CHRONIC KIDNEY DISEASE Asist prof Magdalena Strcea IVth











































- Slides: 80

CHRONIC KIDNEY DISEASE Asist. prof. Magdalena Stârcea IVth Pediatric Clinic

Definitions and terminology • Chronic Kidney Disease (CKD) is defined as the irreversible loss of kidney function, which results in a decrease in glomerular filtration rate.

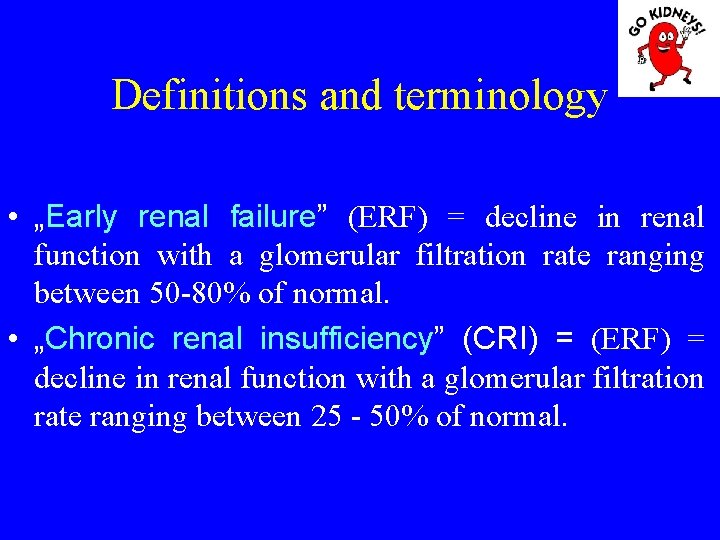
Definitions and terminology • „Early renal failure” (ERF) = decline in renal function with a glomerular filtration rate ranging between 50 -80% of normal. • „Chronic renal insufficiency” (CRI) = (ERF) = decline in renal function with a glomerular filtration rate ranging between 25 - 50% of normal.

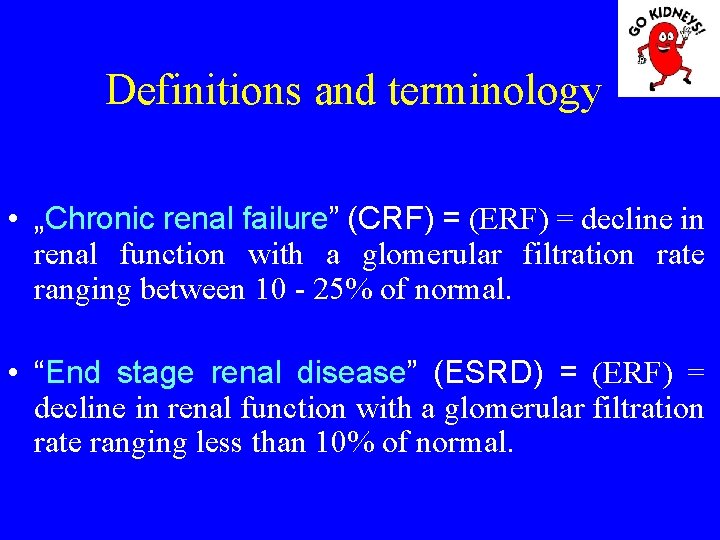
Definitions and terminology • „Chronic renal failure” (CRF) = (ERF) = decline in renal function with a glomerular filtration rate ranging between 10 - 25% of normal. • “End stage renal disease” (ESRD) = (ERF) = decline in renal function with a glomerular filtration rate ranging less than 10% of normal.

Etiology • Urological abnormalities: CKD occur before the age of 6 years, although in some cases it may occur after the age of 8 -12 years. - obstructive abnormalities - renal hypoplasia, - renal dysplasia, - polycystic disease • Chronic glomerulonephritis and other renal diseases progress to renal failure in adolescence.

Pathophysiology IRC Clinical manifestations of the CKD result as a combination of: • failure to maintain fluid and electrolyte balance, • accumulation of toxic metabolites and • lack of renal hormones (erythropoietin and bioactive form 1, 25 dihydroxy - vitamin D 3).

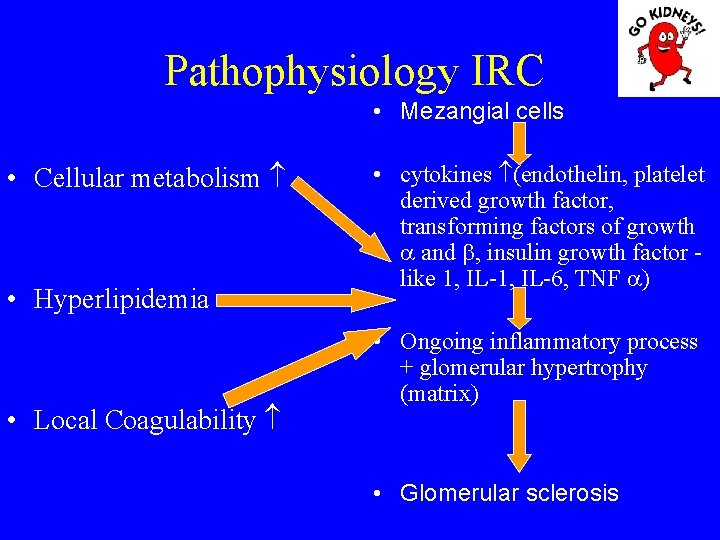
Pathophysiology IRC • Mezangial cells • Cellular metabolism • Hyperlipidemia • Local Coagulability • cytokines (endothelin, platelet derived growth factor, transforming factors of growth and , insulin growth factor - like 1, IL-6, TNF ) • Ongoing inflammatory process + glomerular hypertrophy (matrix) • Glomerular sclerosis

The main pathophysiological syndromes • Growth and development of children with chronic kidney disease In chronic kidney disease is recorded decrease in height. The risk is greater if renal failure early and installed when creatinine clearance falls below 40 ml/minut/1, 73 m 2.


Growth and development of children with chronic kidney disease Factors involved are: - GH decrease - disturbances in the synthesis of somatomedins, insulin-like growth factor (mediates the metabolic effects of GH on cartilage) - P-Ca metabolism disorder that can cause bone deformities

Growth and development of children with chronic kidney disease – Metabolic acidosis, participating both as production osteodystrophy and growth delays. – Anorexia causes decreased caloric intake resulting protein-calorie malnutrition with direct consequences on growth. – Other factors: anemia, hypertension, psychosocial problems. – The duration of action of these factors is verry important.

Malnutrition • Causes: a) Related kidney disease: Anorexia, nausea, vomiting Low energy intake Hipercatabolism increased (coexisting inflammatory condition) The decrease of growth factors

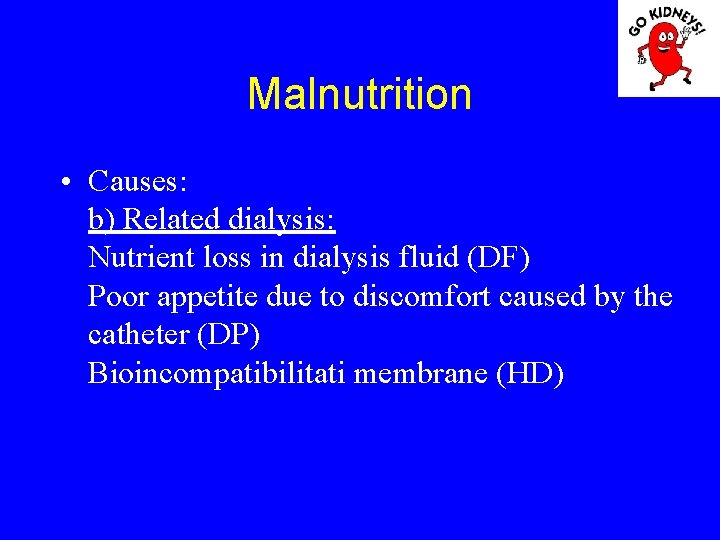
Malnutrition • Causes: b) Related dialysis: Nutrient loss in dialysis fluid (DF) Poor appetite due to discomfort caused by the catheter (DP) Bioincompatibilitati membrane (HD)

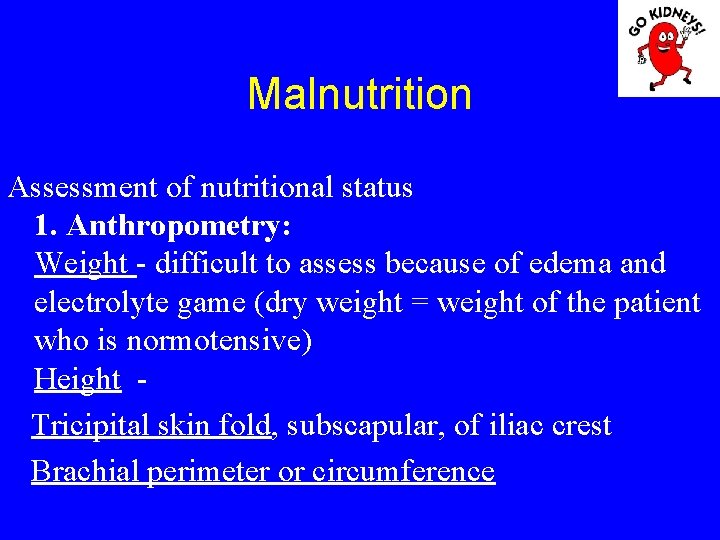
Malnutrition Assessment of nutritional status 1. Anthropometry: Weight - difficult to assess because of edema and electrolyte game (dry weight = weight of the patient who is normotensive) Height - Tricipital skin fold, subscapular, of iliac crest Brachial perimeter or circumference

Malnutrition • Assessment of nutritional status 2. Biological - decreased albumin - transferinemia low - prealbuminele low

Malnutrition • Pathogenic mechanisms: - Inefficient use of protein and use as an alternative energy source branched chain amino acids, with a role in growth (valine, leucine and isoleucine). - Ketoacizi administration improves nutritional status. - L-Carnitine facilitates fatty acid metabolism, improving myocardial contraction.

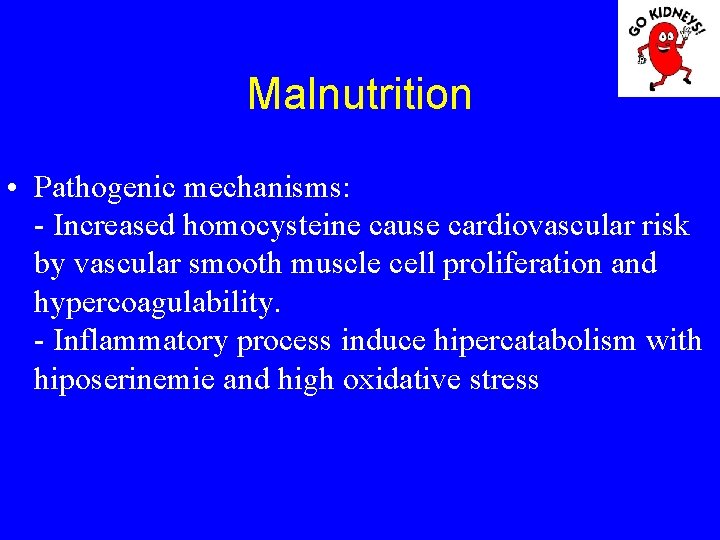
Malnutrition • Pathogenic mechanisms: - Increased homocysteine cause cardiovascular risk by vascular smooth muscle cell proliferation and hypercoagulability. - Inflammatory process induce hipercatabolism with hiposerinemie and high oxidative stress

Malnutrition • Pathogenic mechanisms: - HD induces inflammation through the generation of proinflammatory cytokines. - Oxidative stress with inflammation generates endothelial damage. - Dyslipidemia (increased LDL-cholesterol) increases the risk of atherosclerotic process. - Inflammation increases the risk of calcium deposits in the vessel wall (media), emphasizing compliance disorders and vascular elasticity. Malnutrition increases cardiovascular risk in children with CRF!

Renal osteodystrophy • Pathogenesis 1. Secondary hyperparathyroidism - Hypocalcemia is the main stimulus for triggering hyperparathyroidism in CKD. - Decreased GFR reduce the excretion of phosphorus, which form chemical complexes with Ca, causing calcium loss and the onset of secondary hyperparathyroidism, thus trying to eliminate excess P serum.

Renal osteodystrophy 2. Disorders of vitamin D metabolism in CKD The level of vitamin D is conditioned by the following mechanisms: - synthesis in the skin by the action of sunlight, provitamin D (7 dihidrocolecalciferol); - vitamin D contained in foods undergo hydroxylation in the liver (in position 25) and then kidney (in position 1), resulting in 25 dihydroxy-vitamin D 3, the most active metabolite The vitamion D has the following action: - promoting gastrointestinal absorption of calcium and phosphorus - bone mineralization with keeping normal serum calcium and phosphorus - kidneys - retains calcium and cause phosphaturia

Renal osteodystrophy • In children with CKD, serum metabolite 1, 25 (OH)D 3 are low, especially when GFR reaches <50 ml/minut/1, 73 m 2.


Renal osteodystrophy • Bordier suggested that renal osteodystrophy can evoluate in four successive stages: 1: Stage • Ca, P – normal • High PTH Renal osteodystrophy result of persistent progression and lack hiperparatirodismului 1, 25 (OH) 2 D 3.

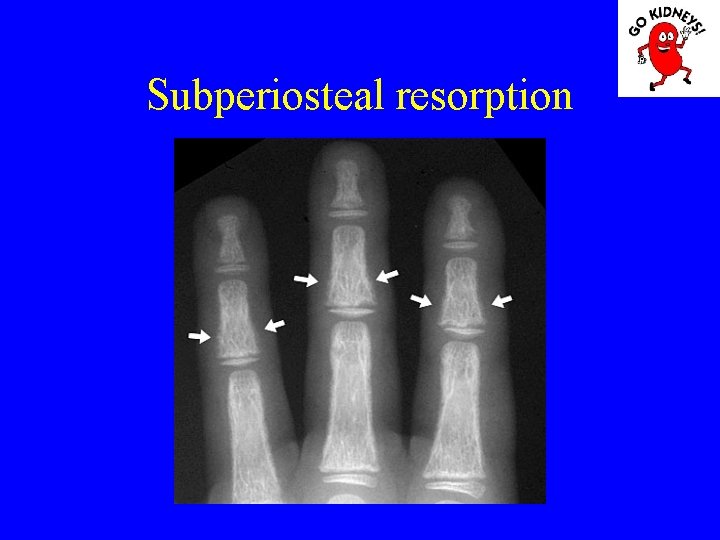
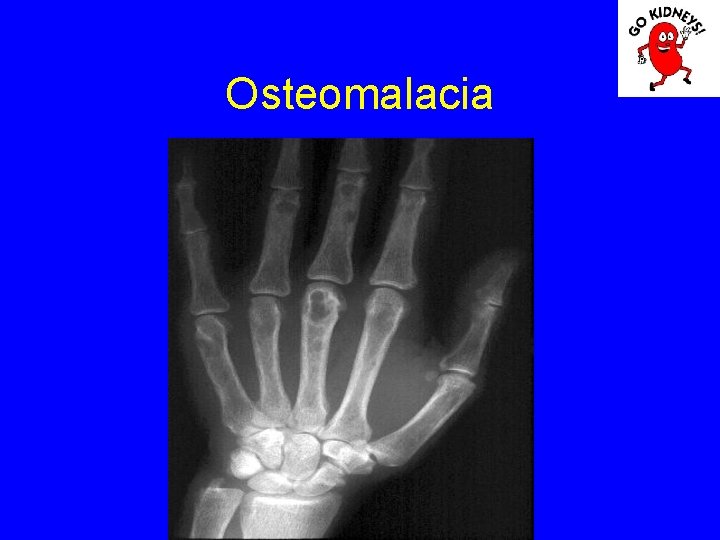
Renal osteodystrophy Stage 2: Despite high PTH can not be obtained P excretion in the kidney resulting increase and decrease calcium phosphoremy. Morphology: deficiency in bone osteoid mineralization (rickets or osteomalacia).

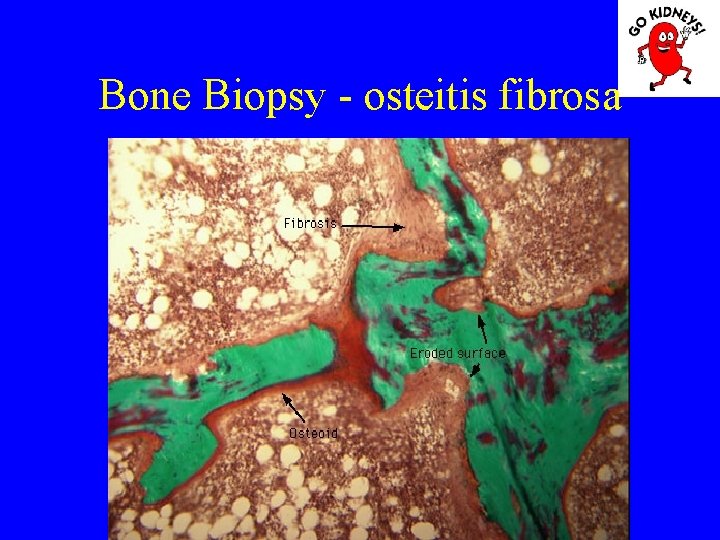
Renal osteodystrophy Stage 3: – Normalization of serum calcium (consequence of bone demineralization caused by secondary hiperparatirodismul), increased PTH and phosphoremy. – Bone morphology shows the presence of osteitis fibrosa.

Renal osteodystrophy Stage 4: Hypercalcemia, hyperparathyroidism and the presence of phosphorus (P) increased serum, produced as Ca x P increases, resulting in metastatic calcification in various organs or soft tissues.

Renal osteodystrophy Clinical signs: - delay in growth - bone pain especially in joints that bear weight (column back injury coxofemoral joints, knees, punches), in particular in the change in position. • epiphyseal dislocation (femur, radius) that induces waddle / limp, limitation of joint movement • bone deformations Children under 4 years make the aspect like lesions of - D sensitive rickets

• Myopathy - emphasizes gait and pain in the muscle groups. • It produces - restriction of activities which affect quality of life. • Autopsy of 120 children with ESRD in HD showed that 60% of them had soft tissue calcification (blood vessels, lung, myocardium, coronary arteries, CNS, gastric mucosa).

• Calcifications have been associated with: - Excessive treatment with Vit. D - Increased Ca x P - Male • Deposition of Ca and P in the conjunctiva - the syndrome of "red eye" - described in 10% of children on dialysis • Corneal calcification - slit lamp • Pulmonary calcifications – respiratory restriction despite effective dialysis • Periarticular calcification - local pain and inflammation • Cardiac calcifications - (sometimes visible in chest ray) * Environmentally renal vessels (Mönckeberg's sclerosis) * Constitutes predictors of death



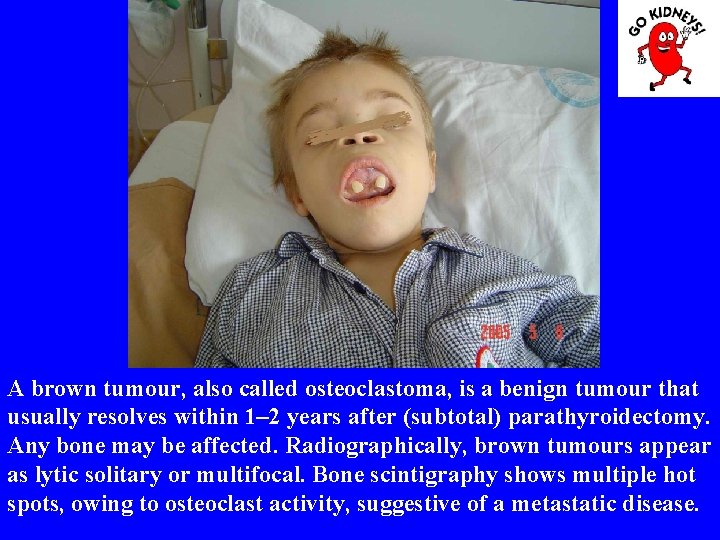
A brown tumour, also called osteoclastoma, is a benign tumour that usually resolves within 1– 2 years after (subtotal) parathyroidectomy. Any bone may be affected. Radiographically, brown tumours appear as lytic solitary or multifocal. Bone scintigraphy shows multiple hot spots, owing to osteoclast activity, suggestive of a metastatic disease.

• Diagnosis of renal osteodystrophy 1. biochemical changes - in early stages of CKD, Ca and P are normal and usually does not correlate with radiological signs and bone morphology. - when GFR <25% appears hiperfosforemia, hypocalcemia, increased alkaline phosphatase (AP), and increased PTH. - hypercalcemia occur spontaneously or after administration of very low doses of vitamin D 3 is an indicator of ODR (stage 4, after Bordier).

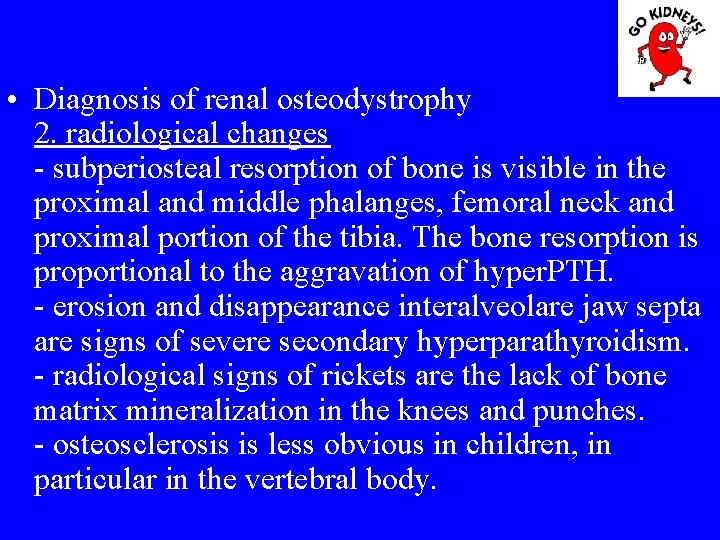
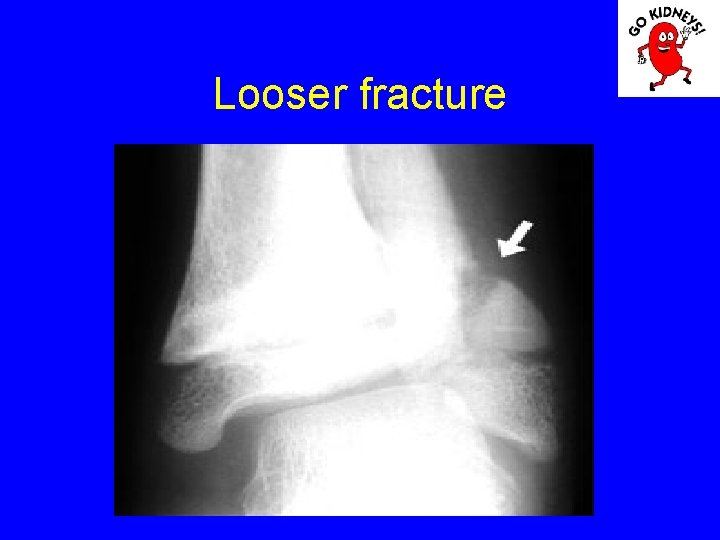
• Diagnosis of renal osteodystrophy 2. radiological changes - subperiosteal resorption of bone is visible in the proximal and middle phalanges, femoral neck and proximal portion of the tibia. The bone resorption is proportional to the aggravation of hyper. PTH. - erosion and disappearance interalveolare jaw septa are signs of severe secondary hyperparathyroidism. - radiological signs of rickets are the lack of bone matrix mineralization in the knees and punches. - osteosclerosis is less obvious in children, in particular in the vertebral body.



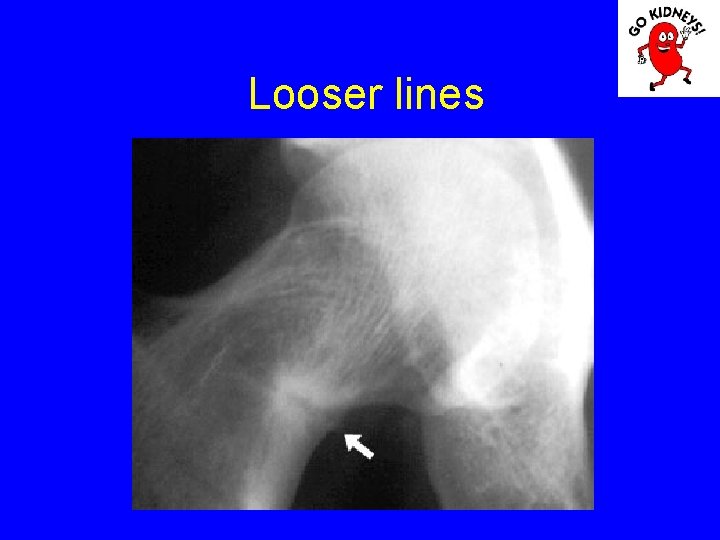
Looser fracture

Subperiosteal resorption

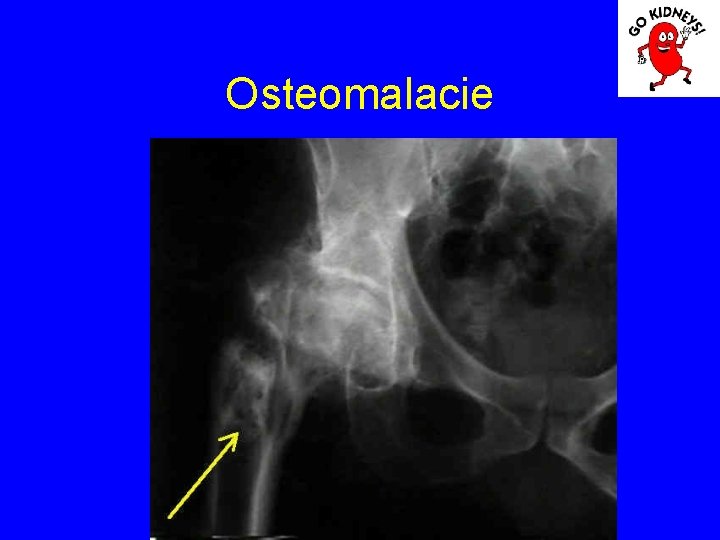
Osteomalacia

Looser lines

Osteomalacie

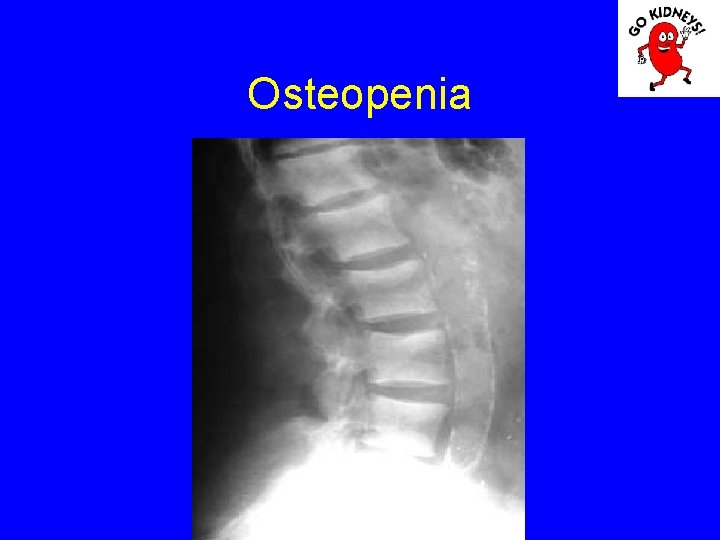
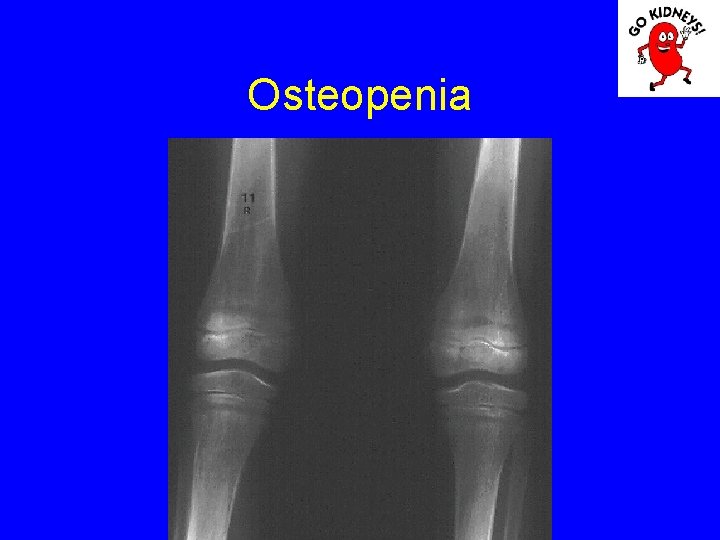
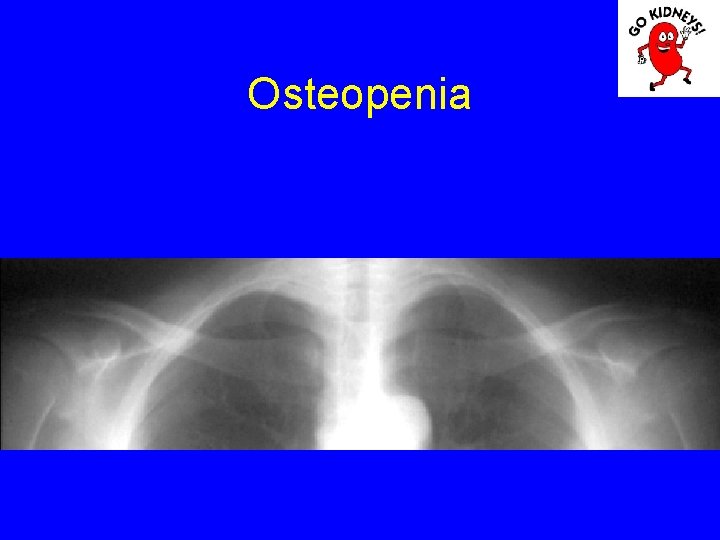
Osteopenia

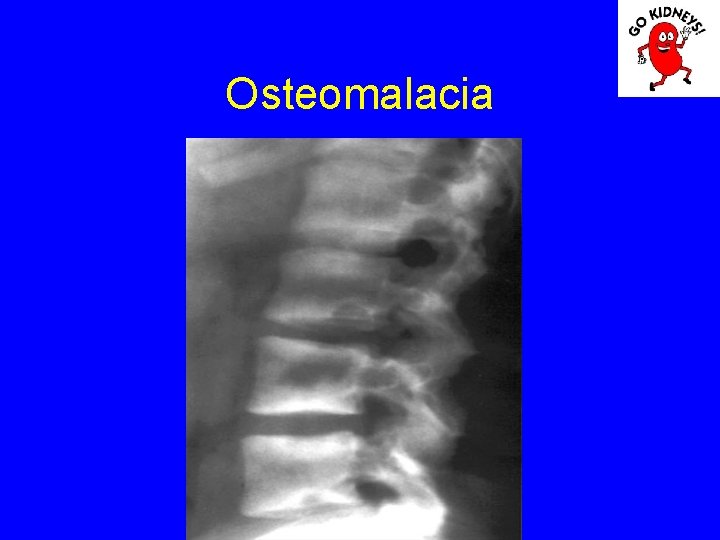
Osteomalacia

Osteopenia

Osteopenia

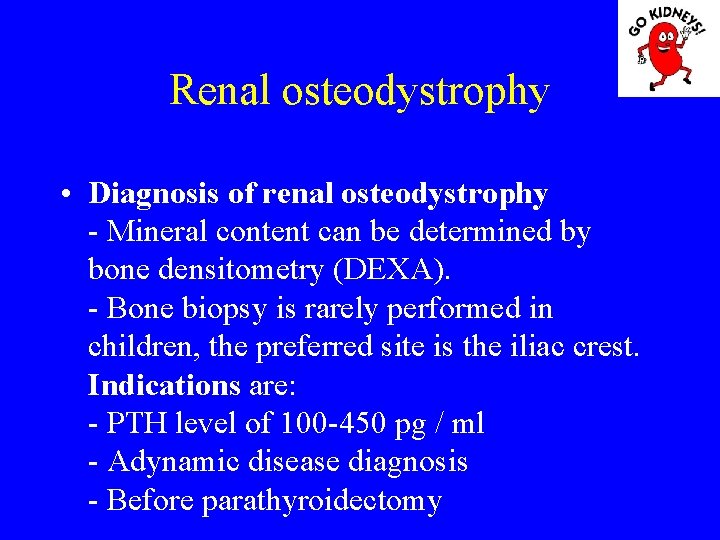
Renal osteodystrophy • Diagnosis of renal osteodystrophy - Mineral content can be determined by bone densitometry (DEXA). - Bone biopsy is rarely performed in children, the preferred site is the iliac crest. Indications are: - PTH level of 100 -450 pg / ml - Adynamic disease diagnosis - Before parathyroidectomy

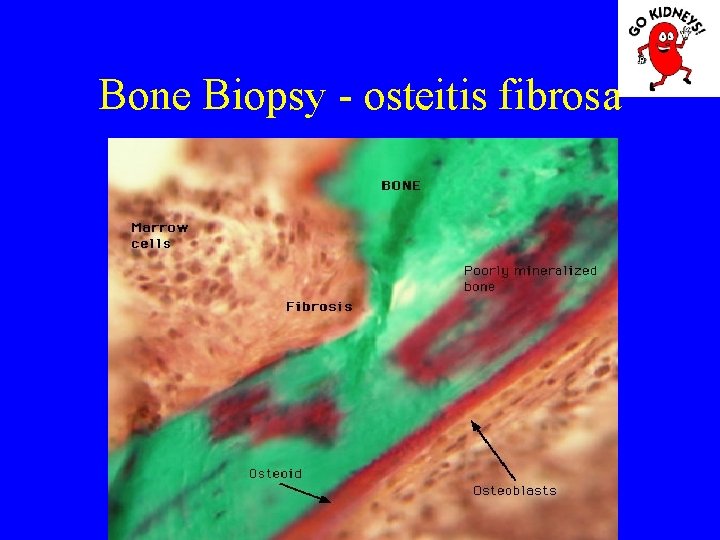
Bone Biopsy - osteitis fibrosa

Bone Biopsy - osteitis fibrosa

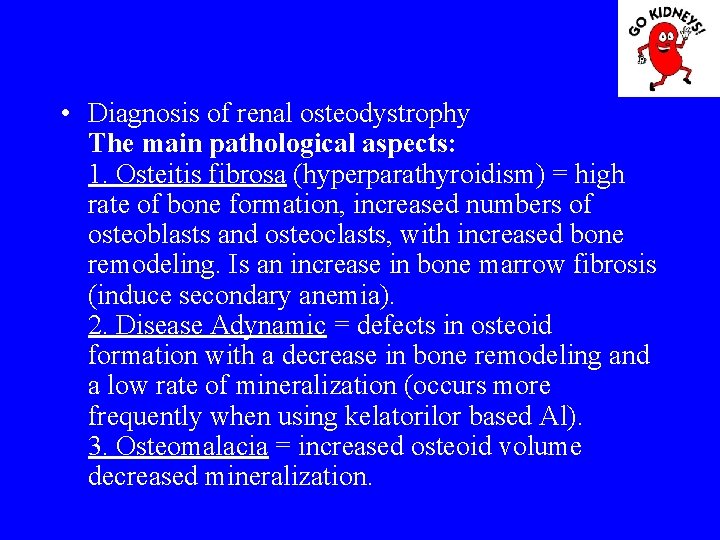
• Diagnosis of renal osteodystrophy The main pathological aspects: 1. Osteitis fibrosa (hyperparathyroidism) = high rate of bone formation, increased numbers of osteoblasts and osteoclasts, with increased bone remodeling. Is an increase in bone marrow fibrosis (induce secondary anemia). 2. Disease Adynamic = defects in osteoid formation with a decrease in bone remodeling and a low rate of mineralization (occurs more frequently when using kelatorilor based Al). 3. Osteomalacia = increased osteoid volume decreased mineralization.

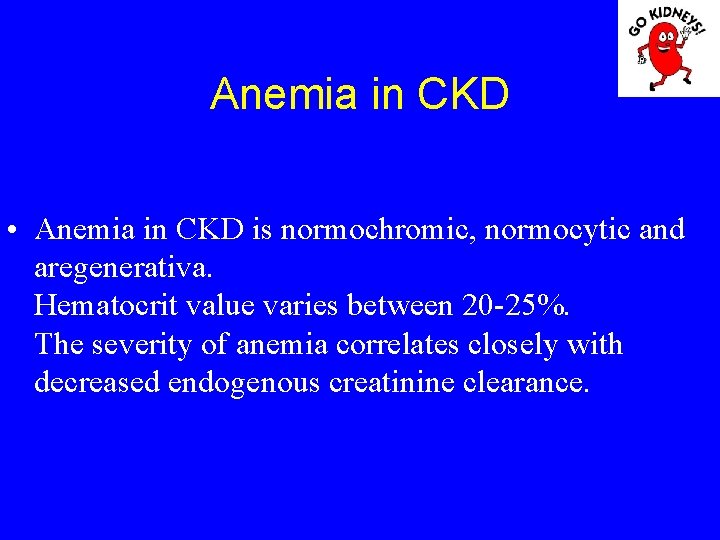
Anemia in CKD • Anemia in CKD is normochromic, normocytic and aregenerativa. Hematocrit value varies between 20 -25%. The severity of anemia correlates closely with decreased endogenous creatinine clearance.

Anemia in CDK Determinants are: - deficit in erythropoietin - shortened red cell life due: uremic toxins, use of phosphorus-based chelator of aluminum (in this case anemia is microcytic, hypochromic, similar to the iron deficiency). Aluminum interferes with the incorporation of iron into heme. - loss of blood during hemodialysis. - Folic acid deficiency, produced mainly in children on dialysis.

Blood disorsers • Advanced CKD is associated with increased bleeding tendency. Common causes involved are: - defect in platelet function - impaired aggregation and reduced production of thromboxane. - adhesion to ADP, collagen and epinephrine is low - decrease platelet factor 3, which cause clot retraction time is increased. Some of these defects is improved dialysis. - defect of FVIII or von Willebrand factor. The correction is done by cryoprecipitate and desmopressin.

Disorders of the electrolytes and acid-base balance – Metabolic acidosis is caused by the inability of hydrogen ion excretion of endogenous acid radicals, due to the impossibility of forming ammonium distal nephron. – Titratable acidity is normal. – About renal bicarbonate loss and retention of phosphorus, sulfur and urate may aggravate acidosis.

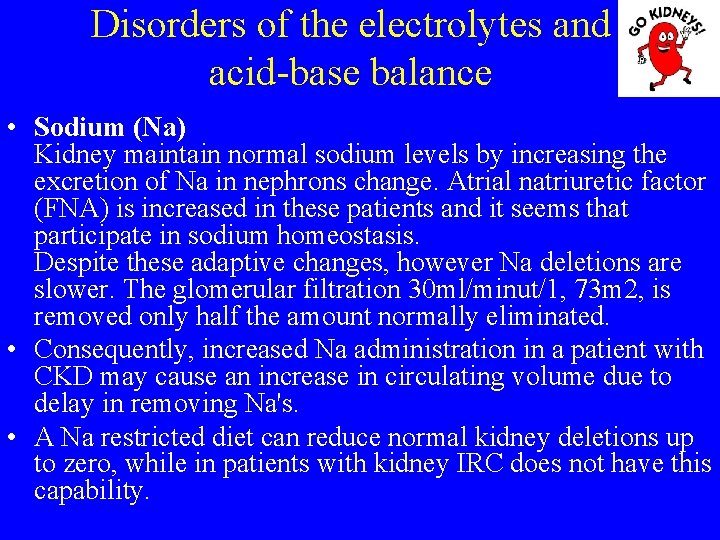
Disorders of the electrolytes and acid-base balance • Sodium (Na) Kidney maintain normal sodium levels by increasing the excretion of Na in nephrons change. Atrial natriuretic factor (FNA) is increased in these patients and it seems that participate in sodium homeostasis. Despite these adaptive changes, however Na deletions are slower. The glomerular filtration 30 ml/minut/1, 73 m 2, is removed only half the amount normally eliminated. • Consequently, increased Na administration in a patient with CKD may cause an increase in circulating volume due to delay in removing Na's. • A Na restricted diet can reduce normal kidney deletions up to zero, while in patients with kidney IRC does not have this capability.

• Potassium (K) In CKD is remarkable inability to eliminate potassium. Homeostasis is maintained by increasing renal and non-renal eliminations. Does an increase in the fractional excretion (secretion of K in the tube) in surviving nephrons and increased non-renal eliminations faeces (over 75% of the potassium intake).

• Fluid balance In IRC there is a shortage of urinary concentration, maximum concentration of 300 m. Osm / l (normal: 350 m. Osm / l). Thus, the density and urinary osmolarity get to be equal to plasma (glomerular ultrafiltrate is not subject to water reabsorption resulting isosthenuria). Isosthenuria is resistant to external administration of antidiuretic hormone, suggesting a tubular defect in response to this hormone. Fluid retention is significant in children with GFR <20%.

Other metabolic disorders • Carbohydrates: In IRC glucose intolerance occurs despite a high level of insulinemie. This is due to peripheral insulin resistance and endogenous uremic toxins (dialysis improves glucose tolerance). • Proteins: - Serum albumin and transferrin levels are normal IRC, unless an associated exudative enteropathy or other causes of hipercatabolism. - Instead levels valine, leucine, isoleucine, histidine and tyrosine are low. - Dialysis does not significantly improve amino acid profile.

• Lipids: CDK is characterized by dyslipidemia, manifested by hyperlipidemia, hypertriglyceridemia and normal levels of total cholesterol, VLDL-cholesterol increased and HDL-cholesterol low. Dyslipidemia may play a role in the progression of renal damage by oxidation of LDL and accelerate atherosclerosis lesions.

Cardiovasculare disorders • High blood pressure (hypertension) may be complicated by hypertensive encephalopathy. High BP can generate gross increases cerebral edema and intracerebral arteriolar necrosis. Clinically manifested by altered sensory headache, tonic-clonic convulsions, coma. Hypertensive crisis may have consequences that -or intraventricular intracerebral hemorrhage, which can lead to severe neurological deficits and death.

• Pathophysiology of hypertension Pathophysiological mechanisms of hypertension is limited to this hipervolemiei and vasoconstriction. Volume overload is the main mechanism of production of hypertension in children with CKD. Circulating blood volume increases due to salt and water retention. • Vasoconstriction may be a consequence of activation of the RAAS, sympathetic nervous system and the release of endothelium-derived vasoconstrictor substances (endothelin-1).

Mechanisms associated with high BP: - Low production of nitric oxide, which amplifies the vasoconstrictor effect - Hyperparathyroidism induce an increase in intracellular calcium ion - Increased vascular stiffness by altering glycosylation of collagen and calcified uremic arteriolopatiei appearance induces vascular compliance to decrease. erythropoietin administration genetic The main effect of hipervolemiei in producing hypertension in many patients with CKD has been shown by Charr in 1996. He obtained a reduction in dose of antihypertensive mortality rate and prolongation of hemodialysis and reducing sodium intake.

• Consequences of hypertension: - Accelerating progression of CDK through hyperfiltration or glomerular ischemia - Acceleration of atherosclerosis vascular lesions - Wall thickening and vascular remodeling (hypertrophy and hyperplasia of the intima and media) - Altered arterial compliance - endothelial dysfunction - LV hypertrophy - LV systolic and diastolic dysfunction - Congestive heart failure

• Pericarditis is a complication of advanced forms of uremia, occurring less frequently in patients on peritoneal dialysis. Determinants are: - retention of uremic toxins, - fluid overload, - infectious agents, - heparinisation on dialysis Clinic is manifested by: - fever - chest pain, - pericardial friction rub (before accumulation of liquid - dry pericarditis). After accumulation of fluid friction rub away, but the heart sounds are deafened.

• Cardiac tamponade occurs mainly in the forms of hemorrhagic pericarditis and is manifested clinically by: - hypotension, - turgid jugular, - paradoxical pulse stands in a decrease in systolic blood pressure below 10 mm Hg and is characterized by decrease or disappearance of peripheral pulse in inspiration. - apex shock subsided, - distant heart sounds, - hepatomegaly, - sometimes peripheral edema.

• Myocardial damage is manifested by decreased exercise tolerance, which increases significantly after correction of anemia with erythropoietin therapy and after hemodialysis. Uremic cardiomyopathy is manifested by an evolving cardiac dysfunction and heart failure due to: - incresed fluids, - anemia, - high BP, - uremic toxins.

• Uremic vasculopathy Coates (1998) introduces the term of uremic calcified arteriolopathie. There are 2 types of calcifications: 1. In the intima common to atherosclerosis in elderly 2. On the average, phospho-related disorders - calcium, frequently described in young people. Consequences: - Loss of elasticity and increased vascular velocity - Increased systolic pressure with the appearance of hypertrophy of LV - Risk of myocardial coronary calcification Cardiovascular disease mortality in children is the result of arteriolopatiei calcified rather than atherosclerosis

Vascular calcification

Peritoneal calcification vessels

Neurologic disorders • Peripheral neuropathy is characterized by: - paresthesia, - muscle necrosis. - weight walking and leg swelling - loss of sensation distal - reducing OTR. • Neuro-uraemic child development: - Head circumference grows more slowly, if uremia occurs in the newborn period. - While severe motor impairments install, we could see EEG changes, progressive encephalopathy in patients who are undergoing hemodialysis program (myoclonic seizures, cerebellar dysfunction and motor retardation, developmental regression).

Immunity to uremic patients – Defense defects occur when GFR falls below 25 ml/minut/1, 73 m 2. It finds tend to lymphopenia although lymphocyte subpopulations (T 4 helper and suppressor T 8) are normal. At the same time there is a decreased response of neutrophils from infectious agents and renal function Fc receptor.

Diagnosis • History of disease - recurrent urinary tract infections, - decreased appetite and physical activity - fatigue, - polyuria or enuresis secondary - polidpsie, - administration of nephrotoxic drugs. The historic track signs of hereditary diseases that could advocate for Alport syndrome or polycystic kidney. A history of renal advocates CNG or CPN

Diagnosis • Physical examination may be noted: - delays in growth - presence of signs of rickets or pathological fractures, - high BP. • Laboratory tests show that: - anemia normochromic, normocytic, aregenerativa. - Nitrogen retention and decreased creatinine clearance - metabolic acidosis, hyperkalemia, hyperphosphatemia, - hypocalcemia, hyponatremia - proteinuria or hematuria urinary sediment suggesting glomerular disease old. - low urinary density suggesting a chronic kidney ailment. • Renal ultrasound may show decreased volume of the kidneys • Bone radiography signs of renal osteodystrophy. • Retrograde cystography may help in the diagnosis of reflux nephropathy. • Radioisotope study can give differentiated data for each kidney function.

Treatment 1. Administration protein Protein intake for reducing the amount of 0, 8 -1, 1 g / kg / day did not significantly influence growth, nor progression to CKD. Featured are high biological value proteins, amino acids, low phosphorus diet. 2. Ingestion of liquid Liquids are liberalized up to a creatinine clearance of 35 -40 ml/minut/1, 73 m 2. If this restriction is imposed oliguria or anuria fluid (urine volume plus insensible losses).

Treatment 3. Administration of Na and K - If GFR not decreased by more than 10% of normal, no restriction is imposed Na and K. - In the management of hypertension and low tolerance of Na, it is reduced to 1 -2 m. Eq / kg / day. We recommend an additional contribution of Na 1 -3 m. Eq / kg / day in patients with tubular damage, renal dysplasia, which eliminates the high amount of Na. The risk of hyperkalemia in patients receiving ACE inhibitors exist, propranolol, or if hiposerinemie, hypoaldosteronism. - Are prohibited citrus fruits, bananas, chocolate, tomatoes, potatoes. We can manage ion exchange resins: Kayexalate 0. 5 - 1 g / kg in three divided doses.

Treatment 4. Correction of acidosis is done by administering Na bicarbonate 1 -5 m. Eq / kg / day 5. Treatment of anemia is based on human erythropoietin administration of 50 -150 IU / kg x 3 / week. 6. Treatment of hypertension include: Na restriction. Furosemide administration (if the patient is still diuresis). antihypertensives (beta blockers, calcium channel).

Treatment 7. Prevention of renal osteodystrophy is by: - phosphorus restriction in the diet (milk, milk products, meat - administration of radioactive phosphorus lock in the gut (eg aluminum hydroxide, rarely used due to its toxicity). - Ca. CO 3 is preferred during lunches: 100 -300 mg / kg / day. - noncalcici chelators have recently been introduced (calcimimetic). 8. Intake of vitamin D is required when the GFR falls below 50% – 1, 25(OH)2 -D 3 = Rocaltrol (0, 25 mg/zi), – Dihidrotahisterol (DMT) = 0, 125 mg/zi, – 1 alfa hidroxi-vitamin D 3 ( 0, 5 - 3 g/zi), D 3 alfa (0, 5 - 3 g/zi).


Treatment • Chronic hemodialysis and continuous peritoneal dialysis • Renal transplantation.

Follow up • Should be monitored: Serum calcium (negative effect is the production of hypercalcemia). P> 70 Ca Fosforemia when produced as � deposition can occur in tissues and vessels. Suppression of PTH may promote disease Adynamic. FA decline and PTH are markers of favorable outcome.

Follow up • The delay in growth requires: improving nutrition STH administration before installing pubescence and improve vascular elasticity Treatment of anemia Fair treatment of osteodystrophy Treatment of comorbid states (infection) • Uremic bleeding requiring: Transfusion of cryoprecipitate, desmopressin (von Willebrand factor induces the release of endogenous) 0. 3 mg / kg or 3 mcg in intranasal.
 Nephrology near atwater
Nephrology near atwater Nih score
Nih score Matrix mitochondria
Matrix mitochondria Albumin kidney disease
Albumin kidney disease Sighns of kidney problems
Sighns of kidney problems Symptomatic polycystic kidney disease
Symptomatic polycystic kidney disease Choronic kidney disease
Choronic kidney disease Albumin kidney disease
Albumin kidney disease Nemo kidney disease
Nemo kidney disease Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease Chronic disease
Chronic disease Jewish chronic disease study
Jewish chronic disease study Developed by ed
Developed by ed Copd lungs images
Copd lungs images Stigmata of portal hypertension
Stigmata of portal hypertension Chronic disease
Chronic disease Chronic granulomatous disease
Chronic granulomatous disease Vijaya's echo criteria
Vijaya's echo criteria Stigmata of chronic liver disease
Stigmata of chronic liver disease Stage 1 cirrhosis
Stage 1 cirrhosis Stigmata of chronic liver disease
Stigmata of chronic liver disease Kate lorig stanford
Kate lorig stanford Communicable disease and non communicable disease
Communicable disease and non communicable disease Magdalena kowalska cern
Magdalena kowalska cern Maria magdalena grigore
Maria magdalena grigore Magdalena sikora
Magdalena sikora Georges de la tour magdalen
Georges de la tour magdalen Magdalena wieczorek feet
Magdalena wieczorek feet Geschwister scholl lebenslauf
Geschwister scholl lebenslauf Magdalena pachut fotografia
Magdalena pachut fotografia Magdalena kowalska cern
Magdalena kowalska cern Magdalena zimová třetí dítě
Magdalena zimová třetí dítě Magdalena definition
Magdalena definition Adam gessler wikipedia
Adam gessler wikipedia Magdalena abakanowicz
Magdalena abakanowicz Magdalena karadža
Magdalena karadža Magdalena jachimowicz
Magdalena jachimowicz Profesor magdalena strus
Profesor magdalena strus Magdalena carmen frida
Magdalena carmen frida Maria magdalena novelda
Maria magdalena novelda Martysz
Martysz Mapa fluvial de colombia
Mapa fluvial de colombia Selesh
Selesh Magdalena kowalska cern
Magdalena kowalska cern Katuturan ng korido
Katuturan ng korido Magdalena abakanowicz życiorys
Magdalena abakanowicz życiorys Sekundarna devijantnost
Sekundarna devijantnost Marzanna pojawa-grajewska
Marzanna pojawa-grajewska Sławomir czetwertyński
Sławomir czetwertyński Magdalena kostka
Magdalena kostka Varlmar
Varlmar Carmen wistan
Carmen wistan Magdalena pala
Magdalena pala Plena in re potestas
Plena in re potestas Cechy wiedzy naukowej
Cechy wiedzy naukowej Magdalena medio
Magdalena medio Que pensaron nick y alex que era raro
Que pensaron nick y alex que era raro Magdalena sopta
Magdalena sopta 4 velika proroka
4 velika proroka Magdalena fitrzyk
Magdalena fitrzyk Magdalena dul
Magdalena dul Magdalena thomas
Magdalena thomas La ultima cena maria magdalena
La ultima cena maria magdalena Magdalena sypek kleiba
Magdalena sypek kleiba Czerwony abakan
Czerwony abakan Magdalena rojek nowosielska
Magdalena rojek nowosielska Maria magdalena grigore
Maria magdalena grigore Magdalena gawron
Magdalena gawron Hospital hilario lugo
Hospital hilario lugo Magdalena ridge observatory interferometer
Magdalena ridge observatory interferometer Magdalena zielińska grajmy w szachy
Magdalena zielińska grajmy w szachy Grupy ryzyka wg spauldinga
Grupy ryzyka wg spauldinga Joanna kaczmarek psycholog
Joanna kaczmarek psycholog Latviešu gleznotājs 1891-1964
Latviešu gleznotājs 1891-1964 Omamy pamięciowe
Omamy pamięciowe Starcea magdalena
Starcea magdalena Magdalena kutnik
Magdalena kutnik Magdalena kachniewska
Magdalena kachniewska Magdalena pietrzykowska
Magdalena pietrzykowska Magdalena carmen frieda kahlo y calderón
Magdalena carmen frieda kahlo y calderón Magdalena burba
Magdalena burba