Empirical and Molecular Formulas Percent Composition I can



































- Slides: 39

Empirical and Molecular Formulas

Percent Composition

I can determine the percent composition for each element in a compound or sample.

Note Expectations: Cell phones and electronics are not in use. You are taking the notes. You are helping the people at your table to answer the questions. You are prepared to answer the questions.

What is a percent? On the mole test, you earned 25 out of 33. What percent did you get on the test? How do you calculate a percent?

What does the formula C 6 H 12 O 6 tell us? How do you find the mass of a compound?

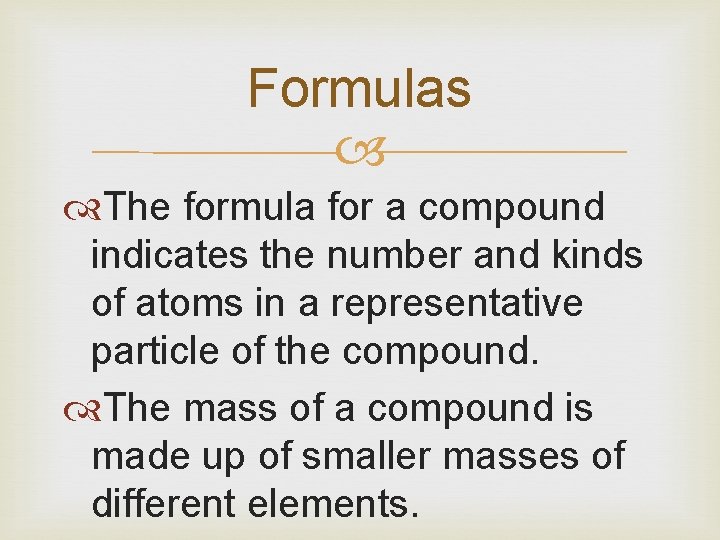
Formulas The formula for a compound indicates the number and kinds of atoms in a representative particle of the compound. The mass of a compound is made up of smaller masses of different elements.

Work as a Table What is the percent of hydrogen and the percent of oxygen in H 2 O? Show you think you would solve this problem.

Percent Composition The percent composition of a compound tells you the percent mass made up by each element in the compound. The percent composition can be determined by taking the mass of individual elements and dividing by the total mass.

Percent Composition Then, you multiply by 100 to get the percent composition.

Example What is the percent composition of H and O in H 2 O 2?

What is the percent composition of N and O if 22. 6 g of N and 54. 4 g of O are present in a 77. 0 gram sample?

Check your work! Once you have found all of the percents, they should add up to 100%, or close to it based on rounding.

Review Questions 1. What is a percent? 2. How do you calculate a percent? You can answer this with an example. 3. What does a formula tell you? 4. What does percent composition tell you? 5. What is the total mass of a compound made of?

Empirical Formulas

I can define empirical formula. I can determine the empirical formula if given the elements in the formula.

Note Expectations: Cell phones and electronics are not in use. You are taking the notes. You are helping the people at your table to answer the questions. You are prepared to answer the questions.

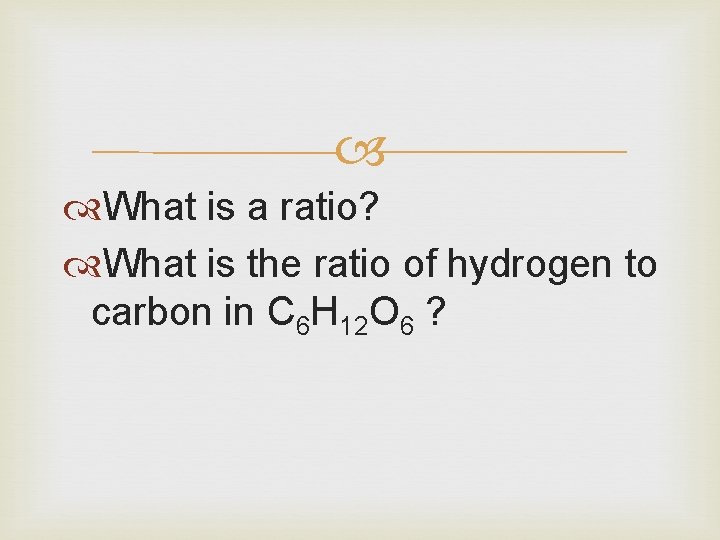
What is a ratio? What is the ratio of hydrogen to carbon in C 6 H 12 O 6 ?

Empirical Formula A formula that gives the simplest whole number ratio of the atoms of the elements is called an empirical formula.

What would the simplest ratio of C 6 H 12 O 6 be?

Formulas The molecular formula for peroxide is H 2 O 2. The empirical formula for peroxide is HO. The empirical formula simply tells you the ratio of atoms, in this case 1: 1.

Procedure To determine the empirical formula based on % composition, you assume 100 g. This will make the math easier. Next, you convert each of the masses to moles using the molar mass of the element.

Review Question How do you convert mass to moles? Where does the mass of an element come from?

Procedure You divide each element by the smallest number of moles. Round your answers to the nearest whole number. These then become the subscripts for each element in the formula. This is your ratio for the empirical formula.

Example A compound has a percent composition of 80% C and 20% H, what is its empirical formula?

If you are given grams instead of percents, you can start by converting to moles.

Example A compound was found to have 13. 5 g Ca, 10. 8 g O, and 0. 675 g H, what is its empirical formula?

Review Questions 1. What is a ratio? 2. What is an empirical formula? 3. Summarize the steps required to calculate an empirical formula.

Calculating Molecular Formulas

I can define molecular formula. I can determine the molecular formula if given the proper information.

Note Expectations: Cell phones and electronics are not in use. You are taking the notes. You are helping the people at your table to answer the questions. You are prepared to answer the questions.

Is the empirical formula the actual formula? How do you think it compares? Could the empirical formula be the actual formula? Why or why not?

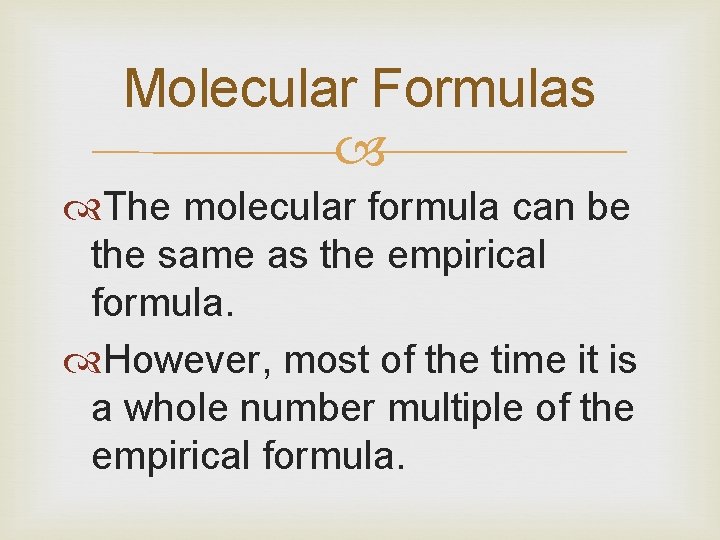
Molecular Formulas The molecular formula can be the same as the empirical formula. However, most of the time it is a whole number multiple of the empirical formula.

Procedure You can determine the molecular formula of a compound if you know its molar mass. First, you find the mass of the empirical formula.

Procedure This is called the empirical formula mass or efm. Then you take the known molar mass and divide by the efm.

Procedure This gives you the number of empirical formula units in one molecule of the compound. Take the number you got and round to the nearest whole number.

Procedure Then, multiply all of the subscripts in the formula by that number. This will give you the molecular formula.

Example Calculate the molecular formula of the compound whose molar mass is 60. 0 g and empirical formula is CH 4 N.

Review Questions What is a molecular formula? How is the molecular formula related to the empirical formula? Summarize the steps to calculate the molecular formula.