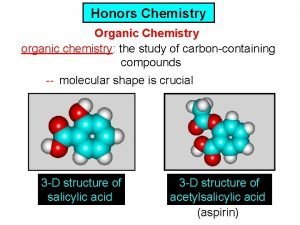
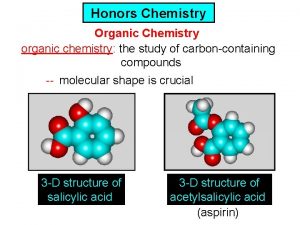
Chemistry What is Chemistry Chemistry is the study














- Slides: 30

Chemistry

What is Chemistry? Chemistry is the study of matter and the changes that place in matter. Matter is anything that takes up space and has mass. Examples?

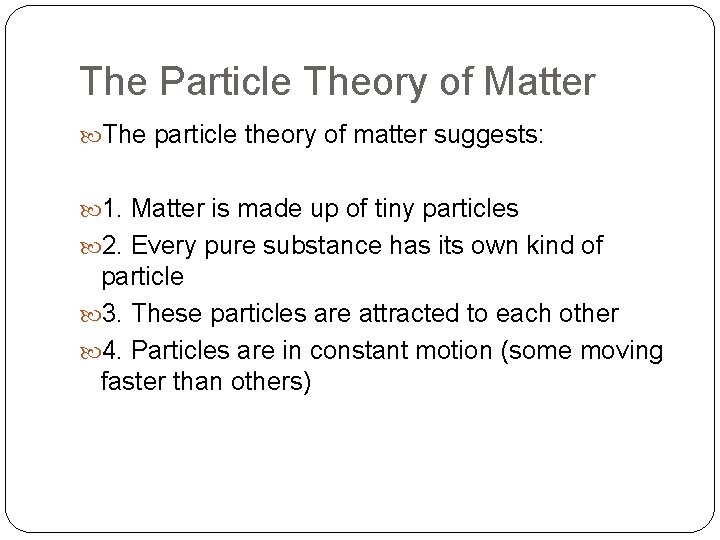
The Particle Theory of Matter The particle theory of matter suggests: 1. Matter is made up of tiny particles 2. Every pure substance has its own kind of particle 3. These particles are attracted to each other 4. Particles are in constant motion (some moving faster than others)


Classification Lab

The Phases of Matter- Solids Particles are closely packed together These particles vibrate in place There is a strong attraction between these molecules They have a definite shape http: //www. harcourtschool. com/activity/states_of_ matter/

Phases of Matter- Liquids Particles are further apart Particles have more movement- they slide around past each other There is some attraction between particles They have no definite shape

Phases of Matter- gases Particles are far apart These particles move freely There is a weak attraction between particles No definite shape

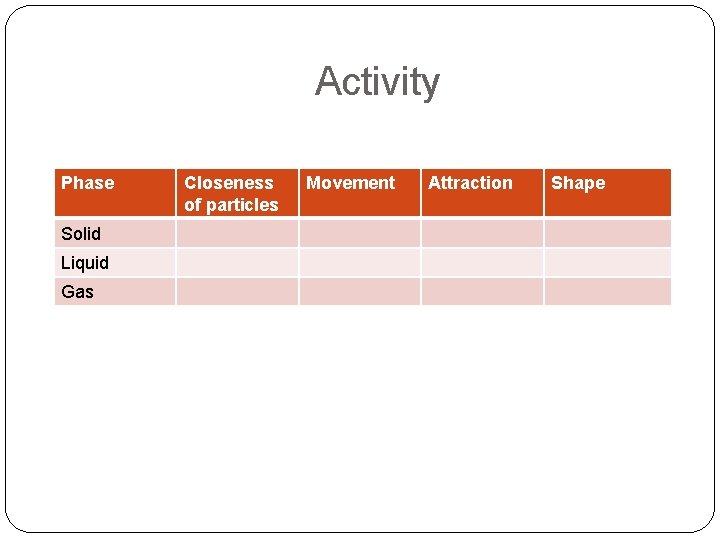
Activity Phase Solid Liquid Gas Closeness of particles Movement Attraction Shape

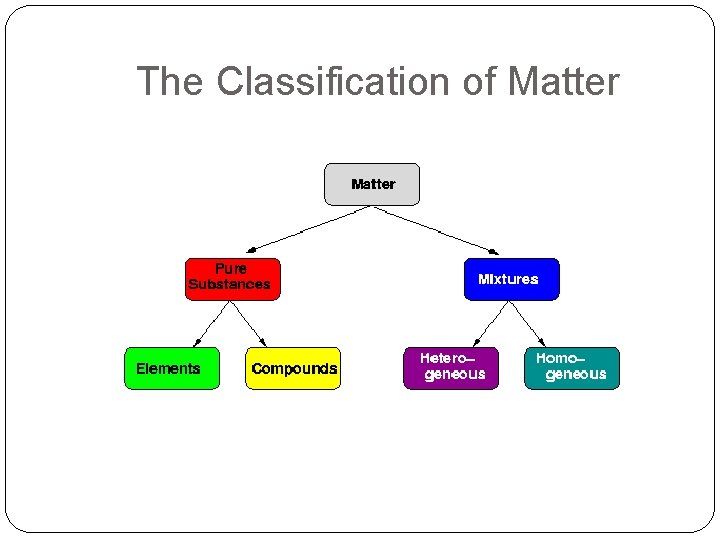
The Classification of Matter

Student Activity. . . With a partner decide: 1. How would you tell the difference between a pure substance and a mixture? 2. How would use the Particle Theory of Matter to support your answer to question #1?

Pure Substances Matter that contains one kind of particle throughout Examples: Hydrogen, Oxygen are elements. Water is a compound and you can’t separate it easily.

Mixtures What are mixtures? Two or more substances mixed together is a mixture There are 2 types of mixtures

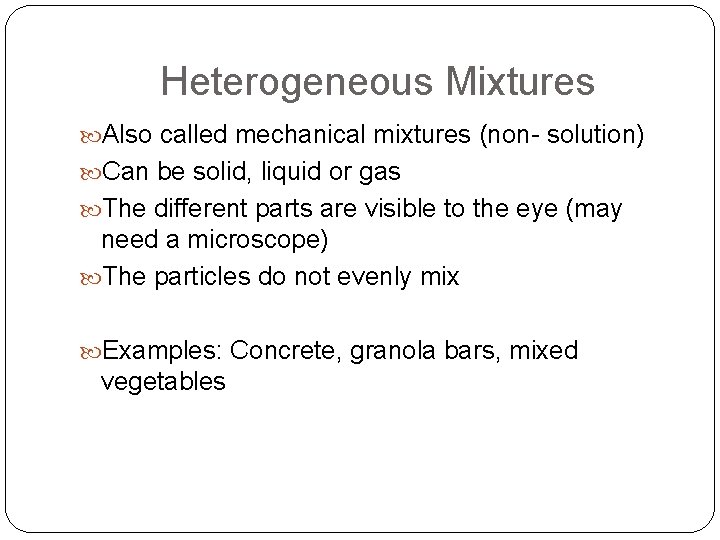
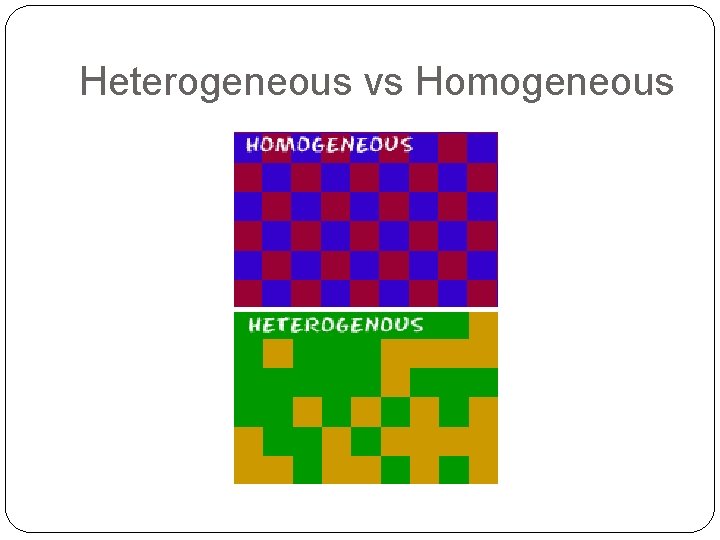
Heterogeneous Mixtures Also called mechanical mixtures (non- solution) Can be solid, liquid or gas The different parts are visible to the eye (may need a microscope) The particles do not evenly mix Examples: Concrete, granola bars, mixed vegetables

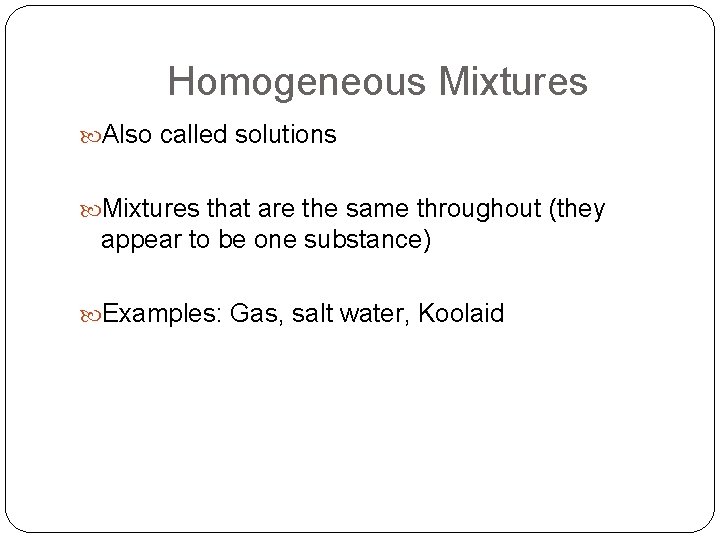
Homogeneous Mixtures Also called solutions Mixtures that are the same throughout (they appear to be one substance) Examples: Gas, salt water, Koolaid

Heterogeneous vs Homogeneous

Create a chart and list the various homogeneous and heterogeneous mixtures in your home.

Solutions

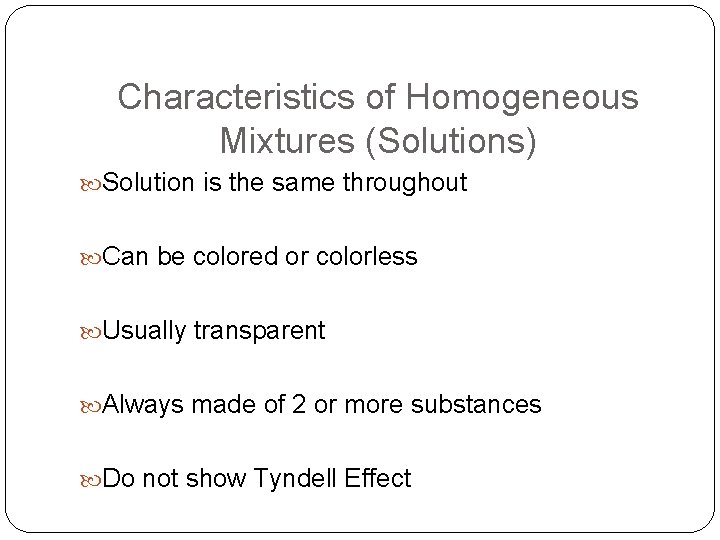
Characteristics of Homogeneous Mixtures (Solutions) Solution is the same throughout Can be colored or colorless Usually transparent Always made of 2 or more substances Do not show Tyndell Effect

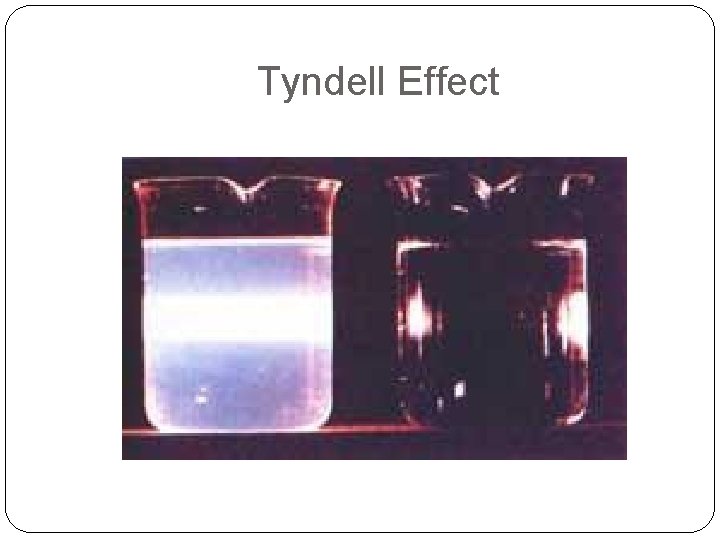
Tyndell Effect

The Tyndall Effect A phenomenon that can be used to distinguish between solutions and what appears to be a solution Cannot be used to distinguish between a solution and a pure liquid In a Solution. . . • Light passes unaffected In a Mechanical Mixture. . . • The light will scatter as it passes through the mixture because all particles are not dissolved

Solutions are made up of two parts 1. The Solute- This is the substance that is being dissolved or the substance of lesser amount. 2. The Solvent- This is the substance that does the dissolving and is usually present in the greatest amount. In a salt water solution, which substance is the solute and which is the solvent?

What Does Soluble Mean? Another way to say sugar dissolves in water is to say sugar is soluble in water. Soluble means able to be dissolved into a particular solvent. Examples?

What Does Insoluble Mean? Another way to say pulp does not dissolve in orange juice is to say pulp is insoluble in orange juice. Insoluble means unable to dissolve into a particular solvent. Examples?

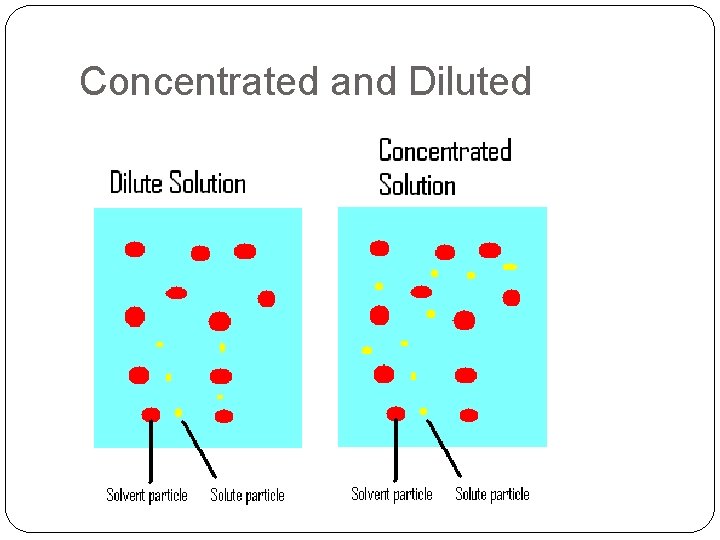
Concentrated and Diluted Solutions with a low concentration of solute are called dilute solutions. Solutions with a high concentration of solute are called concentrated solutions.

Concentrated and Diluted

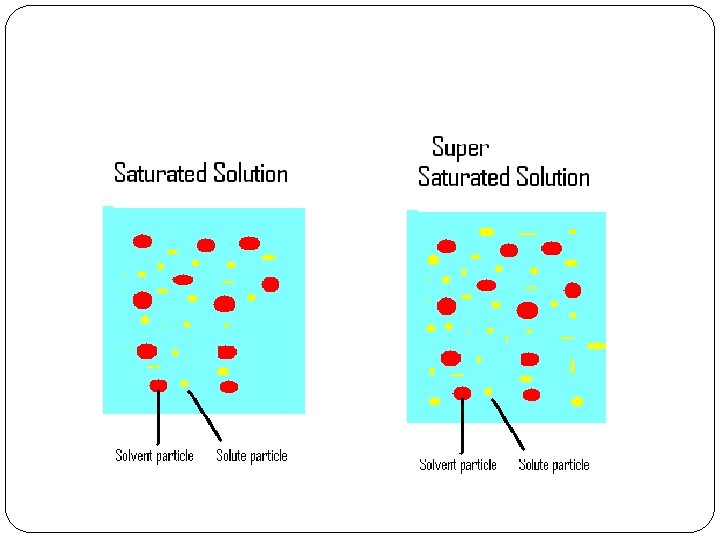
Saturated Solutions An unsaturated solution is a solution that will dissolve more solute at a specific temperature. A saturated solution is a solution that will not dissolve any more solute at the same temperature. A supersaturated solution is a solution that contains more solute than would normally dissolve at a certain temperature.


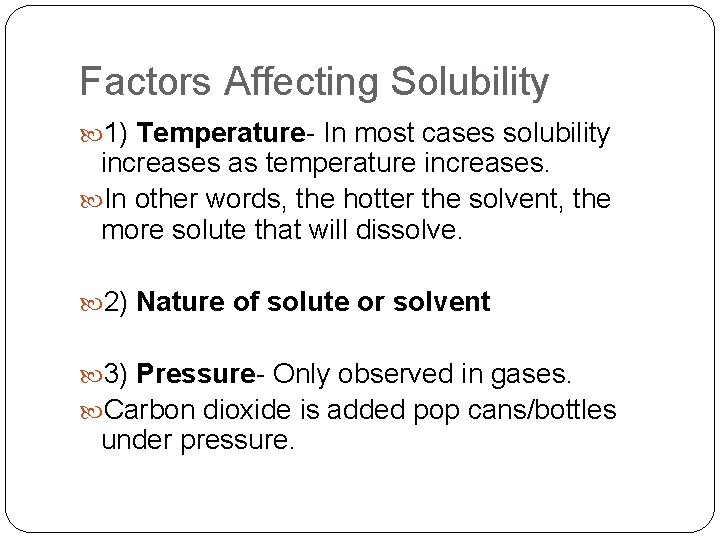
Factors Affecting Solubility 1) Temperature- In most cases solubility increases as temperature increases. In other words, the hotter the solvent, the more solute that will dissolve. 2) Nature of solute or solvent 3) Pressure- Only observed in gases. Carbon dioxide is added pop cans/bottles under pressure.

 Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là