CKS 3 Year 8 Chemistry Unit 3 Whats
















- Slides: 39

CKS 3: Year 8 Chemistry Unit 3 –What's My World Made Of? Part 2: Lesson number: 1. Lesson Title: Atoms, Elements and the Periodic Table Lesson Outcomes How I did Learning Outcome 1: Explain each element is made up of one type of atoms. Level 5 Connector: (Level 4) Write the names of various materials and elements around you. Learning Outcome 2: Explain the structure of an atom. Level 6 Learning Outcome 3: Knowledge of the particle model to represent elements in different states Level 7 Targets TJ

BIG picture Key Question: What is the difference between an element and a compound? • What skills will you be developing this lesson? • HSW- by planning and carrying out an investigation/ Interpreting data/ evaluating an experiment • How is this lesson relevant to every day life? Chemicals and reactions used to work machines for example, for fireworks • Literacy- by writing explanations using correctly spelt keywords and good grammar. Where does this lesson fit in to the rest of the topic? Lesson 1 • Team work- during a practical investigation • Quick Discussion: • What do you already know? • Participation- during a practical activity • Reflection- through self and peer

Keywords: Create sentences using the keywords to show that you know what they mean. Put your hand up if there is any key word from the list that you don’t understand. • • • Phenomena Element Compound Reaction Data Valid Reaction Word Equation Symbol equation.

New Information for Learning Outcome 1 • Visual: Demonstration • Audio: Demonstration • Kinaesthetic: Class experiment

New Information for Learning Outcome 1 Millions of substances There are millions of substances and as a first step to understanding them scientists sort these substances into various types or groups. Here are some of the more common ways of grouping substances. There are far fewer elements than substances. Solid Liquid Natural Synthetic Metal Non-metal Element Compound Gas Mixture In this unit the focus is mainly upon atoms and elements.

New Information for Learning Outcome 1 There are millions of different substances! What are they all made of?

New Information for Learning Outcome 1 All substances are made of very tiny particles called atoms. Many substances are made up of different types of atoms. hydrogen and oxygen atoms carbon and hydrogen atoms iron, aluminium, silicon, oxygen and boron atoms carbon, nitrogen, hydrogen, oxygen and sulphur atoms

New Information for Learning Outcome 1 All substances are made of atoms = tiny particle that all substances are made up of Many substances are made up of different types of atoms.

New Information for Learning Outcome 1 There about one hundred substances that are made up of just one type of atom. These are the elements. carbon helium copper The elements are the simplest substances in the universe. The elements are the building blocks of all other substances.

New Information for Learning Outcome 1 carbon bromine silicon copper chlorine uranium mercury krypton

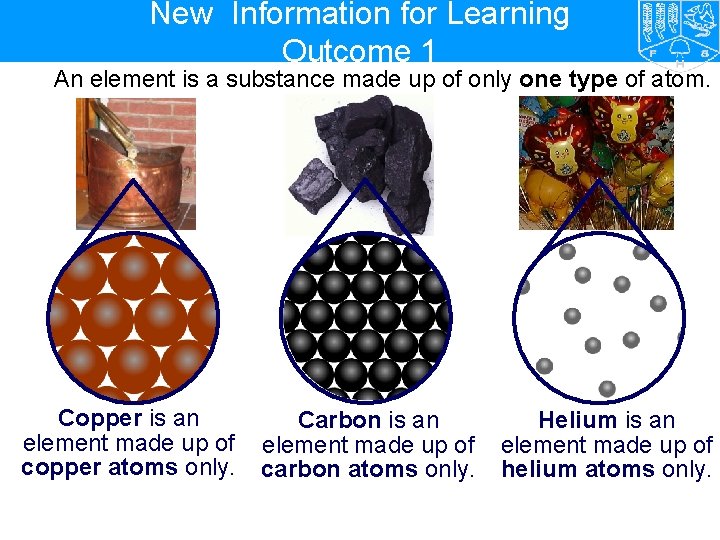
New Information for Learning Outcome 1 An element is a substance made up of only one type of atom. Copper is an element made up of copper atoms only. Carbon is an element made up of carbon atoms only. Helium is an element made up of helium atoms only.

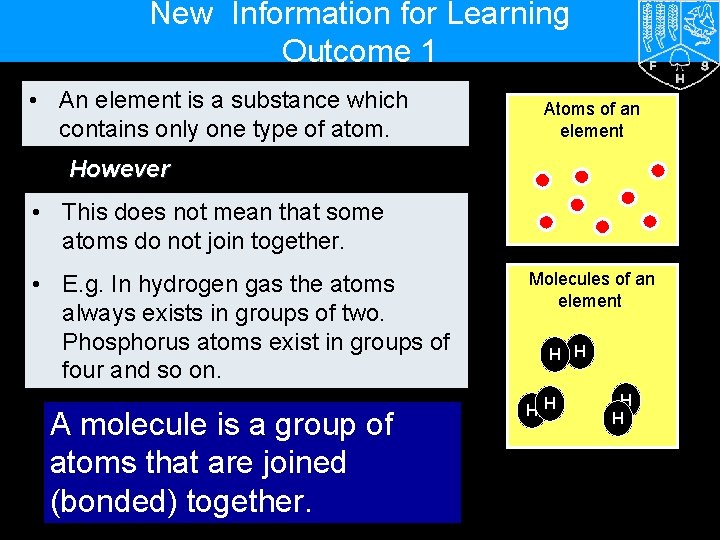
New Information for Learning Outcome 1 • An element is a substance which contains only one type of atom. Atoms of an element However • This does not mean that some atoms do not join together. • E. g. In hydrogen gas the atoms always exists in groups of two. Phosphorus atoms exist in groups of four and so on. A molecule is a group of atoms that are joined (bonded) together. Molecules of an element. H H H

New Information for Learning Outcome 1 Oxygen is an element made up oxygen atoms only. How many atoms are there in an oxygen molecule? Other elements, that contain atoms joined in molecules are hydrogen, nitrogen, chlorine and bromine.

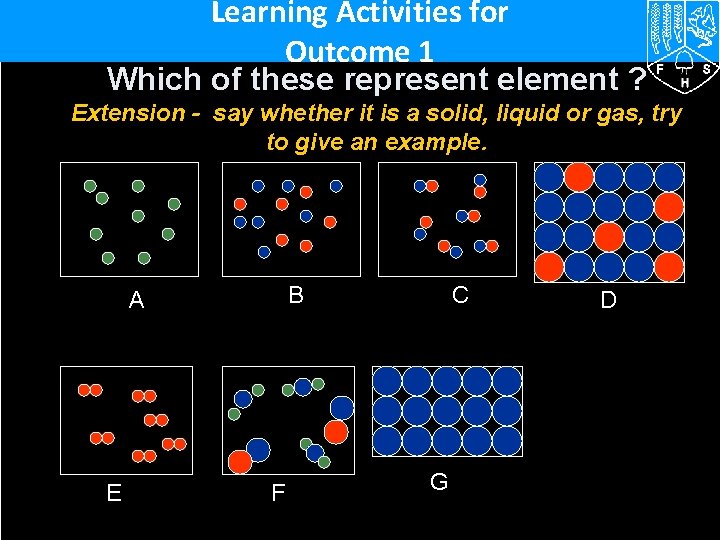
Learning Activities for Outcome 1 Which of these represent element ? Extension - say whether it is a solid, liquid or gas, try to give an example. B A E F C G D

Keywords: Demonstrate your Learning for Outcome 1 I am working at level. . because. . . Create (L 8) Evaluate (L 7) Judge Justify Defend Decide Agree Value Prove Check Criticise Recommend Support Test Create Evaluate Combine construct Develop Imagine Design Change Improve Discuss Create Invent Suppose Put together Make up Synthesise Analyse (L 6) Apply (L 5) Use Build Execute Develop Construct Identify Plan Select Solve Organise Apply Model Analyse Apply Remember (L 3) Understand Who What When Where Why Which How Match Define List Choose Name Spell Tell Describe Remember 18: 58 Take apart Compare Classify Examine List Distinguish Simplify Theme Conclude Motive Discover Understand (L 4) Explain what when where how Rephrase Demonstrate Summarise Contrast Show Predict Compare Clarify Illustrate Categorise

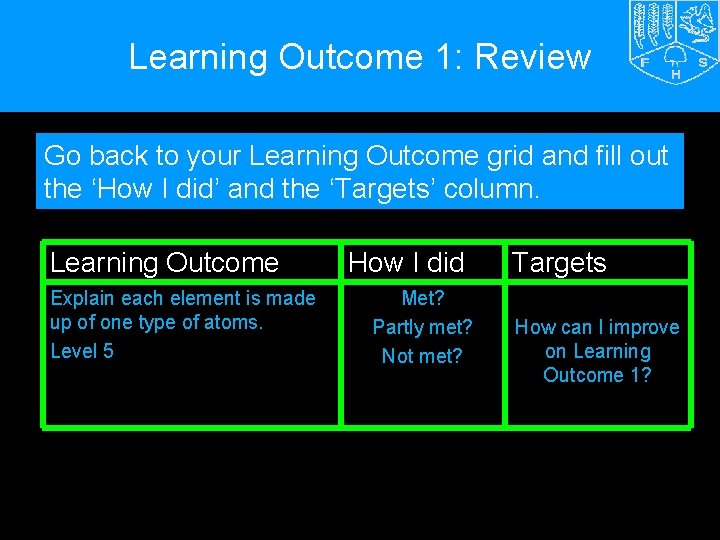
Learning Outcome 1: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcome Explain each element is made up of one type of atoms. Level 5 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 1?

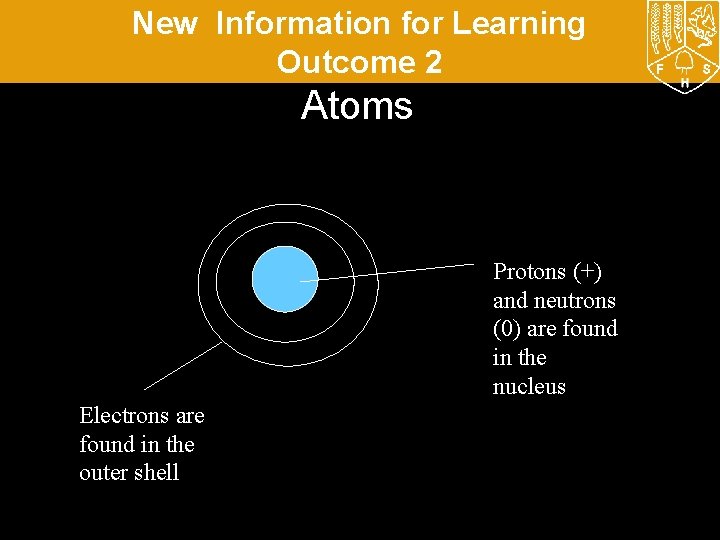
New Information for Learning Outcome 2 Atoms Protons (+) and neutrons (0) are found in the nucleus Electrons are found in the outer shell

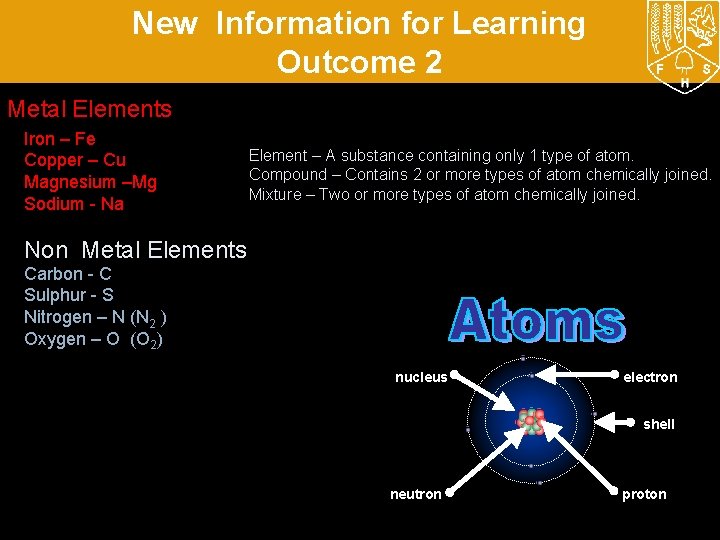
New Information for Learning Outcome 2 Metal Elements Iron – Fe Copper – Cu Magnesium –Mg Sodium - Na Element – A substance containing only 1 type of atom. Compound – Contains 2 or more types of atom chemically joined. Mixture – Two or more types of atom chemically joined. Non Metal Elements Carbon - C Sulphur - S Nitrogen – N (N 2 ) Oxygen – O (O 2) nucleus electron shell neutron proton

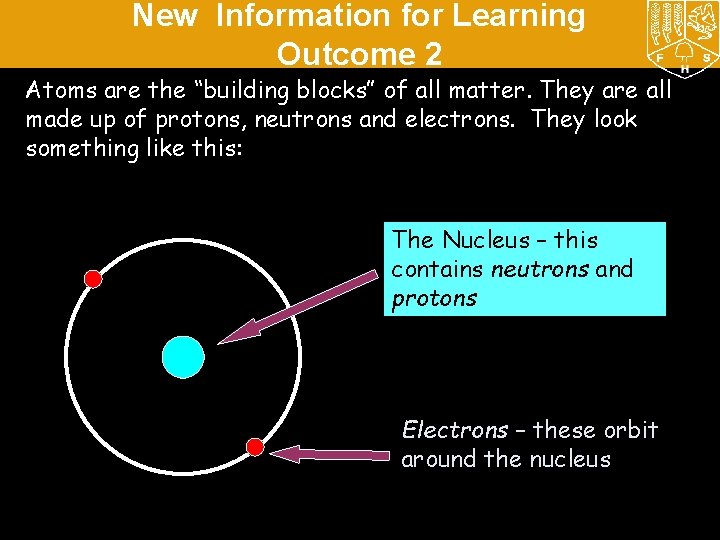
New Information for Learning Outcome 2 Atoms are the “building blocks” of all matter. They are all made up of protons, neutrons and electrons. They look something like this: The Nucleus – this contains neutrons and protons Electrons – these orbit around the nucleus

Learning Activities for Outcome 2 • Draw a model of an atom of Helium • Helium atom contains 2 electrons, 2 protons and 2 neutrons. • Draw lithium, carbon, oxygen, sulphur, chlorine, magnesium

Learning Outcome 2: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Lesson Outcomes Explain the structure of an atom. Level 6 How I did Met? Partly met? Not met? Targets How can I improve on task 2?

Keywords: Demonstrate your Learning for Outcome 2 I am working at level. . because. . . Create (L 8) Evaluate (L 7) Judge Justify Defend Decide Agree Value Prove Check Criticise Recommend Support Test Create Evaluate Combine construct Develop Imagine Design Change Improve Discuss Create Invent Suppose Put together Make up Synthesise Analyse (L 6) Apply (L 5) Use Build Execute Develop Construct Identify Plan Select Solve Organise Apply Model Analyse Apply Remember (L 3) Understand Who What When Where Why Which How Match Define List Choose Name Spell Tell Describe Remember 18: 58 Take apart Compare Classify Examine List Distinguish Simplify Theme Conclude Motive Discover Understand (L 4) Explain what when where how Rephrase Demonstrate Summarise Contrast Show Predict Compare Clarify Illustrate Categorise

New Information for Learning Outcome 3 Scientists use a model to give a mental picture of what is happening in their investigation. A theory is an idea that explains observations Any theory about particles must be able to explain the differences between a solid, a liquid and a gas.

New Information for Learning Outcome 3 The particle model of matter describes: how particles are arranged how much energy particles have and how they move In some matter the particles are held together by forces of attraction. Use the particle model to decide if the forces of attraction are strong, weak or do not exist.

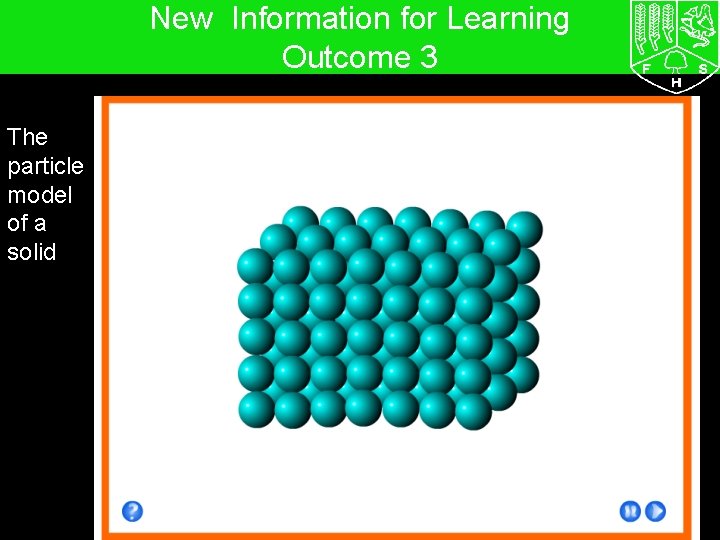
7 G The particle model – Solids, liquids and gases New Information for Learning Outcome 3 The particle model of a solid

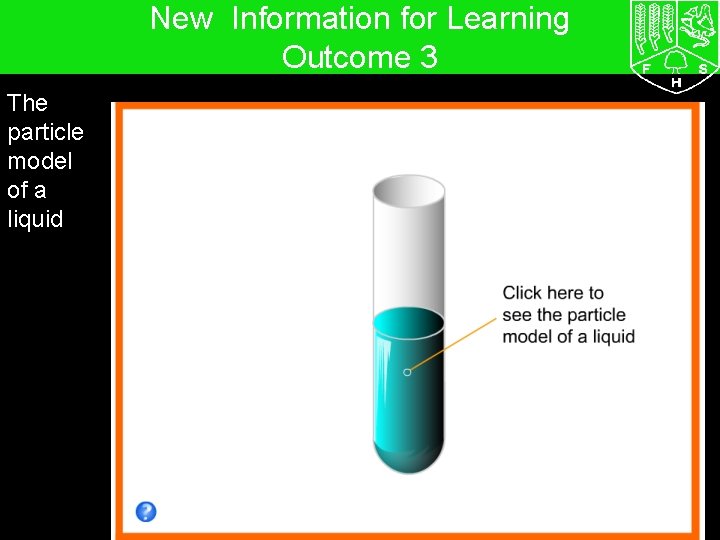
7 G The particle model – Solids, liquids and gases New Information for Learning Outcome 3 The particle model of a liquid

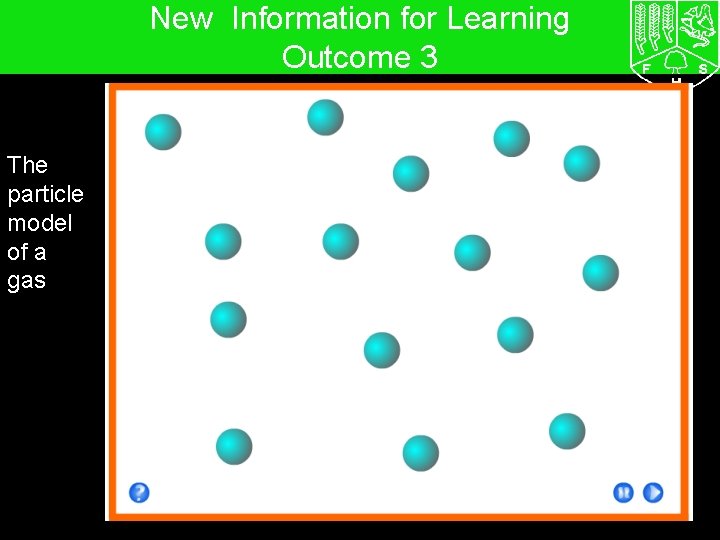
7 G The particle model – Solids, liquids and gases New Information for Learning Outcome 3 The particle model of a gas

7 G The particle model – Solids, liquids and gases New Information for Learning Outcome 3 solid particles… - are very close together in a fixed arrangement - have a small amount of energy - vibrate but do not move liquid particles… - are close together but have no fixed arrangement - more energy than solid particles - vibrate and can move about gas particles - are far apart and have no fixed arrangement - have a large amount of energy - move rapidly in all directions

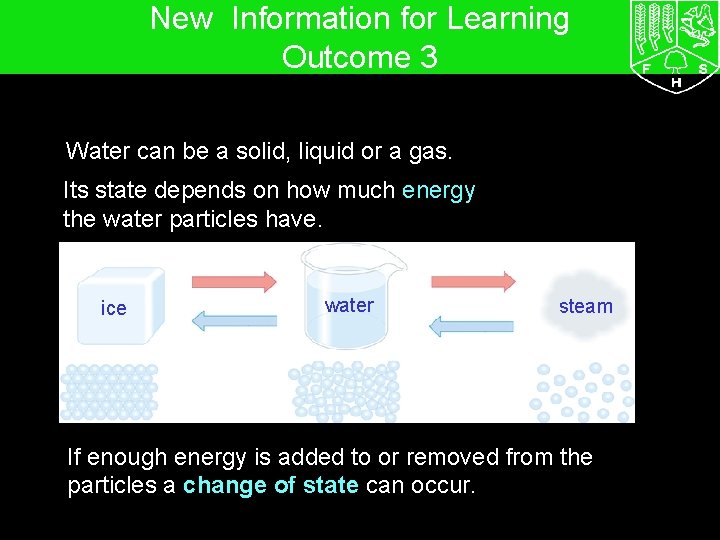
7 G The particle model – Changing state New Information for Learning Outcome 3 Water can be a solid, liquid or a gas. Its state depends on how much energy the water particles have. ice water steam If enough energy is added to or removed from the particles a change of state can occur.

New Information for Learning Outcome 3 a • Experiment • Aim: Investigate the change in temperature of ice when heated. • Prediction: • Method: • Diagram and equipment: • Results:

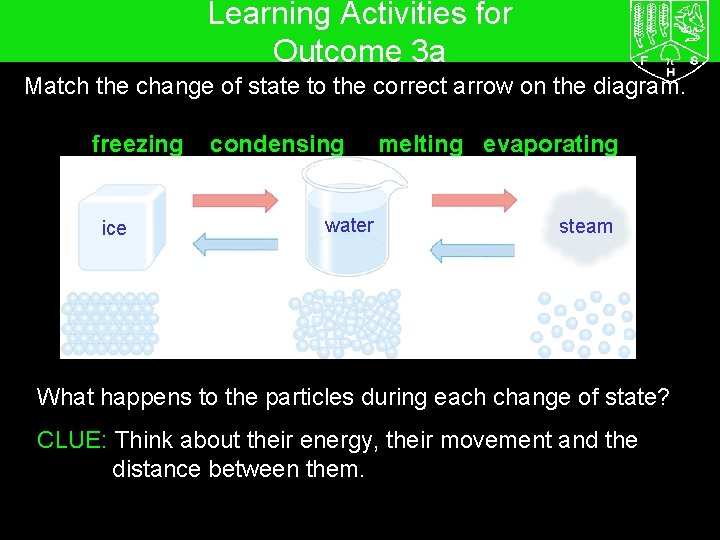
Learning Activities for Outcome 3 a 7 G The particle model – Changing state Match the change of state to the correct arrow on the diagram. freezing ice condensing water melting evaporating steam What happens to the particles during each change of state? CLUE: Think about their energy, their movement and the distance between them.

Learning Activities for Outcome 3 a • Task 3: • Write a conclusion to explain your findings during the investigation and explain how a change in energy affects the particles.

Learning Activities for Outcome 3 b • Draw particle diagrams of the following elements 1. Helium (gas) 2. Mercury (liquid) 3. Copper (solid) 4. Oxygen (gas – it is diatomic) • Extension • If carbon is heated in a limited supply of air, carbon monoxide is produced. Explain why you think this might be a dangerous experiment to do in the laboratory at school

Learning Activities for Outcome 3 b • • Look at these five words about particles. • atom molecule element compound mixture Each word has a different meaning. Match each word to its correct definition as stated below. A contains different kinds of molecules. B the smallest particle in an element. C the smallest particle of a compound. D a substance that contains molecules made from two or more different kinds of atom. E there are 92 of these occurring naturally in nature. Each one consists of the same types of atom.

Task 3: Review Go back to your lesson outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Lesson Outcomes Lesson Outcome 3: Knowledge of the particle model to represent elements in different states Level 7 How I did Met? Partly met? Not met? Targets How can I improve on task 3?

Keywords: Demonstrate your Learning for Outcome 3 I am working at level. . because. . . Create (L 8) Evaluate (L 7) Judge Justify Defend Decide Agree Value Prove Check Criticise Recommend Support Test Create Evaluate Combine construct Develop Imagine Design Change Improve Discuss Create Invent Suppose Put together Make up Synthesise Analyse (L 6) Apply (L 5) Use Build Execute Develop Construct Identify Plan Select Solve Organise Apply Model Analyse Apply Remember (L 3) Understand Who What When Where Why Which How Match Define List Choose Name Spell Tell Describe Remember 18: 58 Take apart Compare Classify Examine List Distinguish Simplify Theme Conclude Motive Discover Understand (L 4) Explain what when where how Rephrase Demonstrate Summarise Contrast Show Predict Compare Clarify Illustrate Categorise

Review for Remembering • Stand up if you have met the lesson outcomes? • If not what do you need to do next in order to meet the outcome? Record this in your diary as part of your homework. • Is there any part of the lesson you think you need to go over again next lesson? • Tell the person next to you three things you have learnt this lesson. • How will you remember this for your exam?

Review for Remembering True or false Every substance is an element All elements are solids. 3 Elements cannot be broken down into anything simpler. 4 An element contains only one type of atom. 5 Helium is an example of an element. 6 Air is a mixture of elements. 7 Atoms of an element cannot join together. 8 There are millions of elements present on earth. 9 Water is an example of an element. 10 A molecule is formed when a group of atoms chemically join. • 1 • 2 • •

Extended Learning task: • Due date: next lesson • Criteria for Level 5: • Criteria for level 6: • Criteria for level 7: