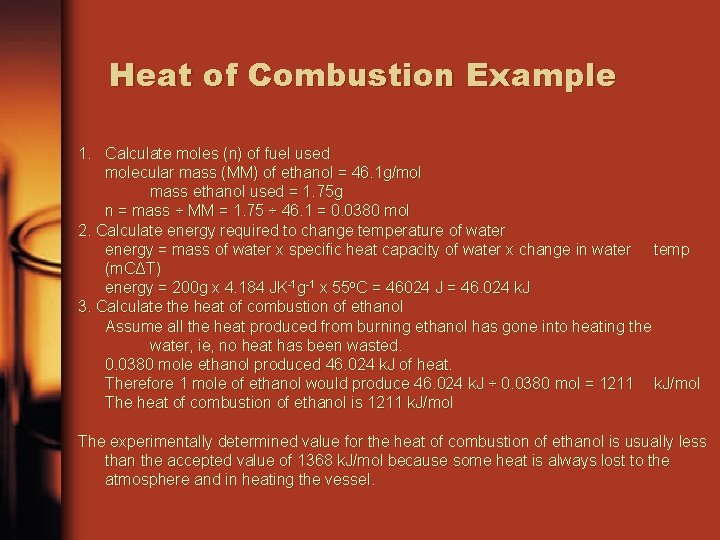
Heat of Combustion Example 1. Calculate moles (n) of fuel used molecular mass (MM) of ethanol = 46. 1 g/mol mass ethanol used = 1. 75 g n = mass ÷ MM = 1. 75 ÷ 46. 1 = 0. 0380 mol 2. Calculate energy required to change temperature of water energy = mass of water x specific heat capacity of water x change in water temp (m. CΔT) energy = 200 g x 4. 184 JK-1 g-1 x 55 o. C = 46024 J = 46. 024 k. J 3. Calculate the heat of combustion of ethanol Assume all the heat produced from burning ethanol has gone into heating the water, ie, no heat has been wasted. 0. 0380 mole ethanol produced 46. 024 k. J of heat. Therefore 1 mole of ethanol would produce 46. 024 k. J ÷ 0. 0380 mol = 1211 k. J/mol The heat of combustion of ethanol is 1211 k. J/mol The experimentally determined value for the heat of combustion of ethanol is usually less than the accepted value of 1368 k. J/mol because some heat is always lost to the atmosphere and in heating the vessel.

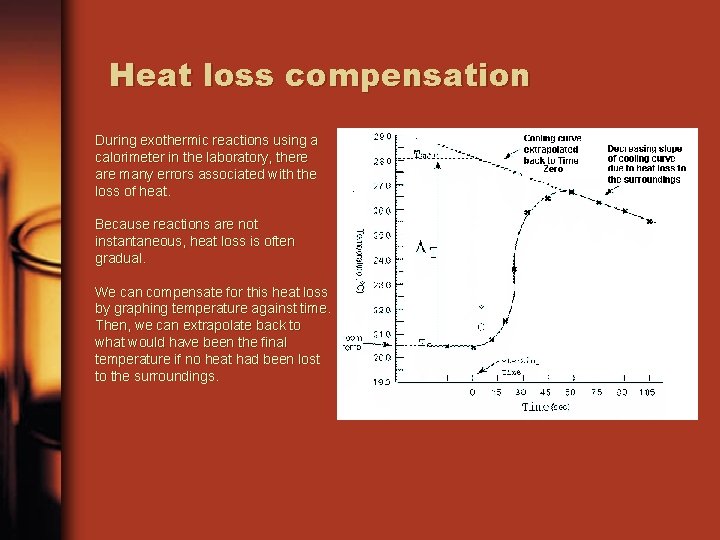
Heat loss compensation During exothermic reactions using a calorimeter in the laboratory, there are many errors associated with the loss of heat. Because reactions are not instantaneous, heat loss is often gradual. We can compensate for this heat loss by graphing temperature against time. Then, we can extrapolate back to what would have been the final temperature if no heat had been lost to the surroundings.

Hess’s Law

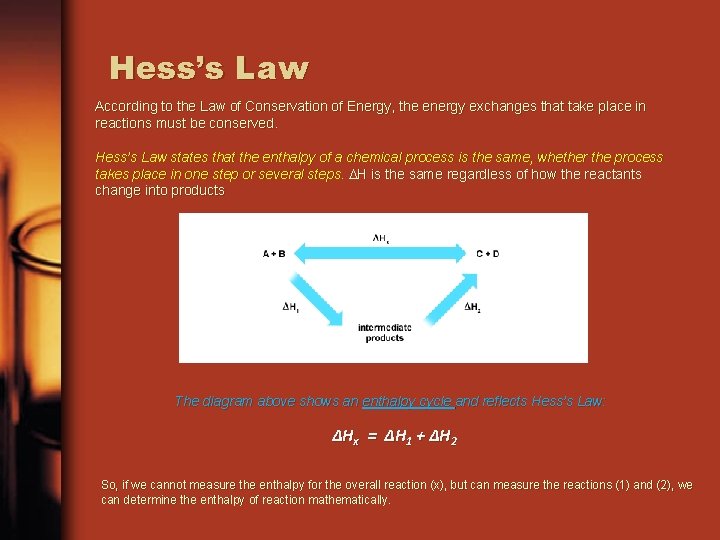
Hess’s Law According to the Law of Conservation of Energy, the energy exchanges that take place in reactions must be conserved. Hess’s Law states that the enthalpy of a chemical process is the same, whether the process takes place in one step or several steps. H is the same regardless of how the reactants change into products The diagram above shows an enthalpy cycle and reflects Hess’s Law: ΔHx = ΔH 1 + ΔH 2 So, if we cannot measure the enthalpy for the overall reaction (x), but can measure the reactions (1) and (2), we can determine the enthalpy of reaction mathematically.

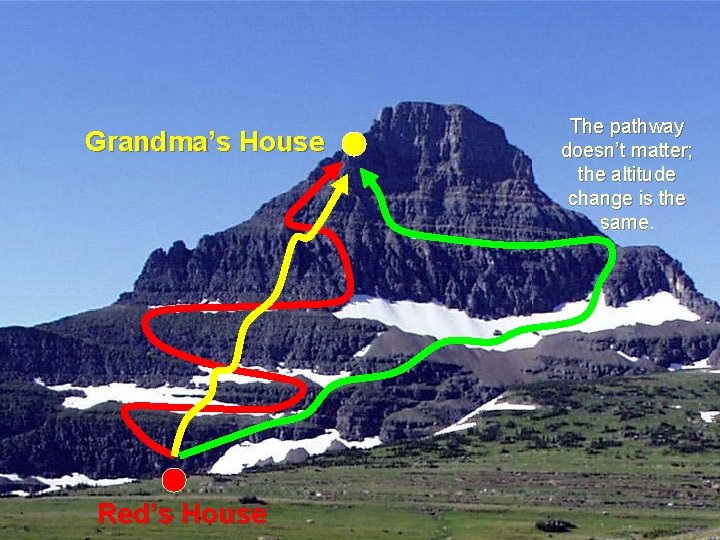
Hess’s Law Analogy Little Red Riding Hood wants to visit her grandmother, who lives at the top of the mountain. Her grandmother’s house is 2000 meters above Red’s house. Regardless of how Red climbs the mountain, her change in altitude will be the same: 2000 meters.

Grandma’s House Red’s House The pathway doesn’t matter; the altitude change is the same.

Hess’s Law Problems Helpful Hints 1. If you reverse a reaction, the sign of H changes. – 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) • H = -196. 4 k. J – 2 H 2 O(l) + O 2(g) 2 H 2 O 2(l) • H = +196. 4 k. J

Hess’s Law Problems Helpful Hints 2. If you multiply the coefficients of a thermochemical equation by some number, you must multiply H by the same number. – 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) • H = -196. 4 k. J – 4 H 2 O 2(l) 4 H 2 O(l) + 2 O 2(g) • H = -392. 8 k. J – H 2 O 2(l) H 2 O(l) + ½O 2(g) • H = -98. 2 k. J

Hess’s Law Problems Helpful Hints 3 Thermochemical equations can be combined to give new thermochemical equations. – N 2 + 2 O 2 2 NO 2 • H = +67. 8 k. J – N 2 O 4 N 2 + 2 O 2 • H = -9. 6 k. J – N 2 + 2 O 2 + N 2 O 4 2 NO 2 + N 2 + 2 O 2 – N 2 O 4 2 NO 2 • H = (67. 8 k. J) + (-9. 6 k. J) = 58. 2 k. J

Hess’s Law Problems summary Below are the steps to follow when performing Hess’s Law problems: 1. Rearrange the balanced chemical equations so that the reactants and products are on the correct sides. If you reverse a chemical equation, then the sign of the enthalpy also changes. 2. Check that you have the correct states, enthalpy changes will be different for species in the solid, liquid and gas states. 3. Assign each rearranged equation the correct ΔH value. Remember, if you need to multiply each species in the chemical equation by 2, then the enthalpy change must also be multiplied by 2. 4. Add the rearranged equations together to give the overall equation for the reaction. Add the ΔH values for these equations to calculate ΔH for the overall reaction.

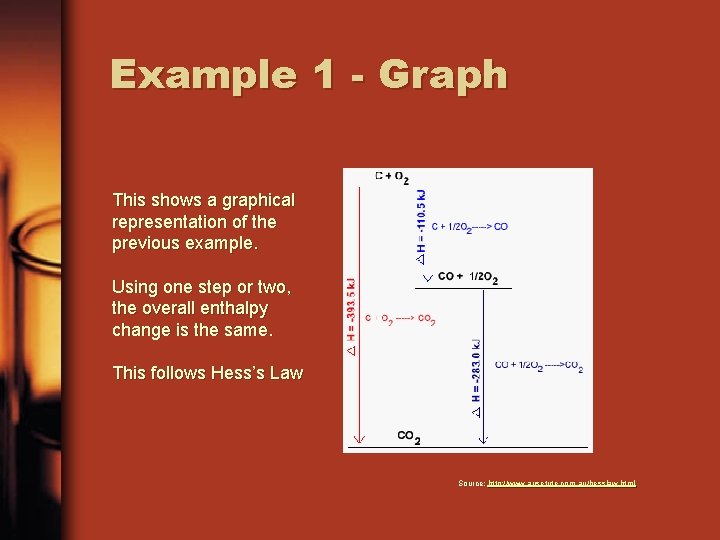
Example 1 When carbon combusts in an excess of oxygen, carbon dioxide is formed and 393. 5 k. J of heat is released per mole of carbon. C(s) + O 2(g) -----> CO 2(g) ΔH = -393. 5 k. J This overall reaction can also be produced as a two stage process: Carbon combusts in limited oxygen producing carbon monoxide: C(s) + ½O 2(g) CO(g) ΔH = -110. 5 k. J Carbon monoxide then combusts in additional oxygen: CO(g) + ½O 2(g) CO 2(g) ΔH = -283. 0 k. J These two equations can be added together to calculate ΔH for the overall reaction: C(s) + ½O 2(g) ---> CO(g) ΔH = -110. 5 k. J CO(g) + ½O 2(g) ---> CO 2(g) ΔH = -283. 0 k. J C(s) + O 2(g) ---> CO 2(g) ΔH = -393. 5 k. J

Example 1 - Graph This shows a graphical representation of the previous example. Using one step or two, the overall enthalpy change is the same. This follows Hess’s Law Source: http: //www. ausetute. com. au/hesslaw. html

Example 2 – you try Calculate ΔH for the reaction: NH 3(g) + HCl(g) NH 4 Cl(s) Given that: ½N 2(g) + 1½H 2(g) NH 3(g) ΔH = -46. 1 k. J ½H 2(g) + ½Cl 2(g) HCl(g) ΔH = -92. 3 k. J ½N 2(g) + 2 H 2(g) + ½Cl 2(g) NH 4 Cl(s) ΔH = -314. 4 k. J

Example 2 - Solution 1. Rearrange each balanced equation with reactants and products on the correct side, ie, NH 3(g) and HCl(g) on the left, NH 4 Cl(s) on the right hand side. NH 3(g) ½N 2(g) + 1½H 2(g) HCl(g) ½H 2(g) + ½Cl 2(g) ½N 2(g) + 2 H 2(g) + ½Cl 2(g) NH 4 Cl(s) 2. Assign correct ΔH values to each equation. NH 3(g) ½N 2(g) + 1½H 2(g) ΔH = +46. 1 k. J HCl(g) ½H 2(g) + ½Cl 2(g) Δ H = +92. 3 k. J ½N 2(g) + 2 H 2(g) + ½Cl 2(g) NH 4 Cl(s) Δ H = -314. 4 k. J 3. Add the rearranged equations together. NH 3(g) ½N 2(g) + 1½H 2(g) ΔH = +46. 1 k. J HCl(g) ½H 2(g) + ½Cl 2(g) Δ H = +92. 3 k. J ½N 2(g) + 2 H 2(g) + ½Cl 2(g) NH 4 Cl(s) Δ H = -314. 4 k. J NH 3(g) + HCl(g) NH 4 Cl(s) 4. Add the ΔH values. NH 3(g) ½N 2(g) + 1½H 2(g) Δ H = +46. 1 k. J HCl(g) ½H 2(g) + ½Cl 2(g) Δ H = +92. 3 k. J ½N 2(g) + 2 H 2(g) + ½Cl 2(g) NH 4 Cl(s) Δ H = -314. 4 k. J NH 3(g) + HCl(g) NH 4 Cl(s) Δ H = -176. 0 k. J

Exercise Calculate the enthalpy change (Δ H°), in k. J, of the reaction: N 2 H 4 + 2 H 2 O 2 N 2 + 4 H 2 O using the following enthalpy of combustion data: a. N 2 H 4 + O 2 N 2 + 2 H 2 O ΔH° = -622 k. J b. H 2 + O 2 H 2 O 2 Δ H° = -188 k. J c. H 2 + ½ O 2 H 2 O Δ H° = -286 k. J To solve this, leave equation (a) alone. Reverse equation (b) and double the coefficients. Then, double the coefficients for equation (c). When that is complete, equations a, b, and c add up to the desired equation and the overall DH° value equals -818 k. J.

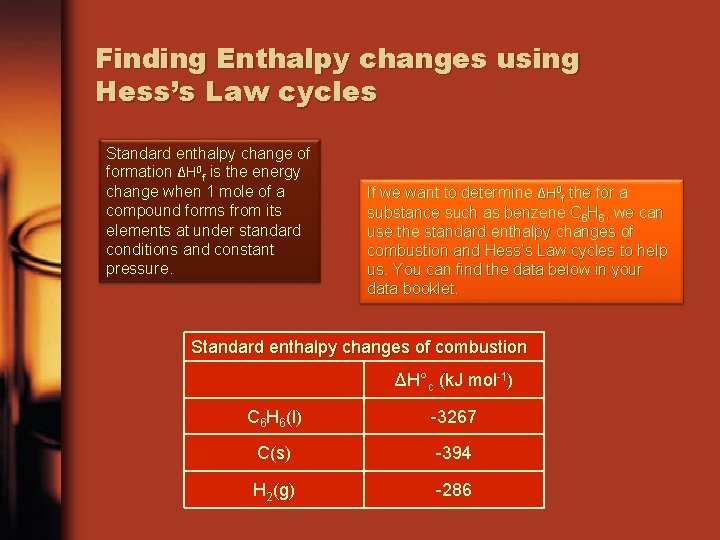
Finding Enthalpy changes using Hess’s Law cycles Standard enthalpy change of formation ΔH 0 f is the energy change when 1 mole of a compound forms from its elements at under standard conditions and constant pressure. If we want to determine ΔH 0 f the for a substance such as benzene C 6 H 6, we can use the standard enthalpy changes of combustion and Hess’s Law cycles to help us. You can find the data below in your data booklet. Standard enthalpy changes of combustion ΔH°c (k. J mol-1) C 6 H 6(l) -3267 C(s) -394 H 2(g) -286

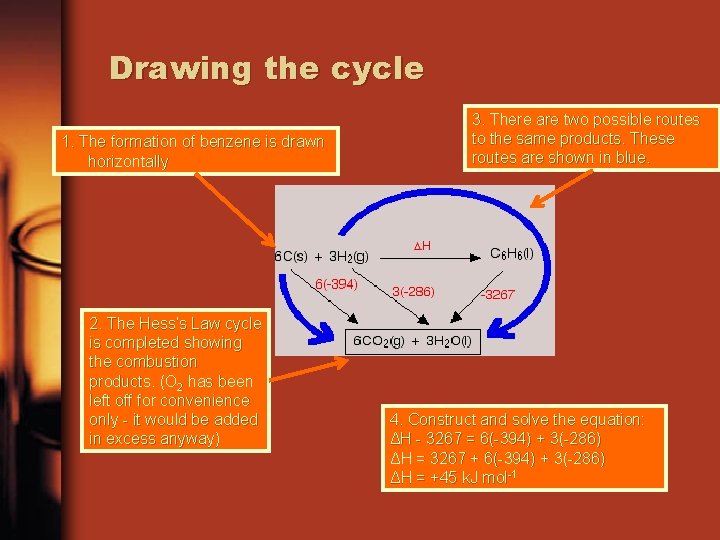
Drawing the cycle 1. The formation of benzene is drawn horizontally 2. The Hess’s Law cycle is completed showing the combustion products. (O 2 has been left off for convenience only - it would be added in excess anyway) 3. There are two possible routes to the same products. These routes are shown in blue. 4. Construct and solve the equation: ΔH - 3267 = 6(-394) + 3(-286) ΔH = 3267 + 6(-394) + 3(-286) ΔH = +45 k. J mol-1

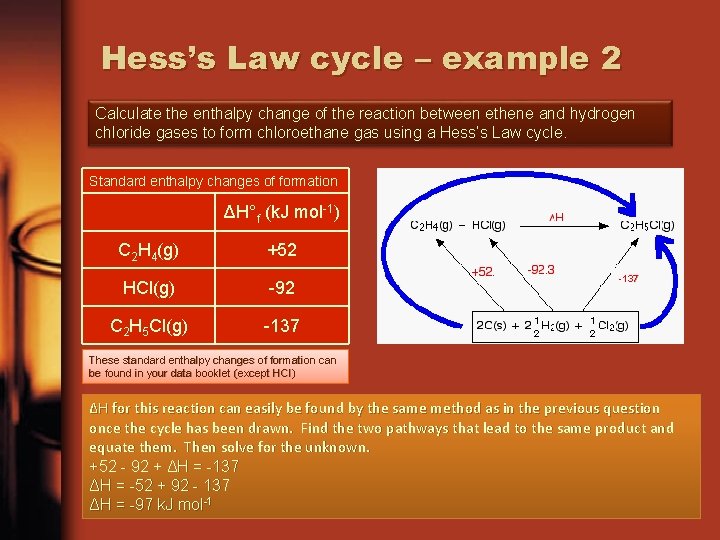
Hess’s Law cycle – example 2 Calculate the enthalpy change of the reaction between ethene and hydrogen chloride gases to form chloroethane gas using a Hess’s Law cycle. Standard enthalpy changes of formation ΔH°f (k. J mol-1) C 2 H 4(g) +52 HCl(g) -92 C 2 H 5 Cl(g) -137 These standard enthalpy changes of formation can be found in your data booklet (except HCl) ΔH for this reaction can easily be found by the same method as in the previous question once the cycle has been drawn. Find the two pathways that lead to the same product and equate them. Then solve for the unknown. +52 - 92 + ΔH = -137 ΔH = -52 + 92 - 137 ΔH = -97 k. J mol-1

Bond Enthalpies

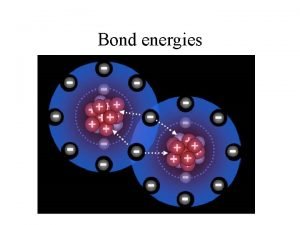
Bond enthalpy Also known as bond energies, this is the enthalpy change required to break a covalent bond when all species are in the gaseous state. These are positive values as breaking bonds is an endothermic process that requires energy. We can use bond enthalpy values to estimate the overall enthalpy changes in reactions. We simply take the sum of the individual bond energies for reactants and subtract the sum of the bond enthalpies for the products (negative because forming bonds releases energy – exothermic) Consider this example: CO(g) + H 2 O(g) CO 2(g) + H 2(g)

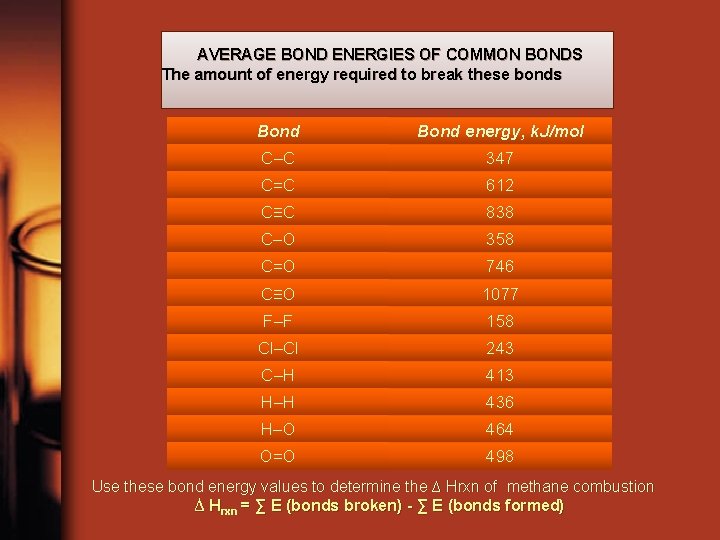
AVERAGE BOND ENERGIES OF COMMON BONDS The amount of energy required to break these bonds Bond energy, k. J/mol C–C 347 C=C 612 C≡C 838 C–O 358 C=O 746 C≡O 1077 F–F 158 Cl–Cl 243 C–H 413 H–H 436 H–O 464 O=O 498 Use these bond energy values to determine the ∆ Hrxn of methane combustion ∆ Hrxn = ∑ E (bonds broken) - ∑ E (bonds formed)

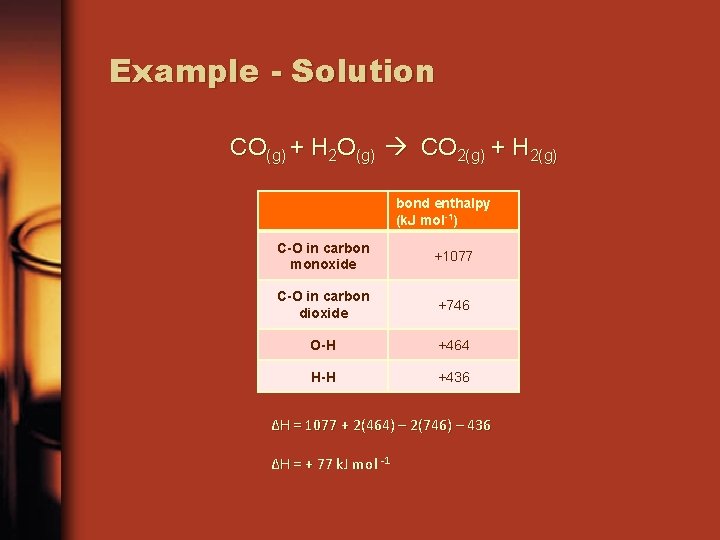
Example - Solution CO(g) + H 2 O(g) CO 2(g) + H 2(g) bond enthalpy (k. J mol-1) C-O in carbon monoxide +1077 C-O in carbon dioxide +746 O-H +464 H-H +436 ΔH = 1077 + 2(464) – 2(746) – 436 ΔH = + 77 k. J mol -1

Energetics Compiled by: Robert Slider (2011) Please share this resource with others
 Exothermic reaction examples
Exothermic reaction examples Anaplerosis
Anaplerosis Hcoh
Hcoh Glycolysis definition
Glycolysis definition Azide electron transport chain
Azide electron transport chain Energetics power tower 180
Energetics power tower 180 Energetics of gluconeogenesis
Energetics of gluconeogenesis Building energetics
Building energetics Ib energetics
Ib energetics Energetics janesville
Energetics janesville Calorimetry 2 chemsheets
Calorimetry 2 chemsheets Crank and slider vex
Crank and slider vex Bevel gear assembly vex
Bevel gear assembly vex Single slider chain
Single slider chain React-native-slider-picker
React-native-slider-picker Kinetic letters handwriting sheet
Kinetic letters handwriting sheet Cold slug well
Cold slug well Agile samurai inception deck
Agile samurai inception deck Kinetic letters families
Kinetic letters families William schroeder slider
William schroeder slider Velocity polygon
Velocity polygon Khdudcm
Khdudcm Excel slider control
Excel slider control King henry slider
King henry slider Kom til mig alle i som er trætte
Kom til mig alle i som er trætte Javafx slider listener
Javafx slider listener How to randomize blocks in qualtrics
How to randomize blocks in qualtrics Contoh abstraction & generalisation
Contoh abstraction & generalisation Kinetic letter families
Kinetic letter families Instagram poll slider
Instagram poll slider Thermic reaction
Thermic reaction Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework Exothermic or endothermic
Exothermic or endothermic Last day of year 6 poem
Last day of year 6 poem Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Exothermic electron affinity trend
Exothermic electron affinity trend Whats the trend for atomic radius
Whats the trend for atomic radius Vaporization endothermic or exothermic
Vaporization endothermic or exothermic Exothermic examples
Exothermic examples Endothermic vs exothermic
Endothermic vs exothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Heating cooling curve
Heating cooling curve Endothermic reaction examples
Endothermic reaction examples Endothermic exothermic venn diagram
Endothermic exothermic venn diagram Kc formula
Kc formula In exothermic reaction temperature
In exothermic reaction temperature Exothermic reaction increase temperature equilibrium
Exothermic reaction increase temperature equilibrium Basic chemistry
Basic chemistry Melting evaporation condensation freezing
Melting evaporation condensation freezing Cellular respiration endothermic or exothermic
Cellular respiration endothermic or exothermic Whats an exothermic reaction
Whats an exothermic reaction Ins
Ins Exothermic or endothermic
Exothermic or endothermic Endothermic vs exothermix
Endothermic vs exothermix Is bond breaking endothermic
Is bond breaking endothermic Ectothermic vs exothermic
Ectothermic vs exothermic