CKS 3 Yr 8 Chemistry Unit 3 Whats














- Slides: 58

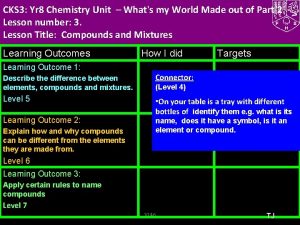
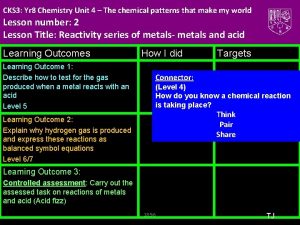
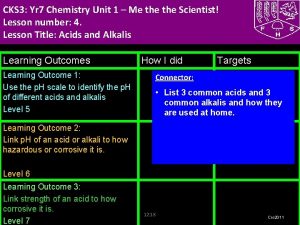
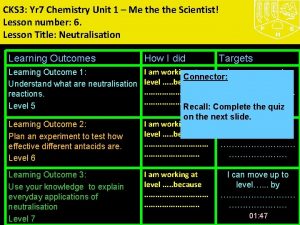
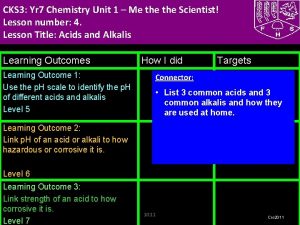
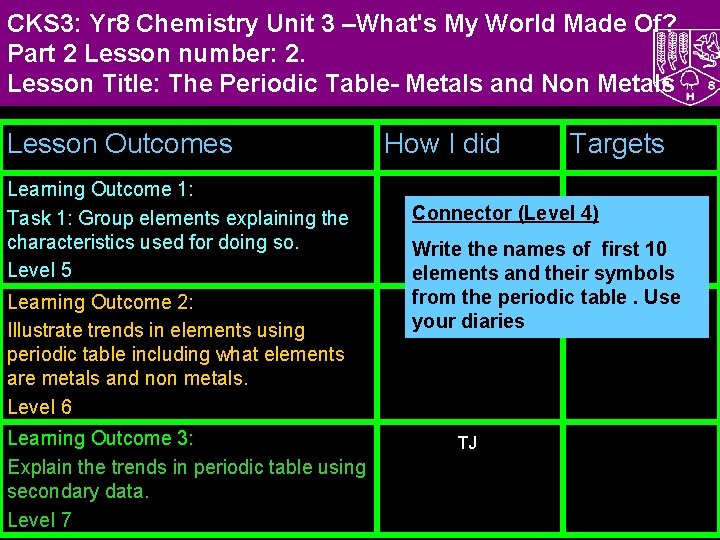
CKS 3: Yr 8 Chemistry Unit 3 –What's My World Made Of? Part 2 Lesson number: 2. Lesson Title: The Periodic Table- Metals and Non Metals Lesson Outcomes Learning Outcome 1: Task 1: Group elements explaining the characteristics used for doing so. Level 5 Learning Outcome 2: Illustrate trends in elements using periodic table including what elements are metals and non metals. Level 6 Learning Outcome 3: Explain the trends in periodic table using secondary data. Level 7 How I did Targets Connector (Level 4) Write the names of first 10 elements and their symbols from the periodic table. Use your diaries TJ

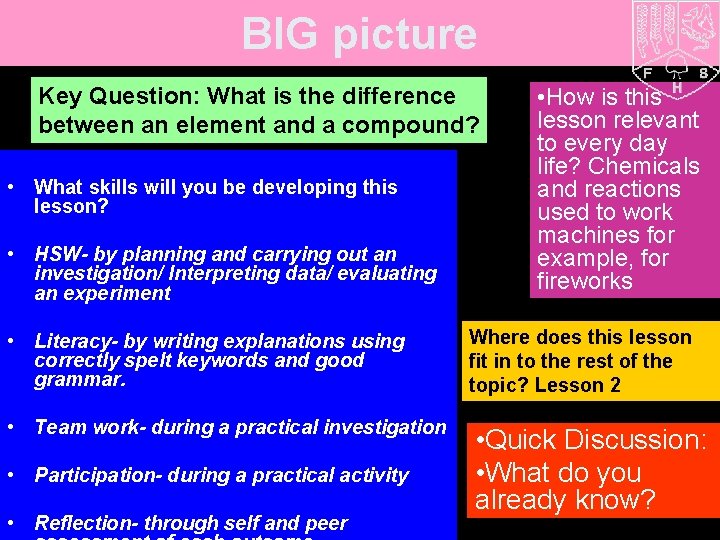
BIG picture Key Question: What is the difference between an element and a compound? • What skills will you be developing this lesson? • HSW- by planning and carrying out an investigation/ Interpreting data/ evaluating an experiment • How is this lesson relevant to every day life? Chemicals and reactions used to work machines for example, for fireworks • Literacy- by writing explanations using correctly spelt keywords and good grammar. Where does this lesson fit in to the rest of the topic? Lesson 2 • Team work- during a practical investigation • Quick Discussion: • What do you already know? • Participation- during a practical activity • Reflection- through self and peer

Keywords: Create sentences using the keywords to show that you know what they mean. Put your hand up if there is any key word from the list that you don’t understand. Periodic table Group Trend Secondary data

New Information for Learning Outcome 1 • Visual: Demonstration • Audio: Demonstration • Kinaesthetic: Class experiment

New Information for Learning Outcome 1 A standard set of symbols is used to represent elements l The symbol for many of the more common elements uses just the first letter of the name. H = hydrogen C = carbon F = fluorine l Others elements have the first two letters. Li = lithium Al = aluminium He = helium O = oxygen N = nitrogen I = iodine l Some of the symbols are not always as you might expect. Pb = lead Au = gold Ag = silver

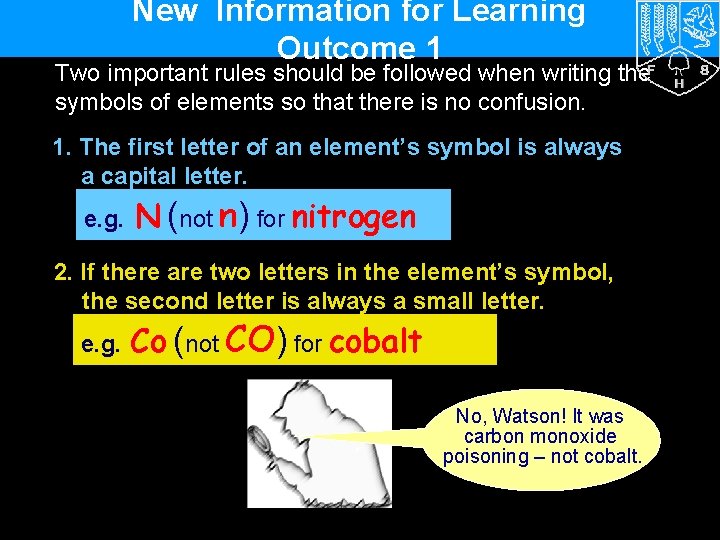
New Information for Learning Outcome 1 Two important rules should be followed when writing the symbols of elements so that there is no confusion. 1. The first letter of an element’s symbol is always a capital letter. e. g. N (not n) for nitrogen 2. If there are two letters in the element’s symbol, the second letter is always a small letter. e. g. Co (not CO) for cobalt No, Watson! It was carbon monoxide poisoning – not cobalt.

New Information for Learning Outcome 1 Elements • Hydrogen Atomic Number Name of element Symbol 1 Hydrogen H

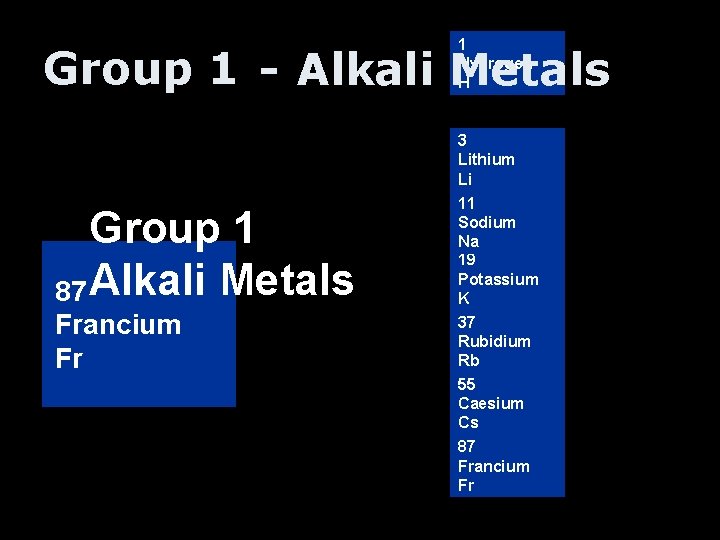
1 Hydrogen H Group 1 - Alkali Metals Group 1 55 3 Alkali Metals 187 11 19 37 Caesium Lithium Sodium Hydrogen Potassium Rubidium Francium Cs Li H Na K Rb Fr 3 Lithium Li 11 Sodium Na 19 Potassium K 37 Rubidium Rb 55 Caesium Cs 87 Francium Fr

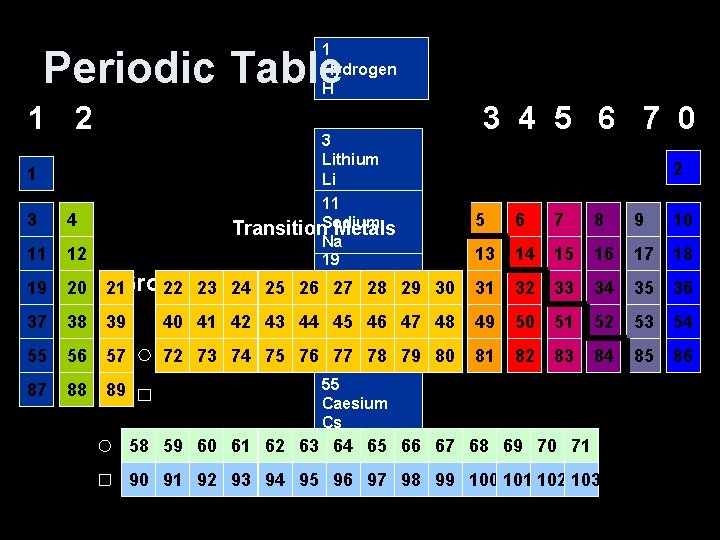
1 Hydrogen H Periodic Table 1 2 1 3 4 11 12 19 20 21 Group 22 1 23 37 38 39 40 41 55 56 57 72 73 87 88 89 58 59 60 90 91 92 3 Lithium Li 11 Transition. Sodium Metals Na 19 Potassium 24 25 26 27 28 29 K 42 43 44 3745 46 47 Rubidium 74 75 76 Rb 77 78 79 55 Caesium Cs 61 62 63 8764 65 66 Francium 93 94 95 Fr 96 97 98 3 4 5 6 7 0 2 5 6 7 8 9 10 13 14 15 16 17 18 30 31 32 33 34 35 36 48 49 50 51 52 53 54 80 81 82 83 84 85 86 67 68 69 70 71 99 100 101 102 103

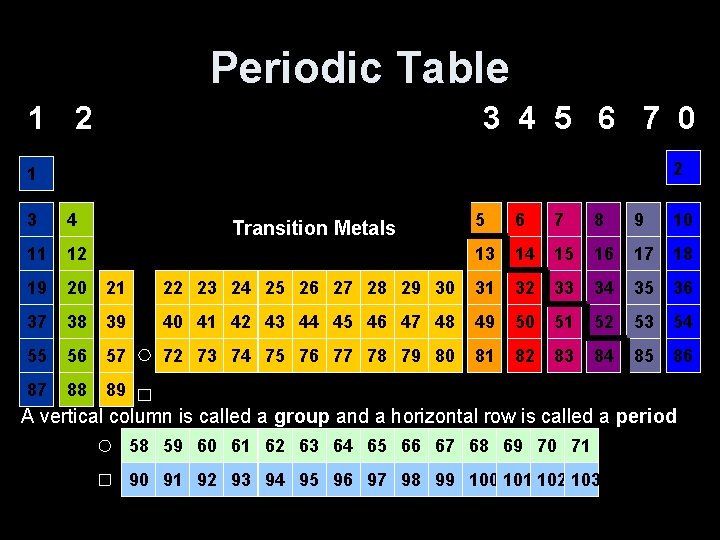
Periodic Table 1 2 3 4 5 6 7 0 2 1 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 Transition Metals A vertical column is called a group and a horizontal row is called a period 58 59 60 61 62 63 64 65 66 67 68 69 70 71 90 91 92 93 94 95 96 97 98 99 100 101 102 103

Learning Activities for Outcome 1 Look at worksheet 1

Keywords: Demonstrate your Learning for Outcome 1 I am working at level. . because. . . Create (L 8) Evaluate (L 7) Judge Justify Defend Decide Agree Value Prove Check Criticise Recommend Support Test Create Evaluate Combine construct Develop Imagine Design Change Improve Discuss Create Invent Suppose Put together Make up Synthesise Analyse (L 6) Apply (L 5) Use Build Execute Develop Construct Identify Plan Select Solve Organise Apply Model Analyse Apply Remember (L 3) Understand Who What When Where Why Which How Match Define List Choose Name Spell Tell Describe Remember 14: 30 Take apart Compare Classify Examine List Distinguish Simplify Theme Conclude Motive Discover Understand (L 4) Explain what when where how Rephrase Demonstrate Summarise Contrast Show Predict Compare Clarify Illustrate Categorise

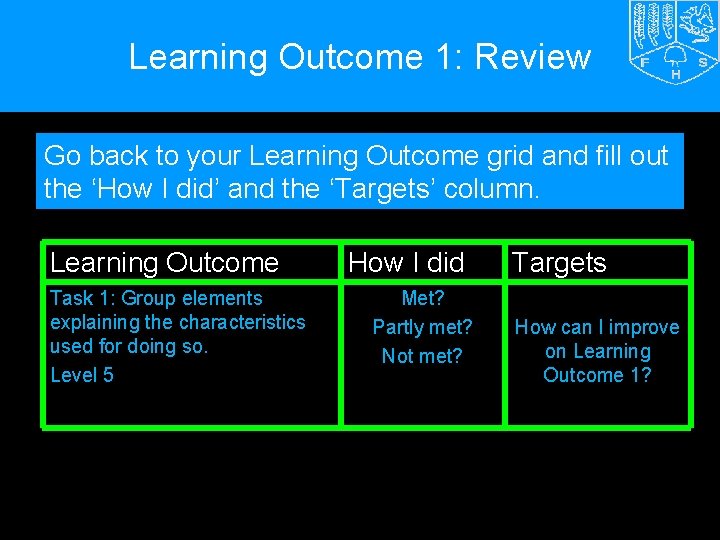
Learning Outcome 1: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcome Task 1: Group elements explaining the characteristics used for doing so. Level 5 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 1?

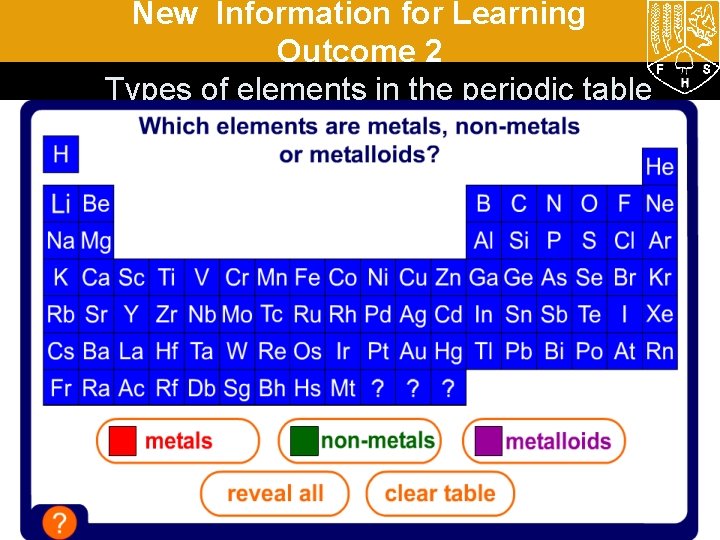
New Information for Learning Outcome 2 Types of elements in the periodic table

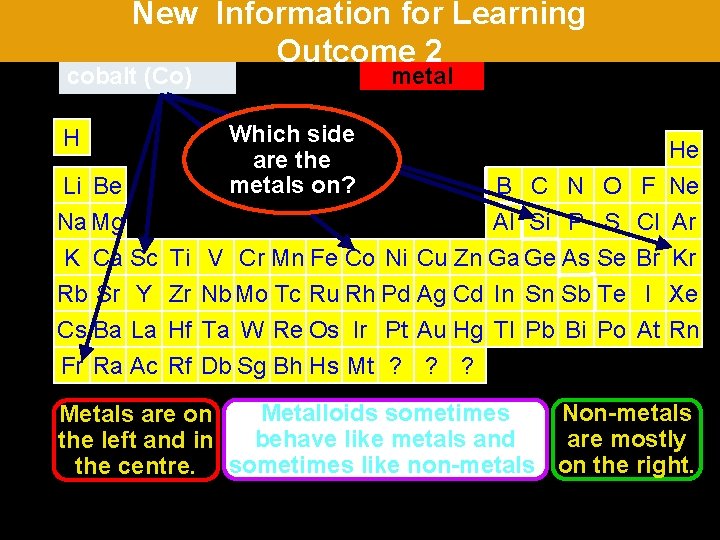
New Information for Learning Outcome 2 francium krypton (Kr) (Fr) silicon(Co) (Si) scandium (Sc) cobalt Which side What areare thethe nonmetalloids? metals on? H Li Be Na Mg K Ca Sc Rb Sr Y Cs Ba La Fr Ra Ac Ti Zr Hf Rf metalloid non-metal B C N O Al Si P S V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po Db Sg Bh Hs Mt ? ? ? F Cl Br I At He Ne Ar Kr Xe Rn Metalloids sometimes Non-metals Metals are on behave like metals and are mostly the left and in the centre. sometimes like non-metals. on the right.

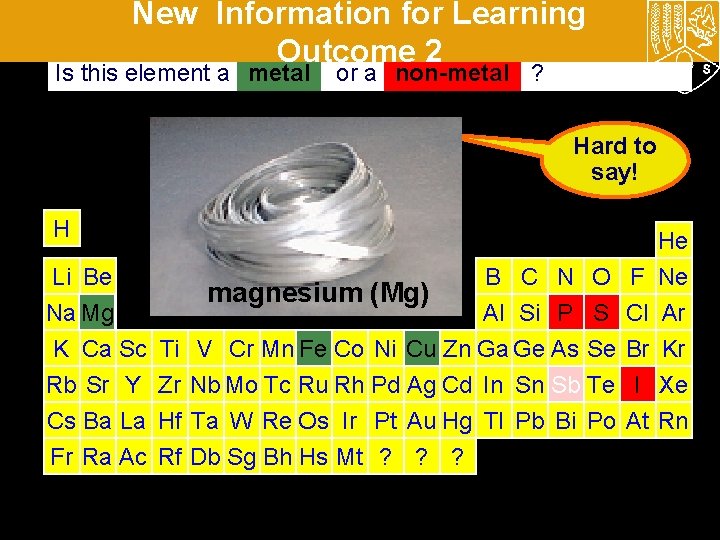
New Information for Learning Outcome 2 Is this element a metal or a non-metal ? Hard to say! H Li Be B C N O iron (Fe) antimony (Sb) iodine (I) copper (Cu) phosphorus (P) sulfur (S)(Mg) magnesium Na Mg Al Si P S K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te F Cl Br I He Ne Ar Kr Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt ? ? ?

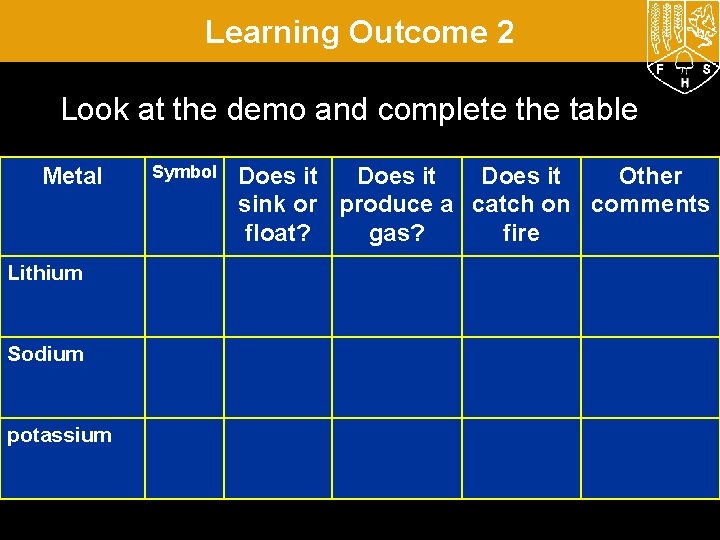
Learning Outcome 2 Look at the demo and complete the table Metal Lithium Sodium potassium Symbol Does it Other sink or produce a catch on comments float? gas? fire

http: //www. theodoregray. com/Periodic. Table/ Alkali. Bangs/index. html

Learning Outcome 2 1. Which metal was the most reactive. Give evidence to support your answer. 2. What gas was produced? 3. How hard were the metals to cut? 4. What is the trend in reactivity as you go down the group? 5. What do you predict rubidium would do in water?

Keywords: Demonstrate your Learning for Outcome 2 I am working at level. . because. . . Create (L 8) Evaluate (L 7) Judge Justify Defend Decide Agree Value Prove Check Criticise Recommend Support Test Create Evaluate Combine construct Develop Imagine Design Change Improve Discuss Create Invent Suppose Put together Make up Synthesise Analyse (L 6) Apply (L 5) Use Build Execute Develop Construct Identify Plan Select Solve Organise Apply Model Analyse Apply Remember (L 3) Understand Who What When Where Why Which How Match Define List Choose Name Spell Tell Describe Remember 14: 30 Take apart Compare Classify Examine List Distinguish Simplify Theme Conclude Motive Discover Understand (L 4) Explain what when where how Rephrase Demonstrate Summarise Contrast Show Predict Compare Clarify Illustrate Categorise

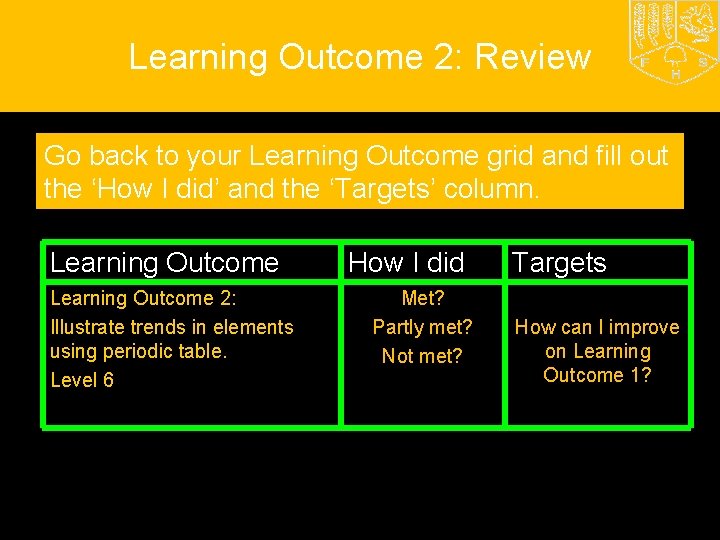
Learning Outcome 2: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcome 2: Illustrate trends in elements using periodic table. Level 6 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 1?

New task for Learning Outcome 1 Choose any one group from • Group 1 • Group 2 • Group 7 • Research the properties of all the elements in that group. • How do these properties vary as you move down the group. Explain your answer.

Keywords: Demonstrate your Learning for Outcome 3 I am working at level. . because. . . Create (L 8) Evaluate (L 7) Judge Justify Defend Decide Agree Value Prove Check Criticise Recommend Support Test Create Evaluate Combine construct Develop Imagine Design Change Improve Discuss Create Invent Suppose Put together Make up Synthesise Analyse (L 6) Apply (L 5) Use Build Execute Develop Construct Identify Plan Select Solve Organise Apply Model Analyse Apply Remember (L 3) Understand Who What When Where Why Which How Match Define List Choose Name Spell Tell Describe Remember 14: 30 Take apart Compare Classify Examine List Distinguish Simplify Theme Conclude Motive Discover Understand (L 4) Explain what when where how Rephrase Demonstrate Summarise Contrast Show Predict Compare Clarify Illustrate Categorise

Task 3: Review Go back to your lesson outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Lesson Outcomes Learning Outcome 3: Explain the trends in periodic table using secondary data. Level 7 How I did Met? Partly met? Not met? Targets How can I improve on task 3?

Review of lesson Look at the following groups of words. In each group find the odd one out (there is more than one correct answer) and give a reason. Gold Sodium Copper Tin Lithium Copper oxide Sodium Magnesium iron potassium iron Iron and sulphur Iron Sulphur oxygen

Review of lesson Alkali metals are found in group ______ of the periodic table. They are the most ____ elements. The reactivity as we go down the group.

Decide whether these elements are metals or non-metals • • Hydrogen Lithium Iron Nitrogen Mercury Copper Fluorine Neon • • Magnesium Sulphur Gold Lead Bromine Phosphorous Aluminium Tin Where are metals and non-metals found in the periodic table?

Review of lesson Chemical symbols game

Question 1 How many different groups are there on the periodic table? 4 8 10 18

Question 2 How many different periods are there on the periodic table? 6 7 8 9

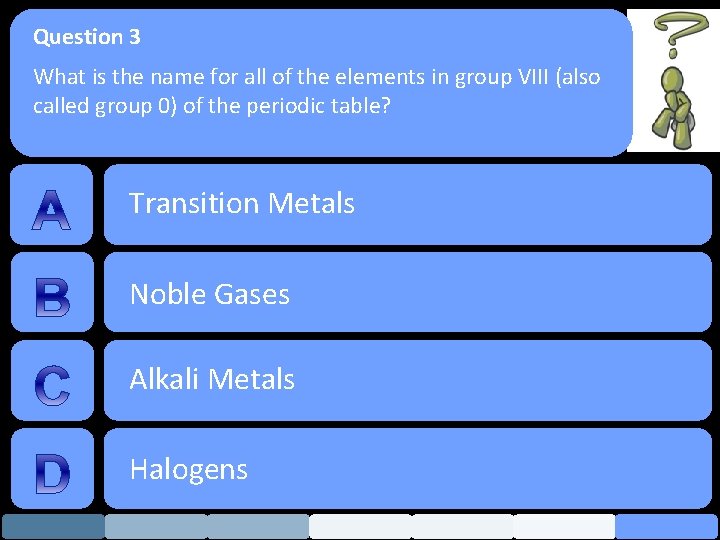
Question 3 What is the name for all of the elements in group VIII (also called group 0) of the periodic table? Transition Metals Noble Gases Alkali Metals Halogens

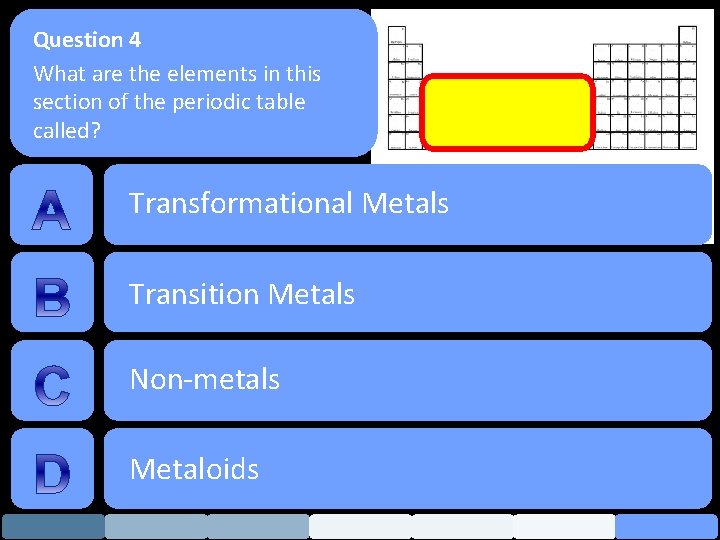
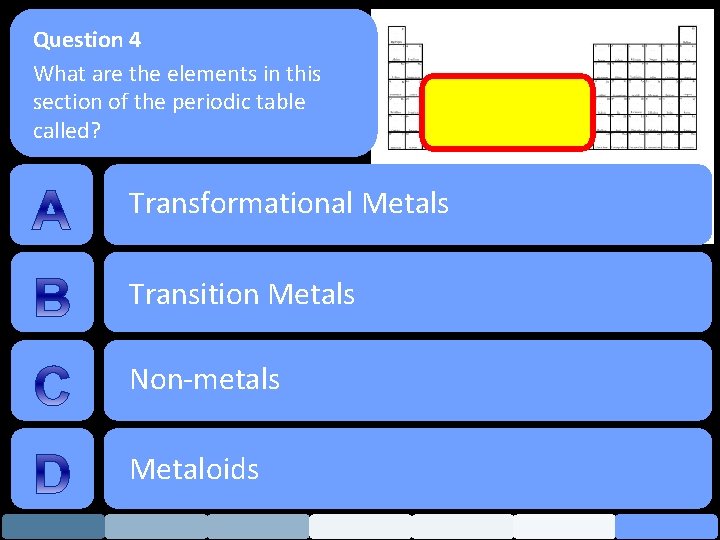
Question 4 What are the elements in this section of the periodic table called? Transformational Metals Transition Metals Non-metals Metaloids

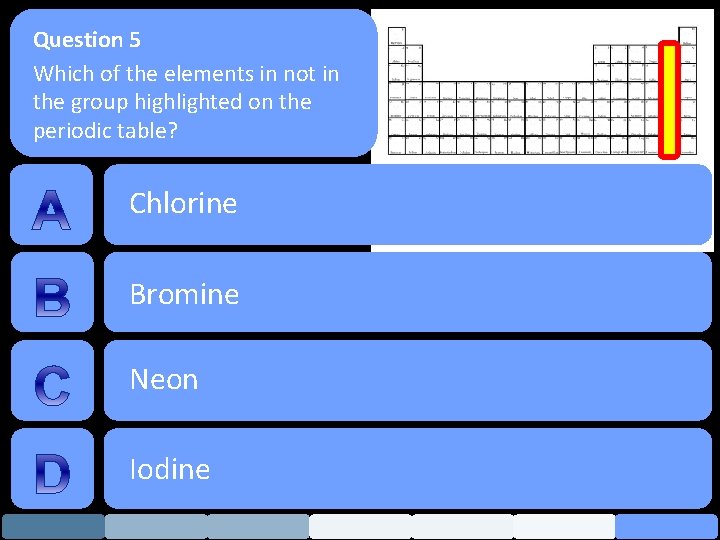
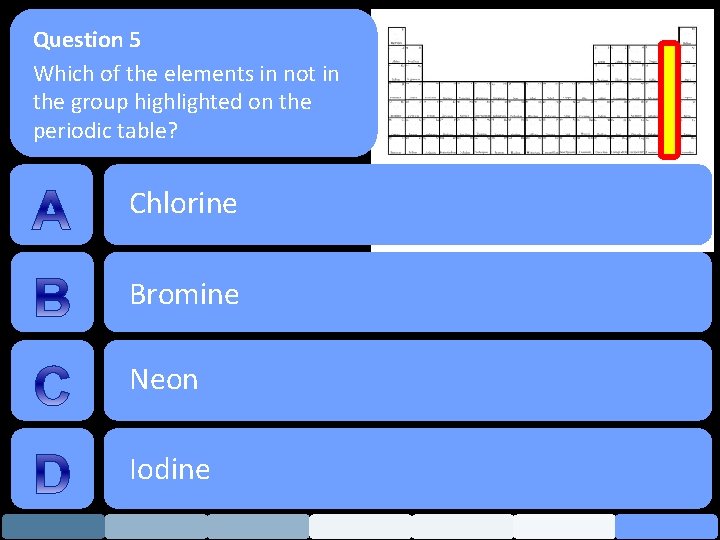
Question 5 Which of the elements in not in the group highlighted on the periodic table? Chlorine Bromine Neon Iodine

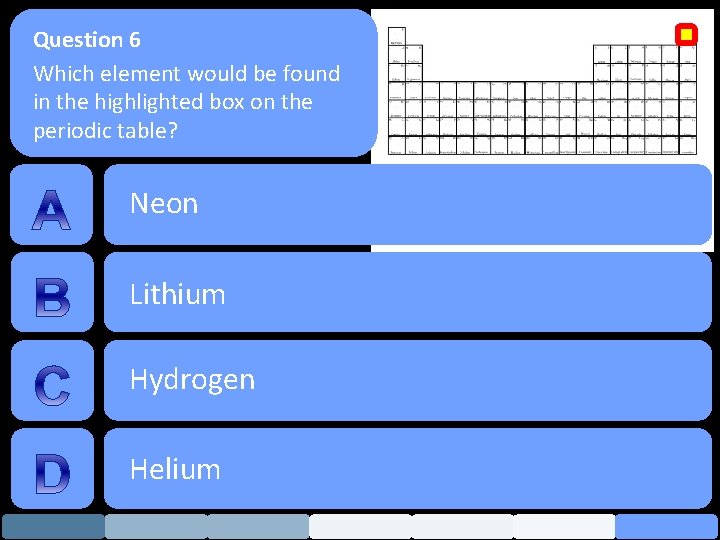
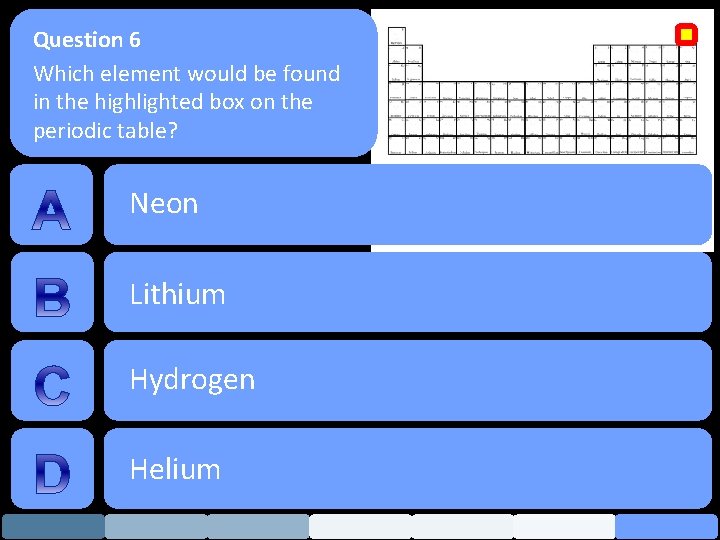
Question 6 Which element would be found in the highlighted box on the periodic table? Neon Lithium Hydrogen Helium

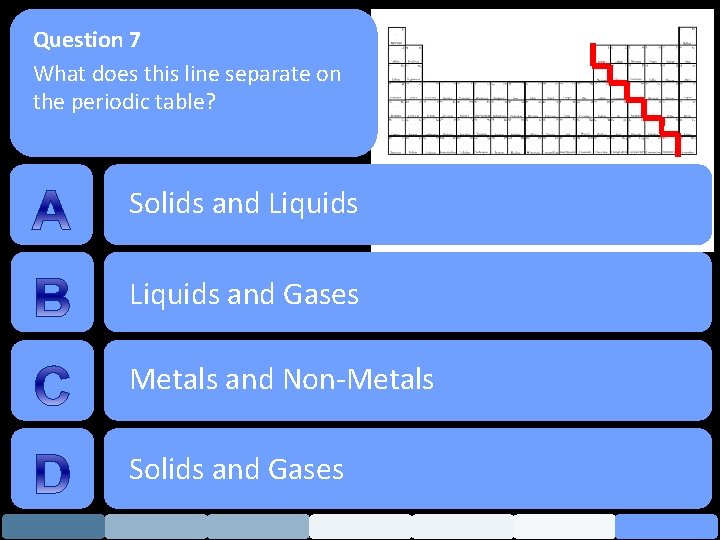
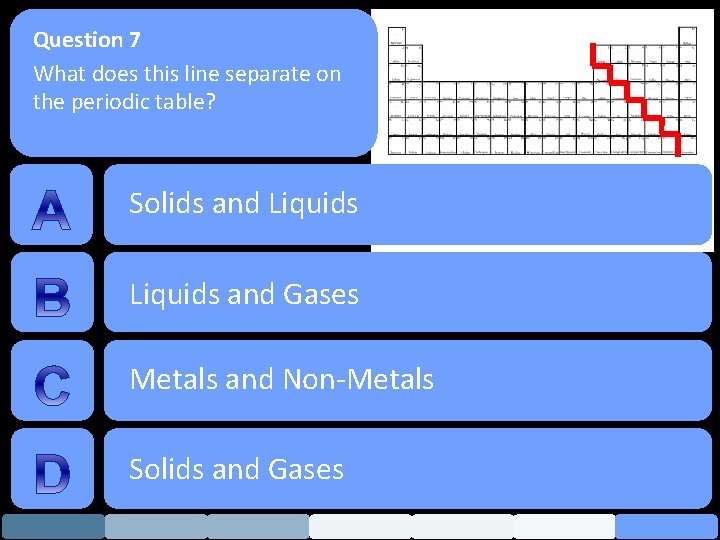
Question 7 What does this line separate on the periodic table? Solids and Liquids and Gases Metals and Non-Metals Solids and Gases

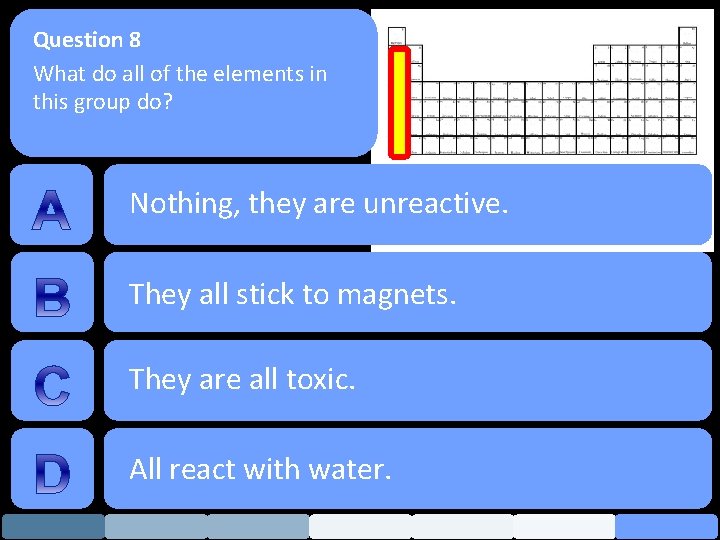
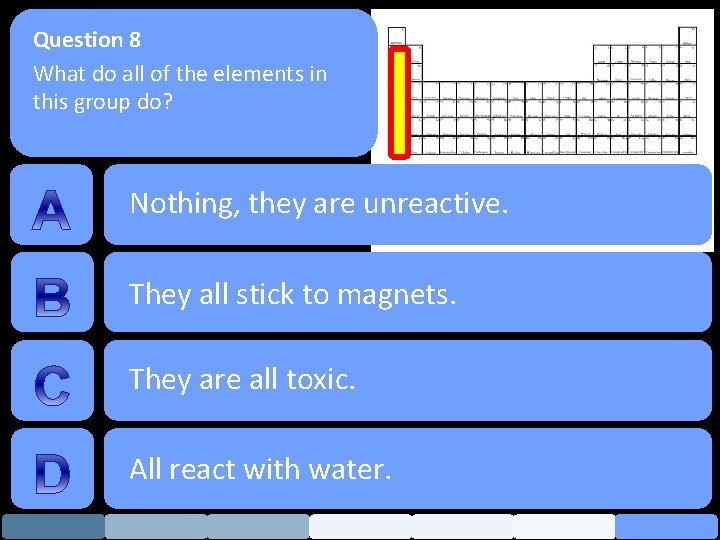
Question 8 What do all of the elements in this group do? Nothing, they are unreactive. They all stick to magnets. They are all toxic. All react with water.

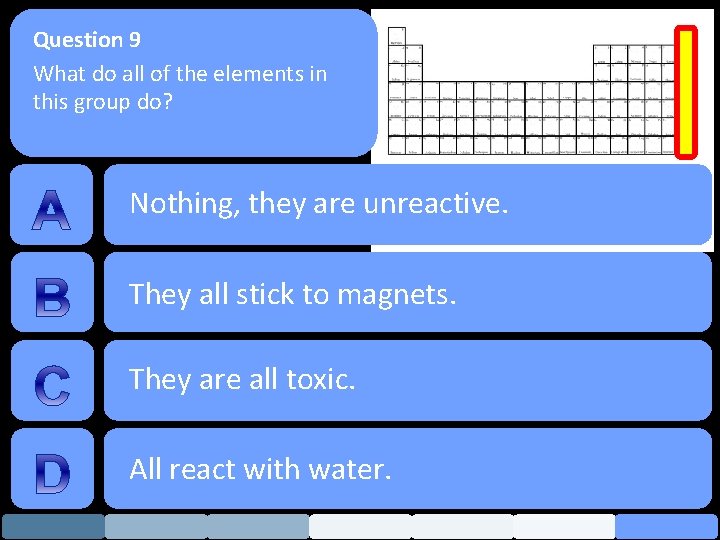
Question 9 What do all of the elements in this group do? Nothing, they are unreactive. They all stick to magnets. They are all toxic. All react with water.

Question 10 Which of these is the biggest group in the periodic table? Solids (at 20 OC) Liquids (at 20 OC) Gases (at 20 OC) Metals

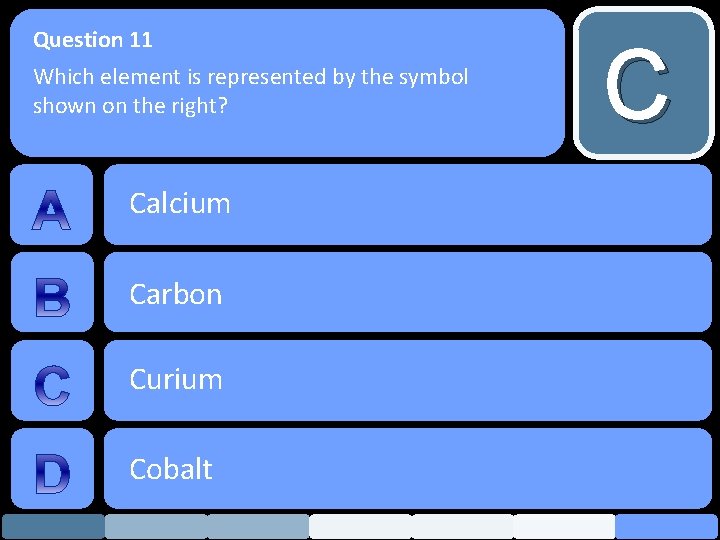
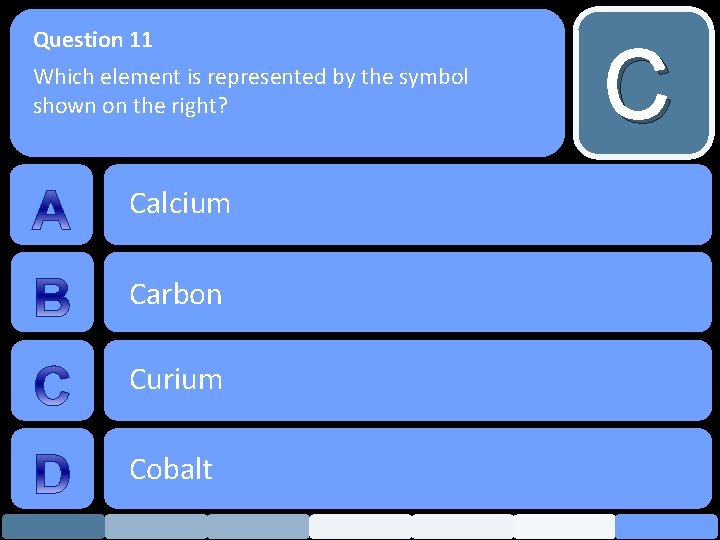
Question 11 Which element is represented by the symbol shown on the right? Calcium Carbon Curium Cobalt C

Question 12 Which element is represented by the symbol shown on the right? Beryllium Boron Berkelium Barium Be

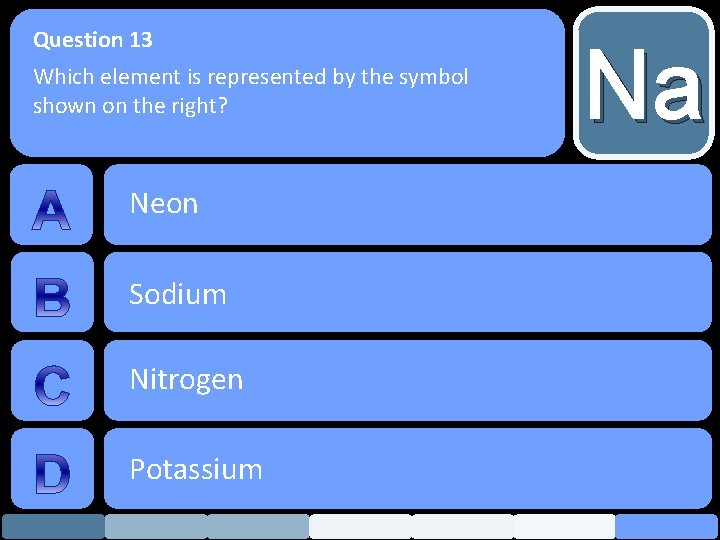
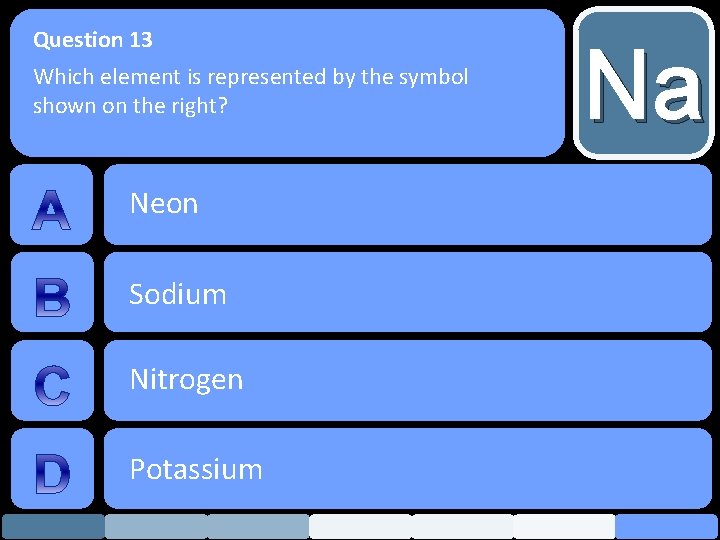
Question 13 Which element is represented by the symbol shown on the right? Neon Sodium Nitrogen Potassium Na

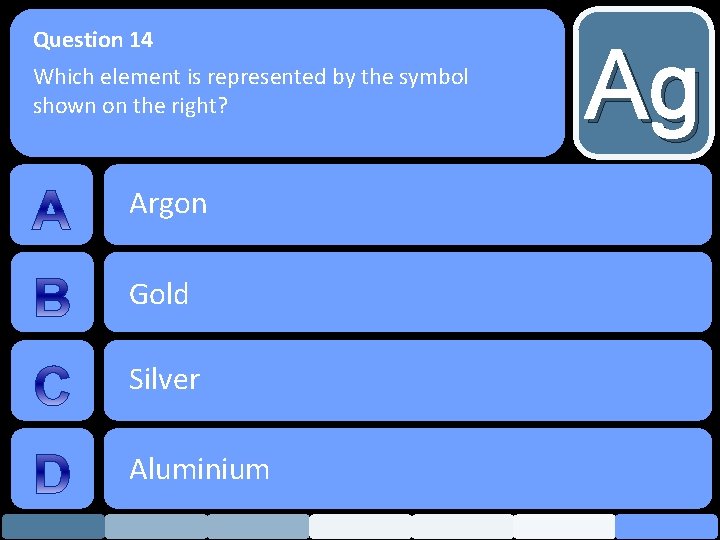
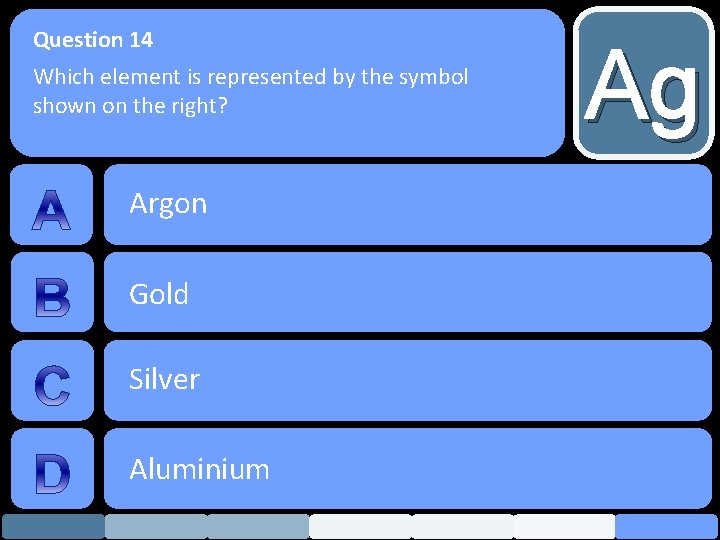
Question 14 Which element is represented by the symbol shown on the right? Argon Gold Silver Aluminium Ag

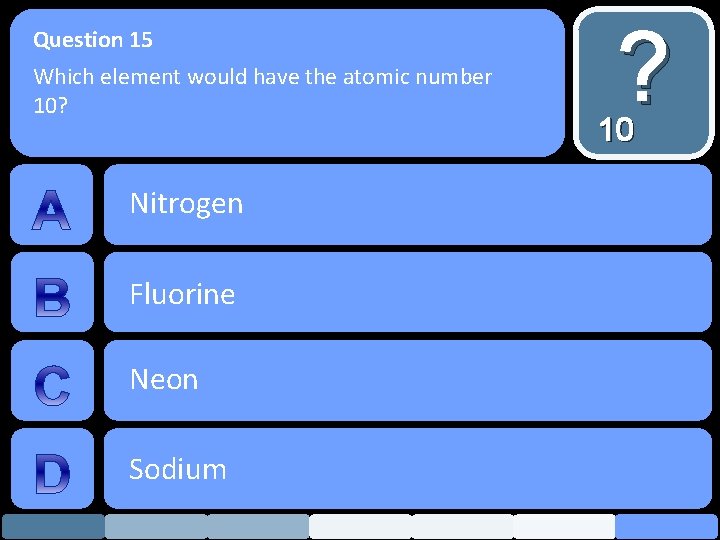
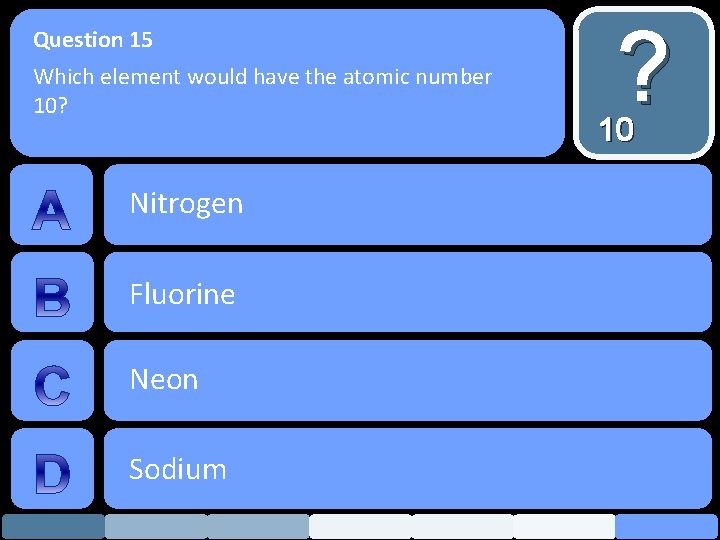
Question 15 Which element would have the atomic number 10? Nitrogen Fluorine Neon Sodium ? 10

Question 1 How many different groups are there on the periodic table? 4 8 10 18

Question 2 How many different periods are there on the periodic table? 6 7 8 9

Question 3 What is the name for all of the elements in group VIII (also called group 0) of the periodic table? Transition Metals Noble Gases Alkali Metals Halogens

Question 4 What are the elements in this section of the periodic table called? Transformational Metals Transition Metals Non-metals Metaloids

Question 5 Which of the elements in not in the group highlighted on the periodic table? Chlorine Bromine Neon Iodine

Question 6 Which element would be found in the highlighted box on the periodic table? Neon Lithium Hydrogen Helium

Question 7 What does this line separate on the periodic table? Solids and Liquids and Gases Metals and Non-Metals Solids and Gases

Question 8 What do all of the elements in this group do? Nothing, they are unreactive. They all stick to magnets. They are all toxic. All react with water.

Question 9 What do all of the elements in this group do? Nothing, they are unreactive. They all stick to magnets. They are all toxic. All react with water.

Question 10 Which of these is the biggest group in the periodic table? Solids (at 20 OC) Liquids (at 20 OC) Gases (at 20 OC) Metals

Question 11 Which element is represented by the symbol shown on the right? Calcium Carbon Curium Cobalt C

Question 12 Which element is represented by the symbol shown on the right? Beryllium Boron Berkelium Barium Be

Question 13 Which element is represented by the symbol shown on the right? Neon Sodium Nitrogen Potassium Na

Question 14 Which element is represented by the symbol shown on the right? Argon Gold Silver Aluminium Ag

Question 15 Which element would have the atomic number 10? Nitrogen Fluorine Neon Sodium ? 10
 Ringworm cks
Ringworm cks Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Mastalgia cks
Mastalgia cks Pesi score
Pesi score Haematospermia cks
Haematospermia cks Nice cks olecranon bursitis
Nice cks olecranon bursitis Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Whats a mole chemistry
Whats a mole chemistry Whats hot whats not
Whats hot whats not Chemistry unit 9 lesson 4
Chemistry unit 9 lesson 4 Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Chemistry unit 7 molarity
Chemistry unit 7 molarity Ap chemistry unit 9
Ap chemistry unit 9 Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Units of concentration
Units of concentration Wjec chemistry unit 2
Wjec chemistry unit 2 Unit 8 ap chemistry
Unit 8 ap chemistry Types of reactions grade 11
Types of reactions grade 11 Chemistry unit 3 study guide
Chemistry unit 3 study guide Chemistry unit review answer key
Chemistry unit review answer key Chemistry grade 10 unit 4
Chemistry grade 10 unit 4 Unit 6 exam chemistry
Unit 6 exam chemistry Ap chemistry molecular geometry
Ap chemistry molecular geometry Cfe higher chemistry
Cfe higher chemistry Wjec chemistry unit 2
Wjec chemistry unit 2 Chemistry unit 6 sticky tape post lab
Chemistry unit 6 sticky tape post lab Living by chemistry unit 2 smells answers
Living by chemistry unit 2 smells answers Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Ap chemistry unit 3
Ap chemistry unit 3 Ap chemistry unit 2
Ap chemistry unit 2 Chemistry unit 4 review answer key
Chemistry unit 4 review answer key Three characteristics of metals
Three characteristics of metals Ap chemistry unit 3
Ap chemistry unit 3 Unit analysis chemistry
Unit analysis chemistry Conversion problems with answers
Conversion problems with answers Chemistry unit 6
Chemistry unit 6 Chemistry
Chemistry Whats a unit rate
Whats a unit rate The smallest unit of matter
The smallest unit of matter Whats unit 7
Whats unit 7 Unit fraction
Unit fraction The smallest unit of an ionic compound is a
The smallest unit of an ionic compound is a Celsius degree symbol
Celsius degree symbol Metode pembiayaan langsung (direct financing method)
Metode pembiayaan langsung (direct financing method) Right triangle trigonometry
Right triangle trigonometry English unit conversion
English unit conversion Unit 1 test algebra 2 answers
Unit 1 test algebra 2 answers Unit cost rekam medis
Unit cost rekam medis Unit process and unit operation
Unit process and unit operation Unit operation and unit process
Unit operation and unit process Setiap unit akuntansi dianggap sebagai unit yang mandiri
Setiap unit akuntansi dianggap sebagai unit yang mandiri Calculating percentage yield
Calculating percentage yield You light up my life chemistry lab answers
You light up my life chemistry lab answers How to find theoretical mass
How to find theoretical mass Criss cross chemistry
Criss cross chemistry Abstract chemistry example
Abstract chemistry example Graphical abstract example
Graphical abstract example