Whats Hot Year 10 Science 2012 Section 1








- Slides: 22

What's Hot? Year 10 Science 2012 Section 1: Energy in Chemical reactions

Energy and Chemical Reactions Many chemical reactions release energy in the form of heat, light, or sound. These are exothermic reactions. There are other chemical reactions that must absorb energy in order to proceed. These are endothermic reactions.

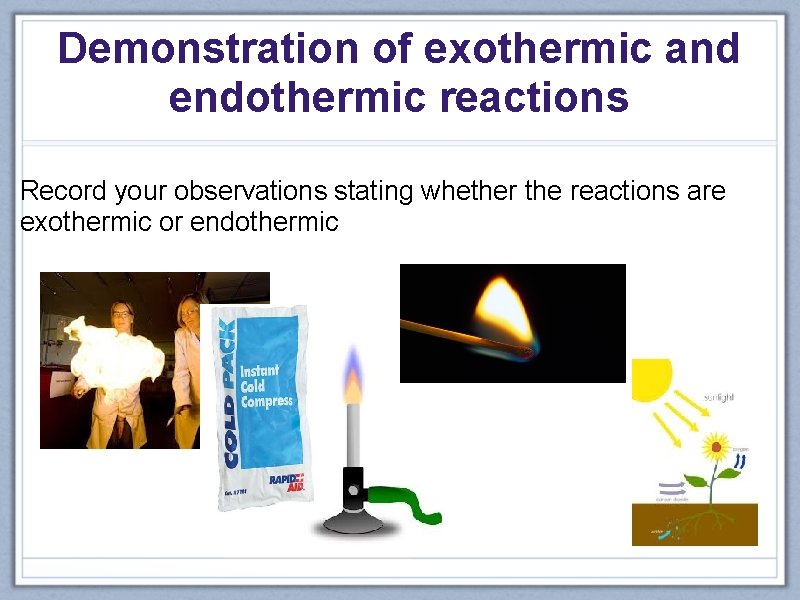
Demonstration of exothermic and endothermic reactions Record your observations stating whether the reactions are exothermic or endothermic

Exothermic Reactions Exothermic reactions may occur spontaneously. Exothermic reactions produce heat or may even be explosive. An exothermic reaction is one which transfers heat energy to the surroundings

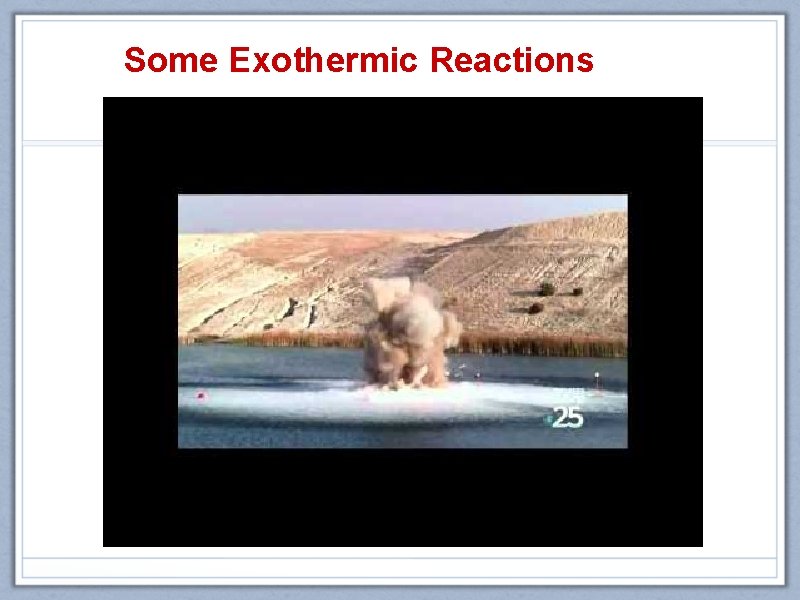
Some Exothermic Reactions

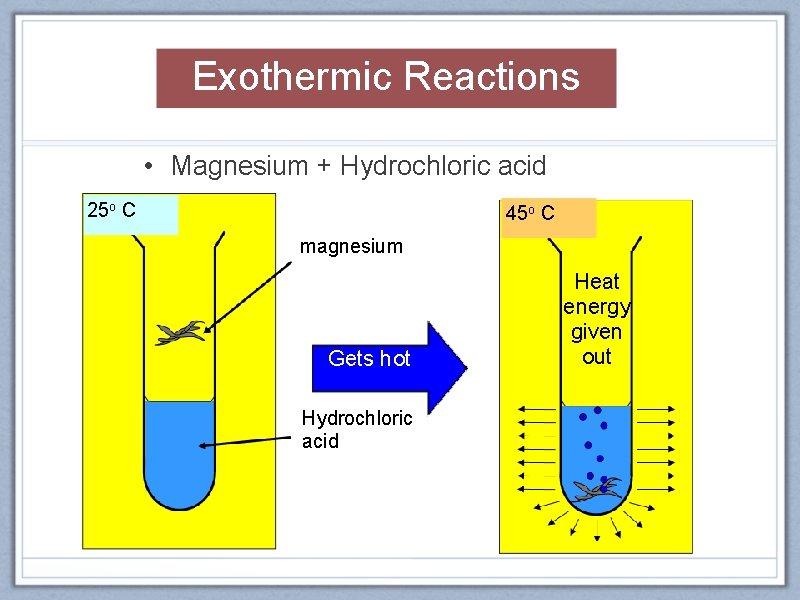
Exothermic Reactions • Magnesium + Hydrochloric acid 25 o C 45 o C magnesium Gets hot Hydrochloric acid Heat energy given out

Examples of Exothermic reactions • Skiers and mountain climbers often use hand warmers inside their gloves. These small heat packs rely on exothermic processes. • Combustion is an example of an exothermic reaction

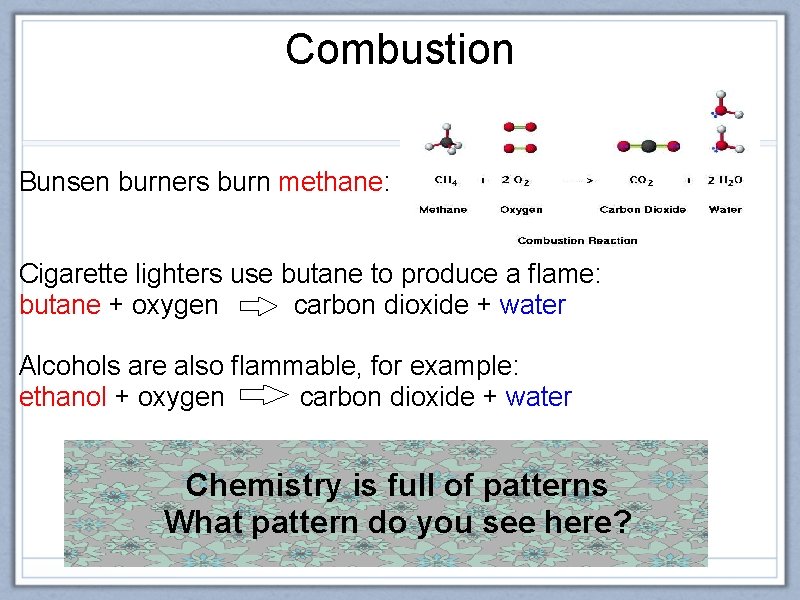
Combustion Bunsen burners burn methane: Cigarette lighters use butane to produce a flame: butane + oxygen carbon dioxide + water Alcohols are also flammable, for example: ethanol + oxygen carbon dioxide + water Chemistry is full of patterns What pattern do you see here?

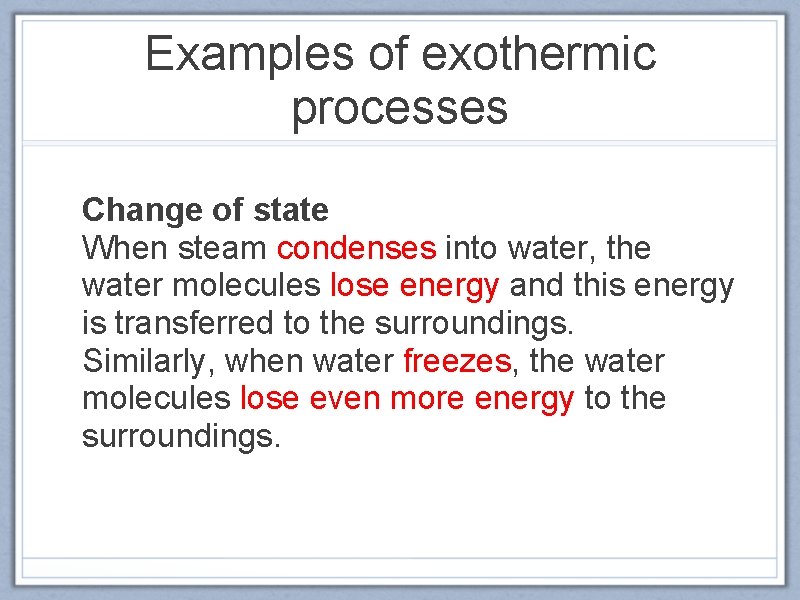
Examples of exothermic processes Change of state When steam condenses into water, the water molecules lose energy and this energy is transferred to the surroundings. Similarly, when water freezes, the water molecules lose even more energy to the surroundings.

• • Exothermic Examples Chemical Reactions o A candle flame o Rusting iron o Burning sugar o Glow sticks o Fire crackers Physical processes o Some hand warmers o Some dissolving o Condensing o Freezing What do you think is the difference between a reaction and process?

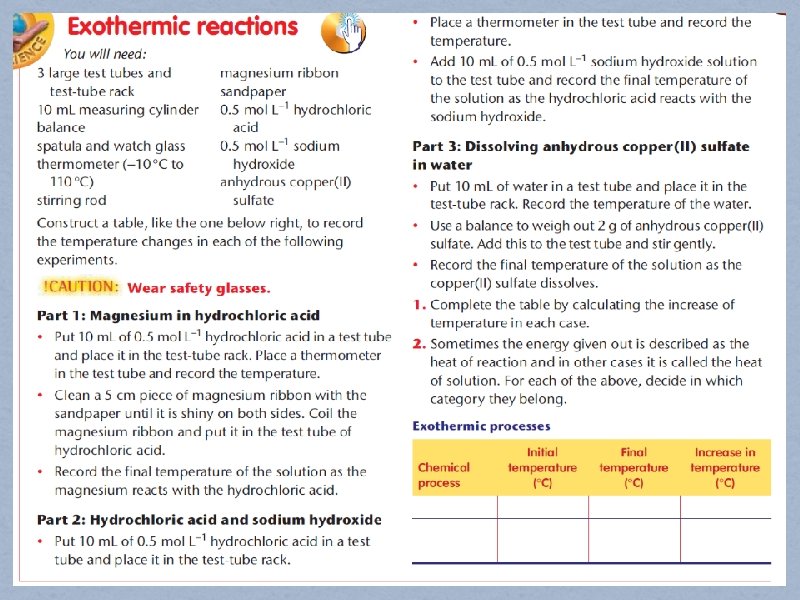
Experiment

Complete page 69 Questions 1 -8

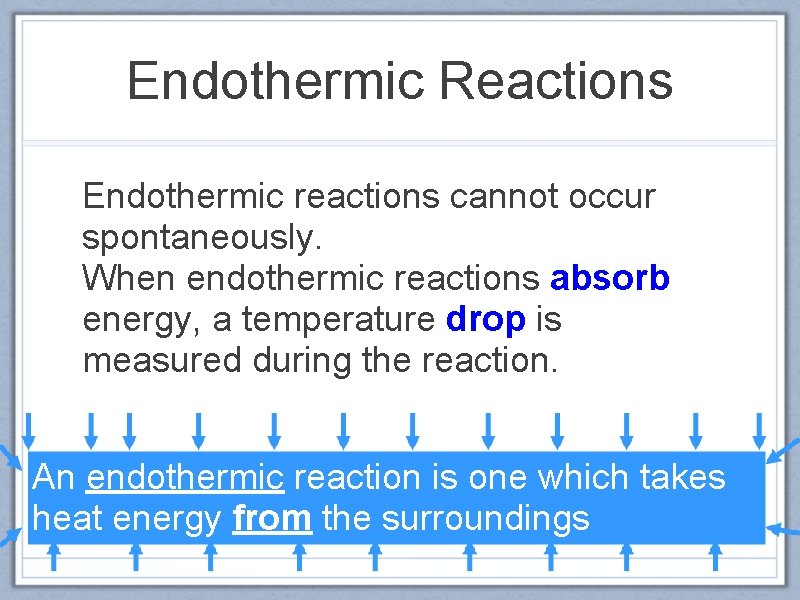
Endothermic Reactions Endothermic reactions cannot occur spontaneously. When endothermic reactions absorb energy, a temperature drop is measured during the reaction. An endothermic reaction is one which takes heat energy from the surroundings

Endothermic Reaction

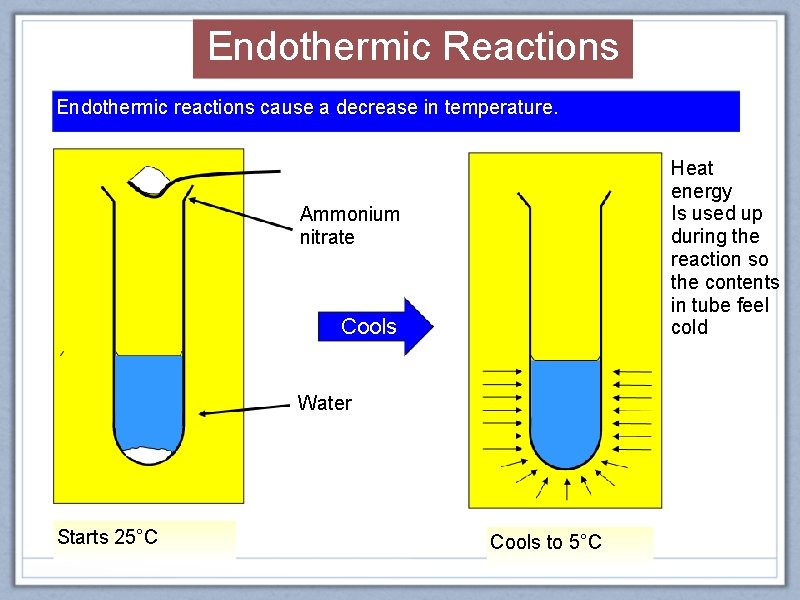
Endothermic Reactions Endothermic reactions cause a decrease in temperature. Heat energy Is used up during the reaction so the contents in tube feel cold Ammonium nitrate Cools Water Starts 25°C Cools to 5°C

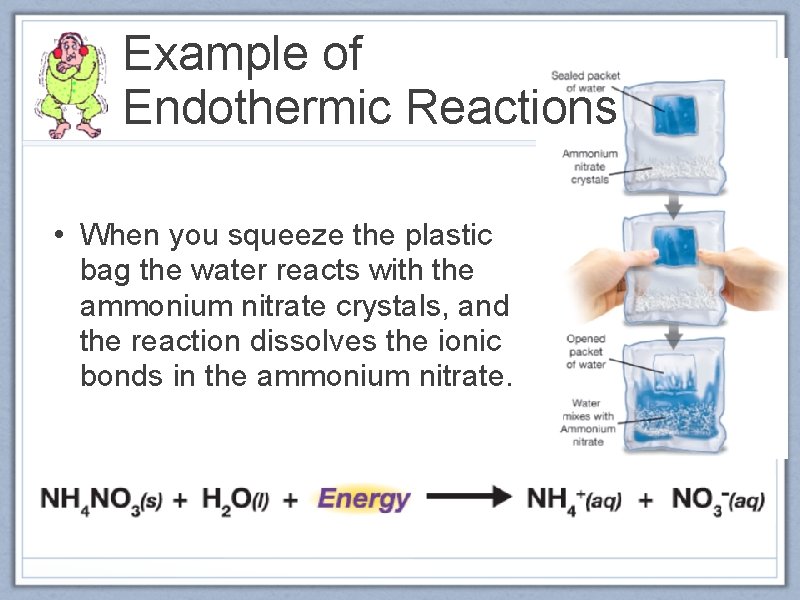
Example of Endothermic Reactions • When you squeeze the plastic bag the water reacts with the ammonium nitrate crystals, and the reaction dissolves the ionic bonds in the ammonium nitrate.

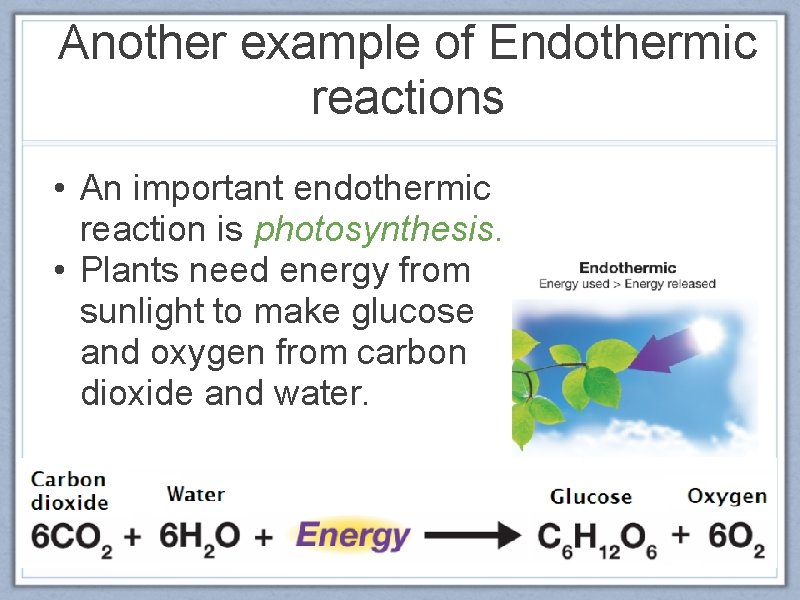
Another example of Endothermic reactions • An important endothermic reaction is photosynthesis. • Plants need energy from sunlight to make glucose and oxygen from carbon dioxide and water.

Endothermic Processes Changes of state For ice to melt, the water molecules must gain energy. For water to boil and turn into steam, the water molecules must gain even more energy. These are both endothermic processes. Energy is needed to make these changes happen.

Endothermic Examples • Chemical Reactions o o Baking bread Cooking an egg o o o Sometimes dissolving Cold packs Melting and boiling • Physical Processes


Complete Page 71 questions 15
