CKS 3 Yr 8 Chemistry Unit 4 The













- Slides: 27

CKS 3: Yr 8 Chemistry Unit 4 – The chemical patterns that make my world Lesson number: 2 Lesson Title: Reactivity series of metals- metals and acid Learning Outcomes Learning Outcome 1: Describe how to test for the gas produced when a metal reacts with an acid Level 5 How I did Targets Connector: (Level 4) How do you know a chemical reaction is taking place? Think Pair Share Learning Outcome 2: Explain why hydrogen gas is produced and express these reactions as balanced symbol equations Level 6/7 Learning Outcome 3: Controlled assessment: Carry out the assessed task on reactions of metals and acid (Acid fizz) 18: 56 TJ

BIG picture Key Question: Why is hydrogen gas produced when an acid reacts with a metal? • What skills will you be developing this lesson? • HSW- by planning and carrying out an investigation/ Interpreting data/ evaluating an experiment • Literacy- by writing explanations using correctly spelt keywords and good grammar. • Team work- during a practical investigation • Participation- during a practical activity • Reflection- through self and peer assessment of each outcome • How is this lesson relevant to every day life? Where does this lesson fit in to the rest of the topic? Lesson 2 • Quick Discussion: • What do you already know?

Keywords: Create sentences using the keywords to show that you know what they mean. Put your hand up if there is any key word from the list that you don’t understand. • Hydrogen • Copper • Metal • Zinc • Chemical reactions • Lighted splint • ‘Squeaky pop’ • Reactivity • Magnesium • Iron

New Information for Learning Outcome 1 • Visual: Demonstration • Audio: Demonstration • Kinaesthetic: Class experiment

New Information for Task 1 What happens when we put Magnesium into hydrochloric acid?

New Information for Task 1 Many metals react with acids. When this happens the metal fizzes as bubbles are produced. hydrogen burning splint What do the bubbles mean? Do all the metals react A gas is produced. equally to acids? magnesium + acid How can you test to find out if the gas produced is hydrogen? Place a burning splint next to the mouth of test tube. A ‘squeaky pop’ as the gas ignites shows that hydrogen is the gas produced in this reaction.

Learning Activities for Outcome 1 • Your task is to carry out the reaction between acid and metal. • You will also carry out a test for the reaction to show that it has taken place.

Learning Activities for Outcome 1 • What equipment will you need? • • 4 test tubes Test tube rack Zinc, copper, magnesium and iron Spatula Splint Hydrochloric acid Bunsen burner Heat proof mat

Keywords: Demonstrate your Learning for Outcome 1 I am working at level. . because. . . Create Evaluate Apply (L 5) Using the information from the practical, can you place the metals in order from most reactive to the least reactive justifying your choice Analyse (L 6) Can you construct a general word equation for the reaction Apply Understand Remember 18: 57 Understand (L 4) Describe what happened when acid was added to the metals and explain the test for the gas produced

Learning Activities for Outcome 1 • Order of reactivity : magnesium zinc iron copper 1. Which acid is a stronger acid? How do you know?

Learning Outcome 1: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcome 1: Describe how to test for the gas produced when a metal reacts with an acid Level 5 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 1?

New Information for Learning Outcome 2 Why is Hydrogen gas produced when a metal reacts with an acid?

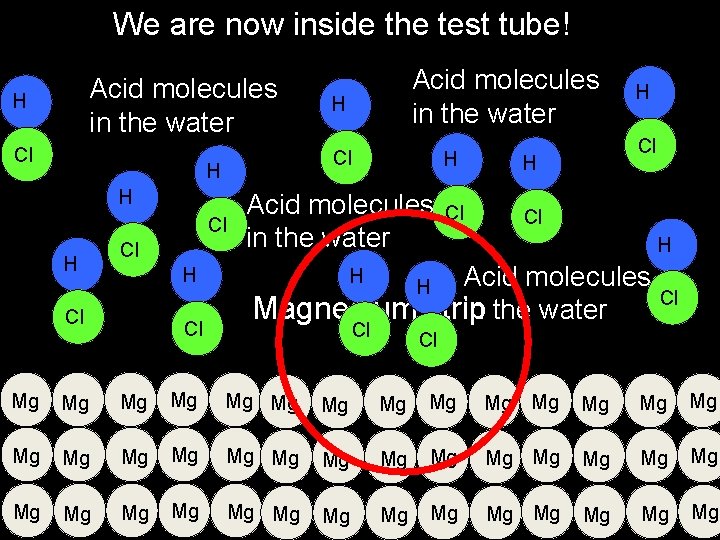
New Information for Learning Outcome 2 Zooming into a microscopic level…. . We are going to look at the surface of the magnesium in hydrochloric acid

We are now inside the test tube! Acid molecules in the water H Cl Cl H H Acid molecules in the water Cl Cl H H Acid molecules in the water H Cl H Acid molecules Cl Magnesium strip in the water H H Cl Cl Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg

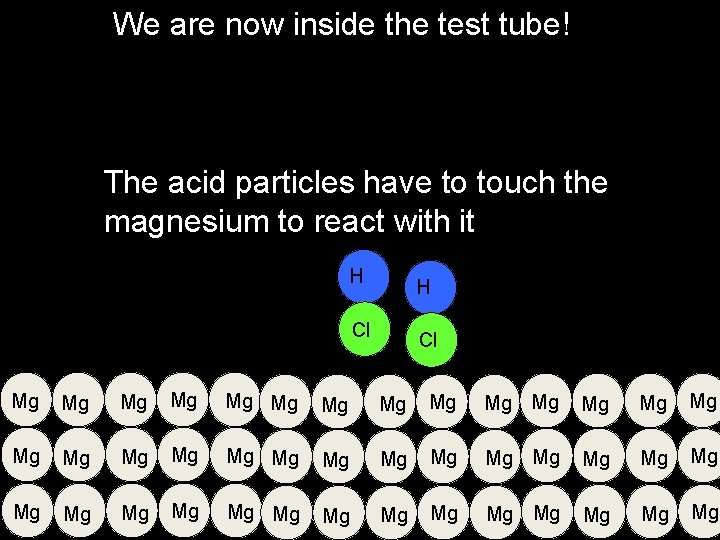
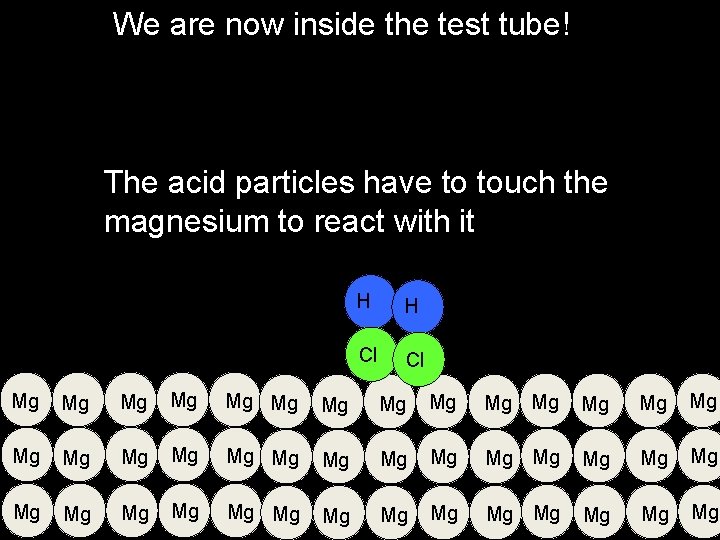
We are now inside the test tube! The acid particles have to touch the magnesium to react with it H H Cl Cl Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg

We are now inside the test tube! The acid particles have to touch the magnesium to react with it H H Cl Cl Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg

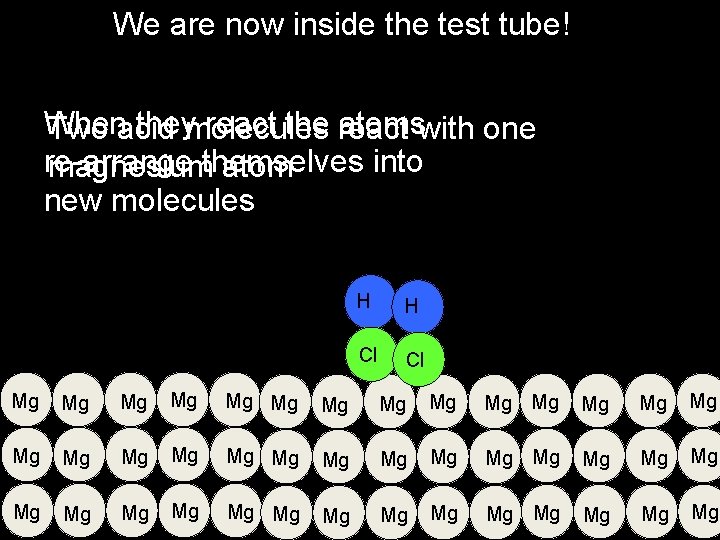
We are now inside the test tube! When theymolecules react the react atomswith one Two acid re-arrange into magnesiumthemselves atom new molecules H H Cl Cl Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg Mg

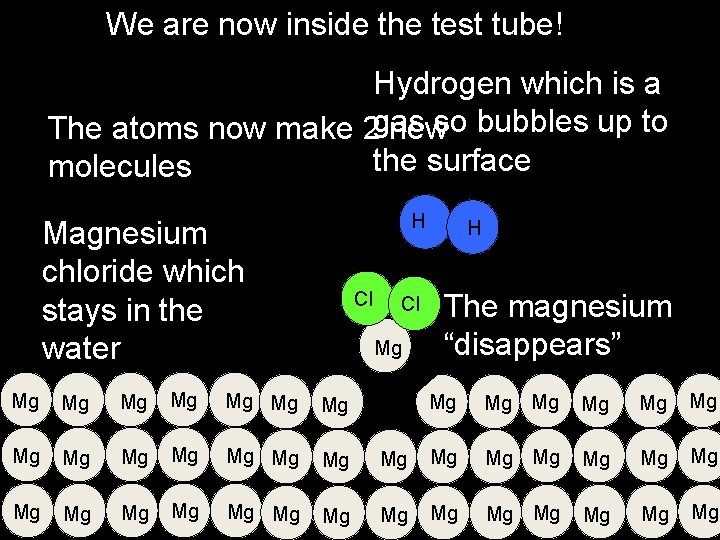
We are now inside the test tube! Hydrogen which is a The atoms now make 2 gas newso bubbles up to the surface molecules H Magnesium chloride which stays in the water Cl Cl Mg Mg Mg Mg Mg Mg H The magnesium “disappears” Mg Mg Mg Mg Mg

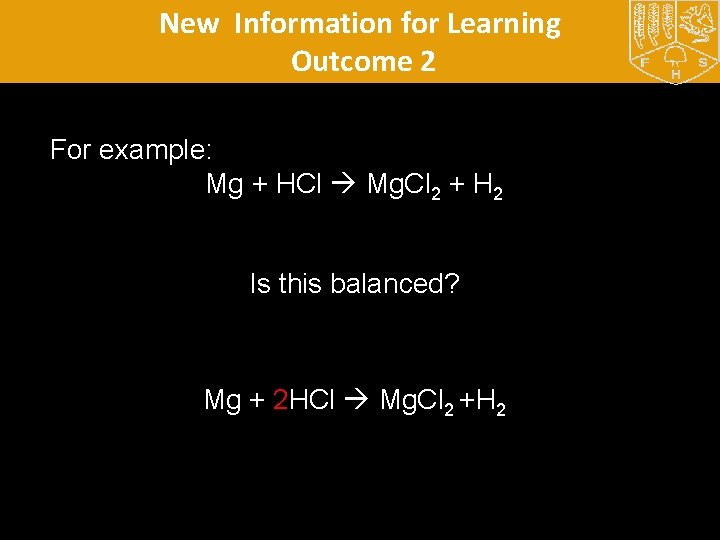
New Information for Learning Outcome 2 What is the general equation for when an acid reacts with a metal? Metal + acid salt + Hydrogen Can you use this to write equations for what the observation exercise? Can you write symbol equations for the reactions of magnesium, iron, zinc and copper with acid?

New Information for Learning Outcome 2 For example: Mg + HCl Mg. Cl 2 + H 2 Is this balanced? Mg + 2 HCl Mg. Cl 2 +H 2

Keywords: Demonstrate your Learning for Outcome 2 I am working at level. . because. . . Create Evaluate Apply (L 5) Using a model, explain why hydrogen gas is produced? Analyse Apply Understand Remember 18: 57 Analyse (L 6/7) Can you write symbol equations for the successful reactions?

Learning Outcome 2: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcome 2: Demonstrate the concept of acid reactions with metal carbonates by making bath bombs Level 6 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 2?

Learning Activities for Task 3 You will carry out an controlled assessment (acid fizz) Aim: complete cartoon strip and explain each step

Learning Activities for Task 3 To get level You might have: 5 Drawn diagrams and described what happened in the reaction. Drawn simple particle diagrams to represent the chemicals in the reaction. Identified the elements and compounds. Described the appearance and properties of the materials before and after the reaction (classified as metals or non-metals). 6 Drawn accurate particle diagrams to describe the reaction. Written the word equation for the reaction. Used some symbols to represent some elements. Described why the substances can be classified as elements or compounds. Explained if the mass of the beaker and its contents will stay the same throughout the reaction. Carry out peer/self assessment using this criteria 7 Drawn detailed and accurate particle diagrams to explain the reaction. Accurately written the word and balanced symbol equation. Explained if the mass of the beaker and its contents will stay the same throughout the reaction. Used these key words accurately: atom, molecule, element, compound. Used knowledge of chemical reactions to make generalisations and described other similar reactions.

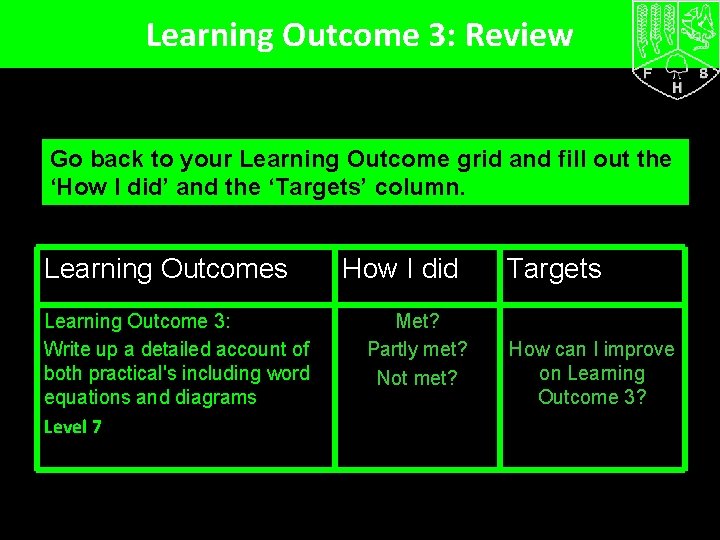
Learning Outcome 3: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcomes Learning Outcome 3: Write up a detailed account of both practical's including word equations and diagrams Level 7 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 3?

Review for Remembering What have I learnt today? ? When a ball is thrown at you, you need to say one thing you have learnt form today’s lesson

Science Department Lesson plan Teacher information 4 test tubes Test tube rack Metals: Zinc, Iron, Magnesium and Copper Spatula Pipette Hydrochloric acid Splints Bunsen burner Heat proof mat Provision: 1) EAL: 2) SEN: Role of TA: 1) 2) 3)