Chemistry for Changing Times Thirteenth Edition Lecture Outlines






- Slides: 70

Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chapter 16 Biochemistry John Singer, Jackson Community College © 2013 Pearson Education, Inc.

The Living Cell Biochemistry is the chemistry of living things and life processes. © 2013 Pearson Education, Inc. Chapter 16 2

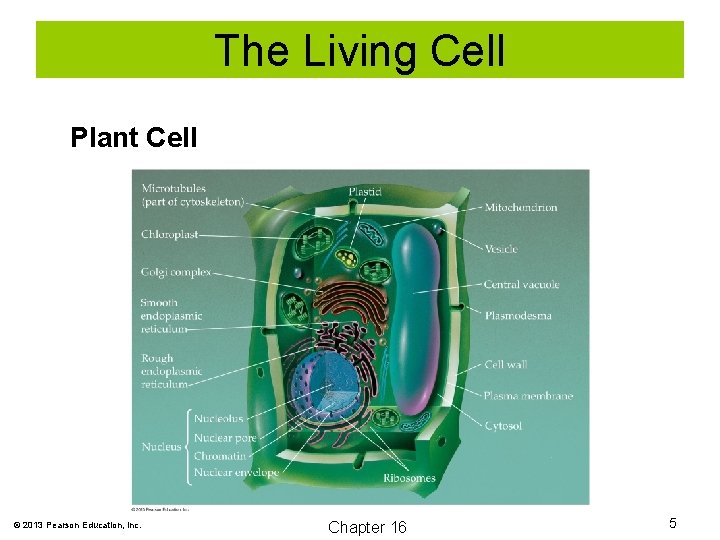
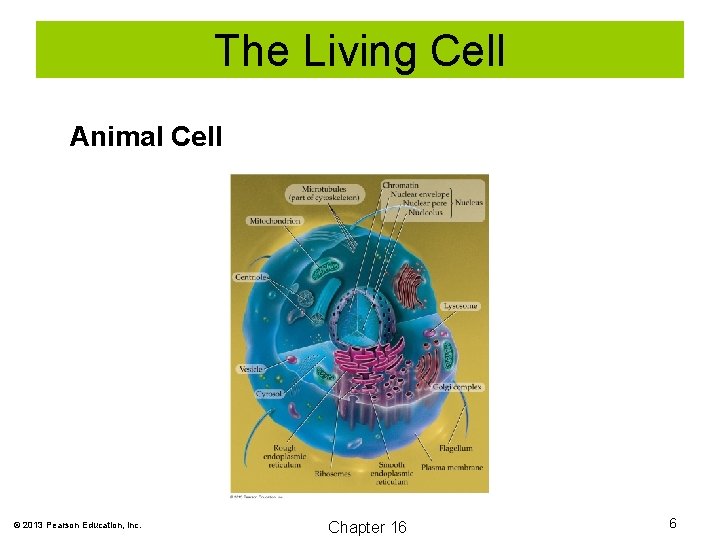
The Living Cell The basic structural unit of all living organisms is the cell. All cells are enclosed in a cell membrane, which regulates the passage of nutrients and wastes. In addition to a cell membrane, plant cells are surrounded by a cell wall composed of cellulose. © 2013 Pearson Education, Inc. Chapter 16 3

The Living Cell Nucleus: The nucleus contains the genetic material that controls heredity. Ribosomes: The structure where protein synthesis occurs. Mitochrondria: The cell structure where energy production occurs. Chloroplasts: Found only in plant cells. In the chloroplasts, photosynthesis occurs. © 2013 Pearson Education, Inc. Chapter 16 4

The Living Cell Plant Cell © 2013 Pearson Education, Inc. Chapter 16 5

The Living Cell Animal Cell © 2013 Pearson Education, Inc. Chapter 16 6

Energy in Biological Systems Green plants contain chloroplasts, which are capable of taking the radiant energy of the sun and storing it as chemical energy in glucose molecules. 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2 Plant cells can also convert carbohydrate molecules to fat molecules, and some are even capable of converting them to proteins. Animals cannot produce their own energy. They must obtain such energy by eating plants or other animals that eat plants. © 2013 Pearson Education, Inc. Chapter 16 7

Energy in Biological Systems Metabolism is defined as the series of chemical reactions that keep a cell alive. Metabolic reactions are divided into two categories: 1. Catabolism: The process of breaking down molecules to produce energy. 2. Anabolism: The process of synthesizing molecules. © 2013 Pearson Education, Inc. Chapter 16 8

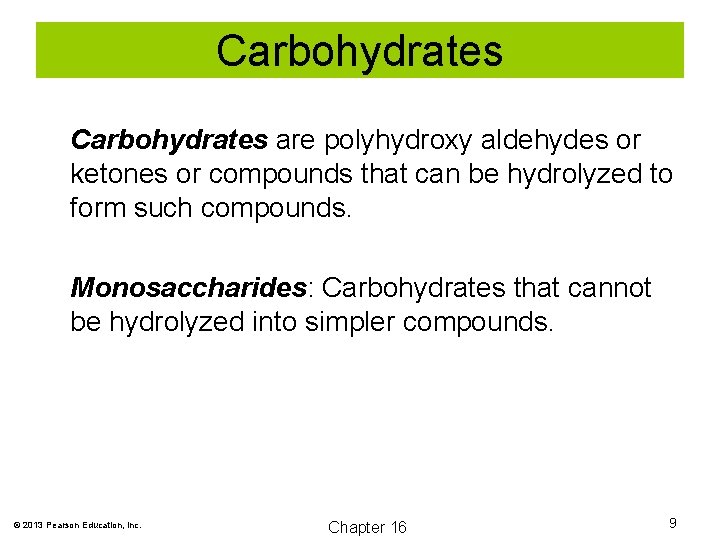
Carbohydrates are polyhydroxy aldehydes or ketones or compounds that can be hydrolyzed to form such compounds. Monosaccharides: Carbohydrates that cannot be hydrolyzed into simpler compounds. © 2013 Pearson Education, Inc. Chapter 16 9

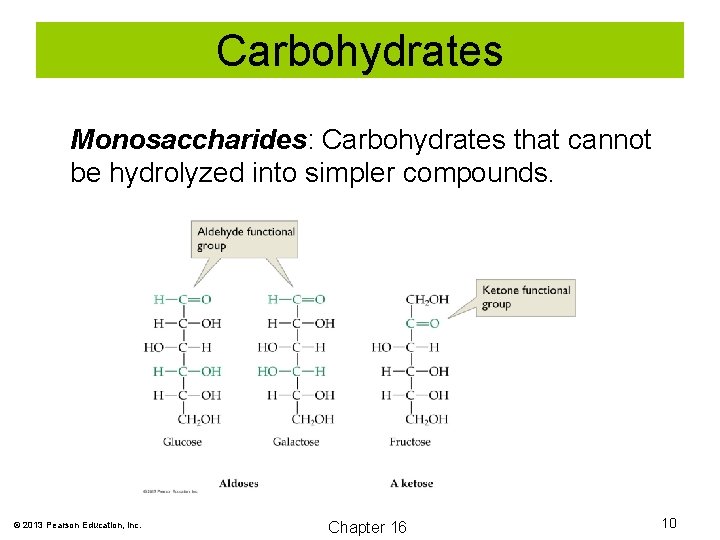
Carbohydrates Monosaccharides: Carbohydrates that cannot be hydrolyzed into simpler compounds. © 2013 Pearson Education, Inc. Chapter 16 10

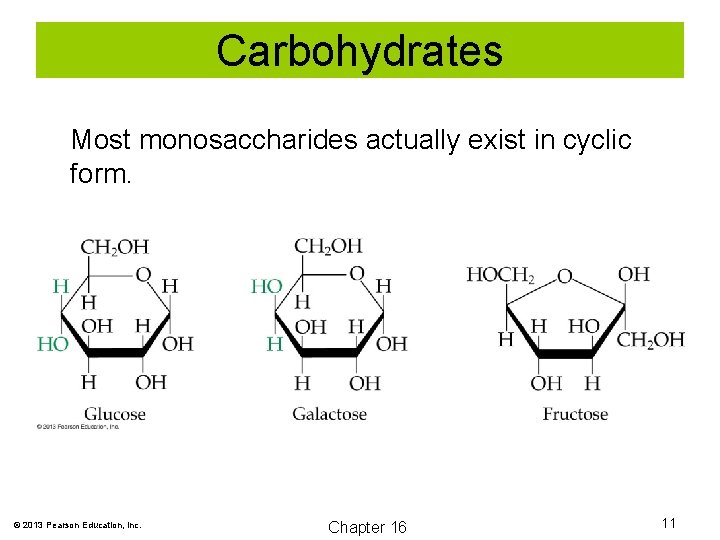
Carbohydrates Most monosaccharides actually exist in cyclic form. © 2013 Pearson Education, Inc. Chapter 16 11

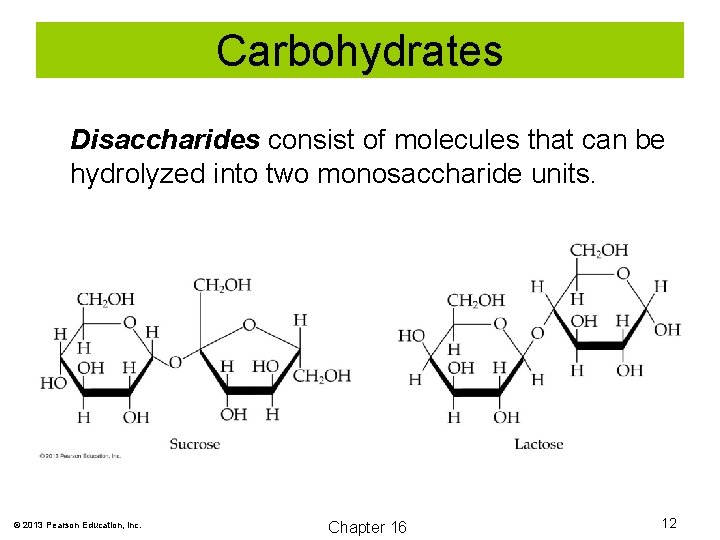
Carbohydrates Disaccharides consist of molecules that can be hydrolyzed into two monosaccharide units. © 2013 Pearson Education, Inc. Chapter 16 12

Carbohydrates Polysaccharides are composed of large molecules that can be hydrolyzed into many monosaccharide units. Examples include starch, cellulose, and glycogen. © 2013 Pearson Education, Inc. Chapter 16 13

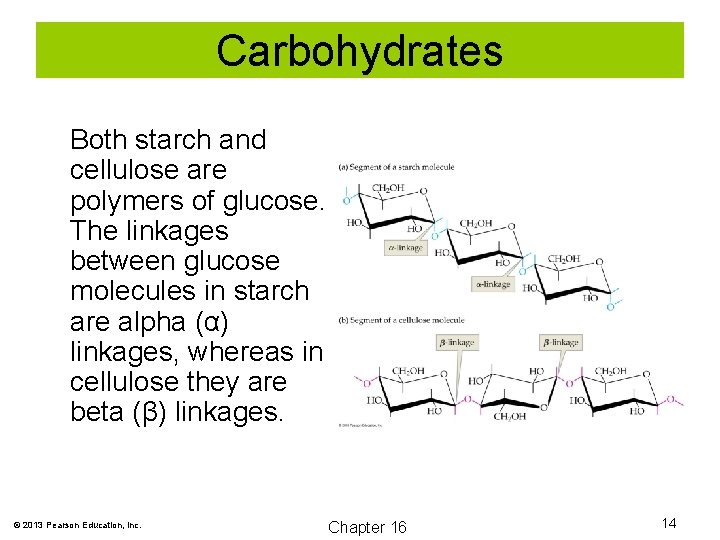
Carbohydrates Both starch and cellulose are polymers of glucose. The linkages between glucose molecules in starch are alpha (α) linkages, whereas in cellulose they are beta (β) linkages. © 2013 Pearson Education, Inc. Chapter 16 14

Carbohydrates Cellulose makes up the structural units of plants. Cellulose chains are composed of parallel bundles called fibrils. © 2013 Pearson Education, Inc. Chapter 16 15

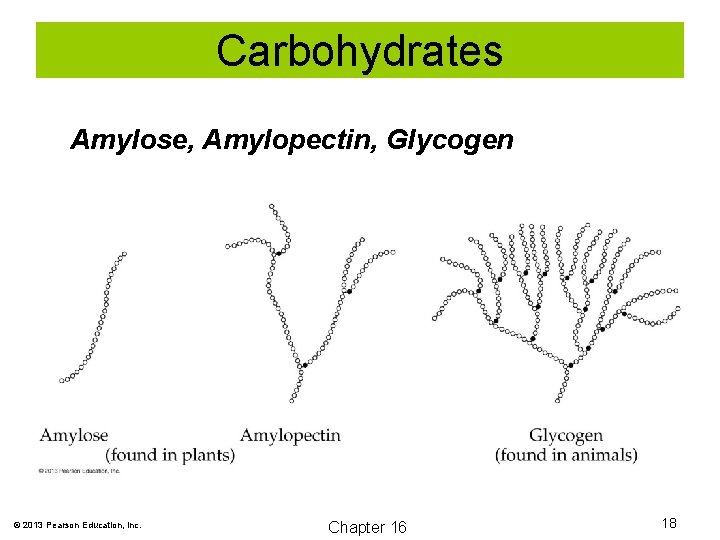
Carbohydrates Starch is composed of two polymers, amylose and amylopectin. In amylose, the glucose molecules are connected in long parallel chains. In amylopectin, the chains are branched. © 2013 Pearson Education, Inc. Chapter 16 16

Carbohydrates Glycogen is known as animal starch. It is similar to amylopectin in that the glucose polymers are branched. © 2013 Pearson Education, Inc. Chapter 16 17

Carbohydrates Amylose, Amylopectin, Glycogen © 2013 Pearson Education, Inc. Chapter 16 18

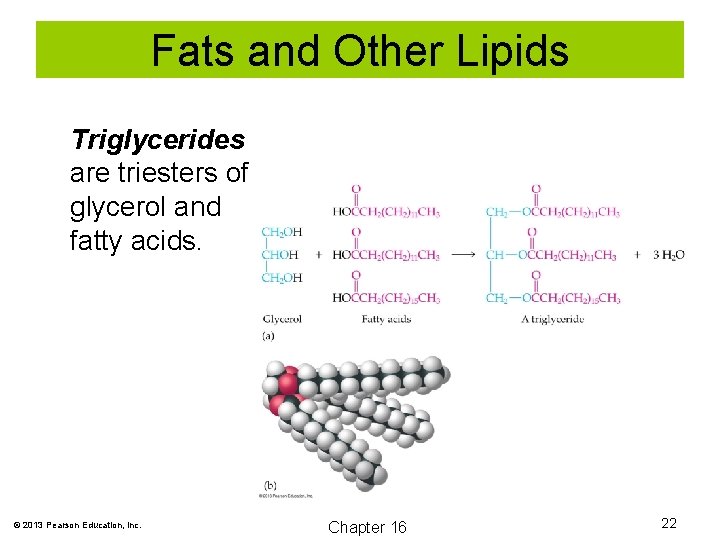
Fats and Other Lipids are biological molecules that are insoluble in water, but are soluble in nonpolar organic solvents. Fats are esters of long-chain fatty acids and glycerol. Fats are often called triglycerides or triacylglycerols. © 2013 Pearson Education, Inc. Chapter 16 19

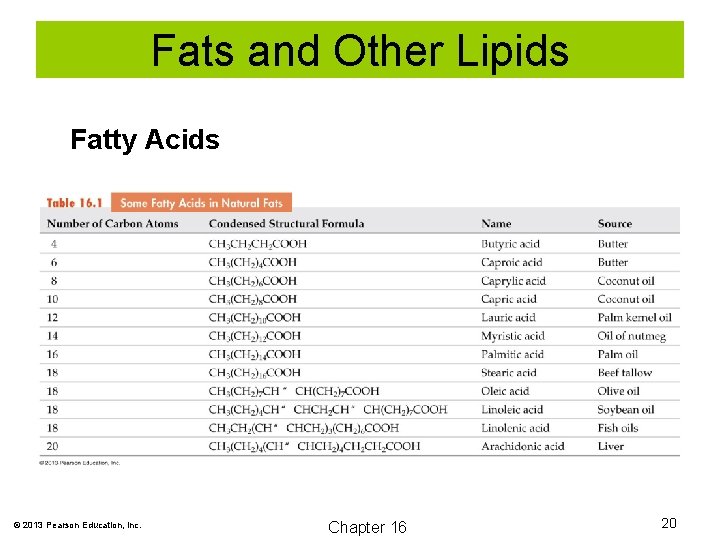
Fats and Other Lipids Fatty Acids © 2013 Pearson Education, Inc. Chapter 16 20

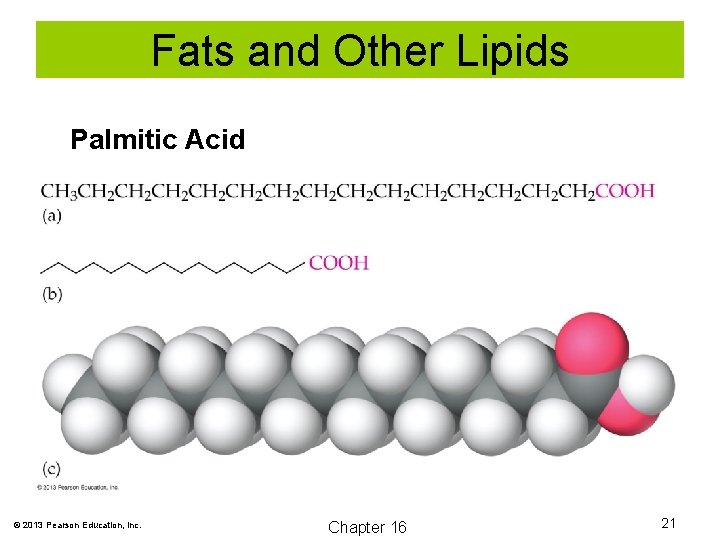
Fats and Other Lipids Palmitic Acid © 2013 Pearson Education, Inc. Chapter 16 21

Fats and Other Lipids Triglycerides are triesters of glycerol and fatty acids. © 2013 Pearson Education, Inc. Chapter 16 22

Fats and Other Lipids Saturated fatty acids have no carbon-to-carbon double bonds. Monounsaturated fatty acids have one carbonto-carbon double bond. Polyunsaturated fatty acids have two or more carbon-to-carbon double bonds. © 2013 Pearson Education, Inc. Chapter 16 23

Fats and Other Lipids Solid fats have a high proportion of saturated fatty acids. Liquid oils have only unsaturated fatty acids. © 2013 Pearson Education, Inc. Chapter 16 24

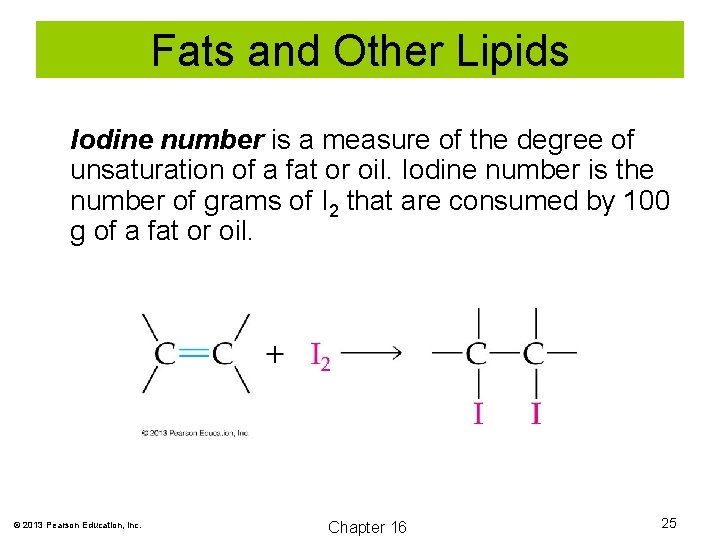
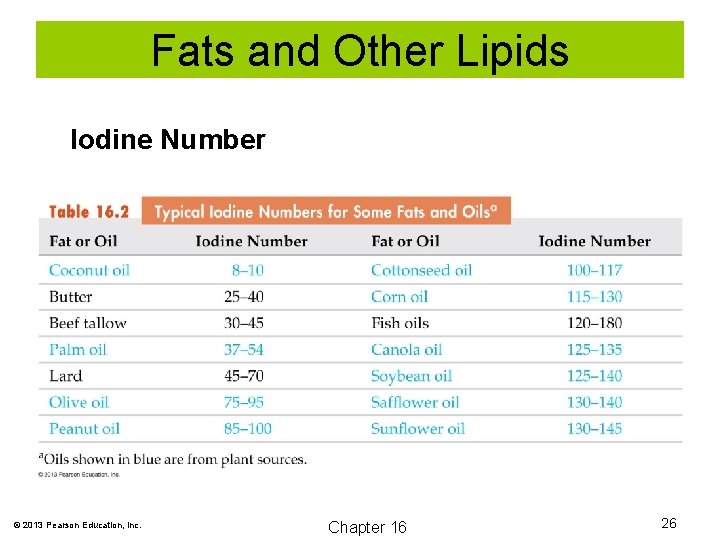
Fats and Other Lipids Iodine number is a measure of the degree of unsaturation of a fat or oil. Iodine number is the number of grams of I 2 that are consumed by 100 g of a fat or oil. © 2013 Pearson Education, Inc. Chapter 16 25

Fats and Other Lipids Iodine Number © 2013 Pearson Education, Inc. Chapter 16 26

Proteins are a vital component of all living things. © 2013 Pearson Education, Inc. Chapter 16 27

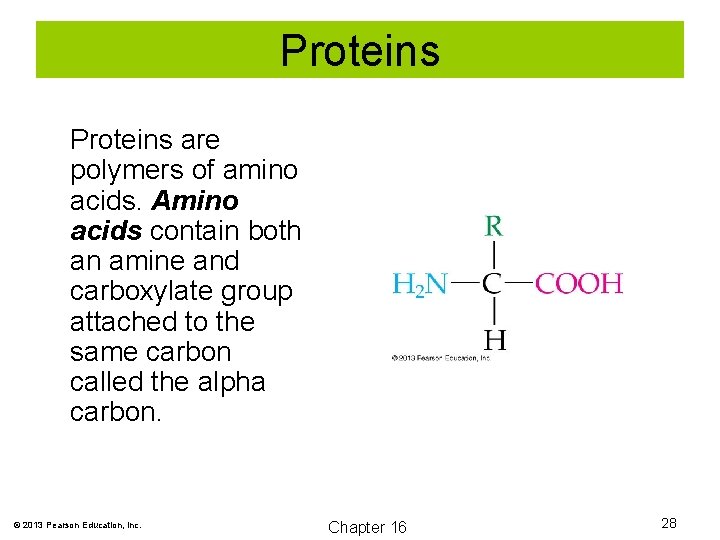
Proteins are polymers of amino acids. Amino acids contain both an amine and carboxylate group attached to the same carbon called the alpha carbon. © 2013 Pearson Education, Inc. Chapter 16 28

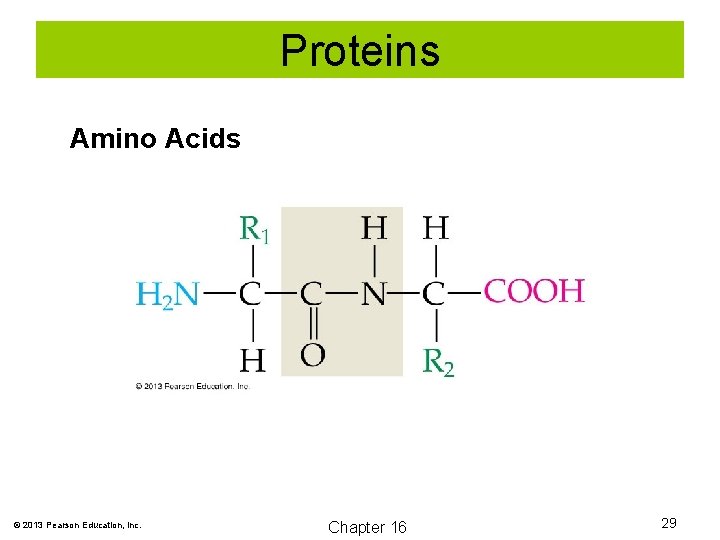
Proteins Amino Acids © 2013 Pearson Education, Inc. Chapter 16 29

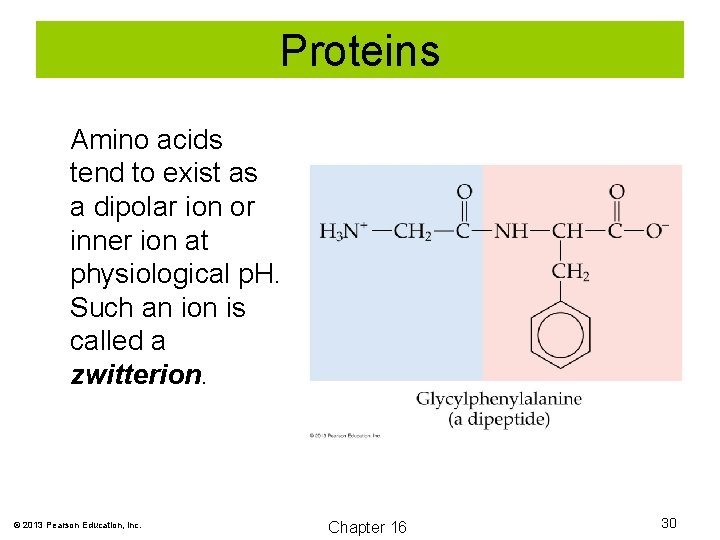
Proteins Amino acids tend to exist as a dipolar ion or inner ion at physiological p. H. Such an ion is called a zwitterion. © 2013 Pearson Education, Inc. Chapter 16 30

Proteins Plants can synthesize proteins from carbon dioxide, water, and minerals contained in compounds like nitrates or sulfates. Animals must consume proteins as part of their diet. Humans can synthesize some amino acids, but must obtain essential amino acids in a normal diet. © 2013 Pearson Education, Inc. Chapter 16 31

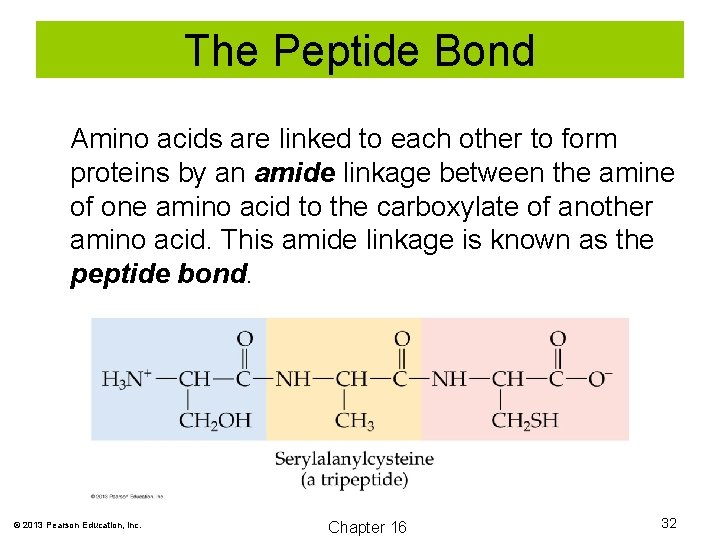
The Peptide Bond Amino acids are linked to each other to form proteins by an amide linkage between the amine of one amino acid to the carboxylate of another amino acid. This amide linkage is known as the peptide bond. © 2013 Pearson Education, Inc. Chapter 16 32

The Peptide Bond A dipeptide is formed when two amino acids are joined. Tripeptides contain three amino acid units. Polypeptides contain ten or more amino acid units. Proteins may contain 10, 000 or more amino acid units. © 2013 Pearson Education, Inc. Chapter 16 33

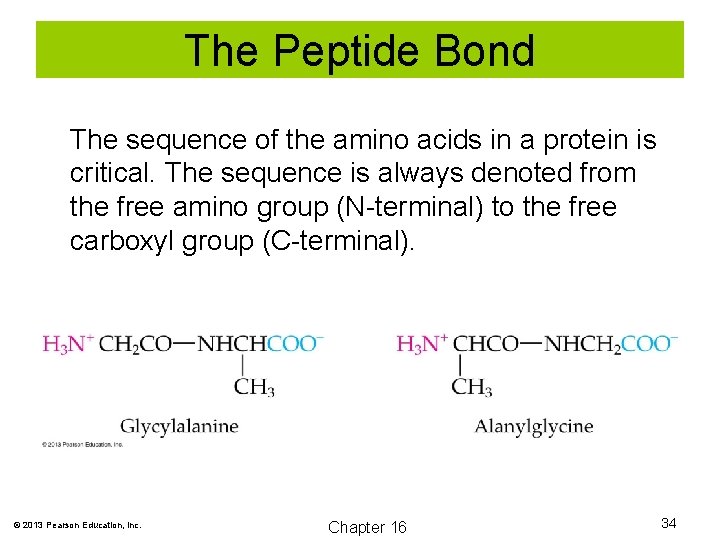
The Peptide Bond The sequence of the amino acids in a protein is critical. The sequence is always denoted from the free amino group (N-terminal) to the free carboxyl group (C-terminal). © 2013 Pearson Education, Inc. Chapter 16 34

Structure of Proteins Primary structure: The primary structure of a protein is simply the sequence of amino acids from N-terminal to C-terminal. Example: The primary structure of angiotensin II is Asp-Arg-Val-Tyr-Ile-His-Pro-Phe © 2013 Pearson Education, Inc. Chapter 16 35

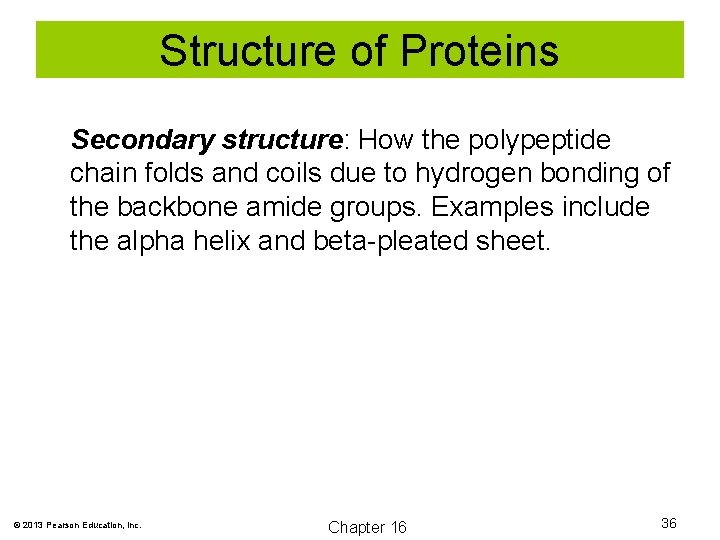
Structure of Proteins Secondary structure: How the polypeptide chain folds and coils due to hydrogen bonding of the backbone amide groups. Examples include the alpha helix and beta-pleated sheet. © 2013 Pearson Education, Inc. Chapter 16 36

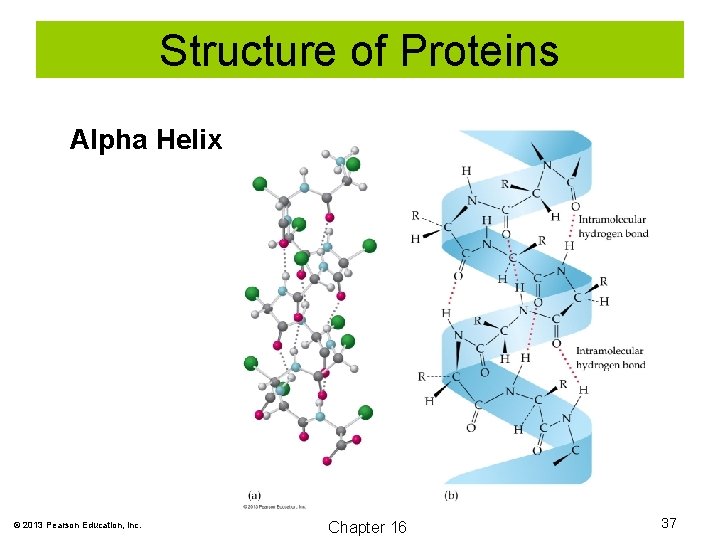
Structure of Proteins Alpha Helix © 2013 Pearson Education, Inc. Chapter 16 37

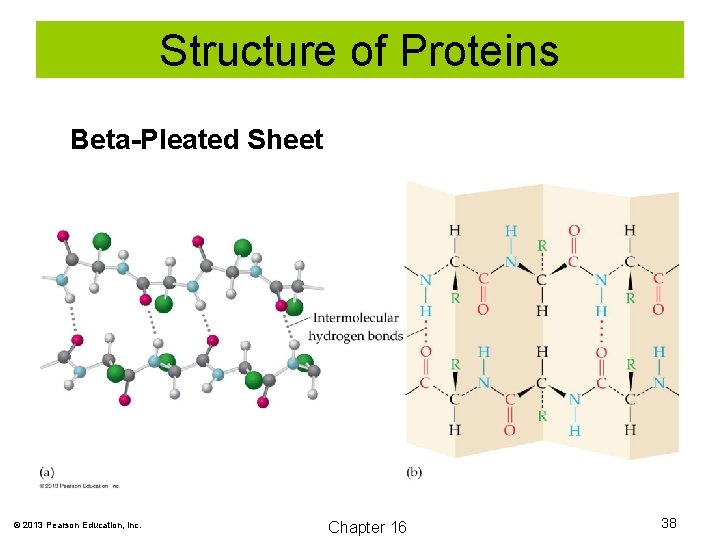
Structure of Proteins Beta-Pleated Sheet © 2013 Pearson Education, Inc. Chapter 16 38

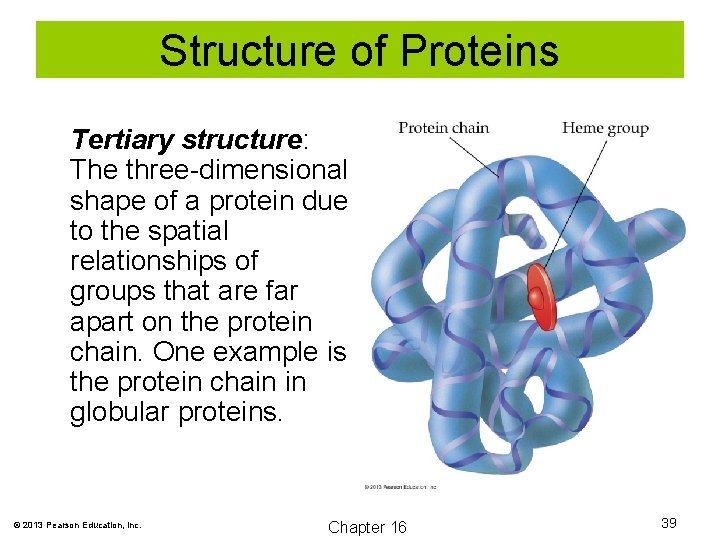
Structure of Proteins Tertiary structure: The three-dimensional shape of a protein due to the spatial relationships of groups that are far apart on the protein chain. One example is the protein chain in globular proteins. © 2013 Pearson Education, Inc. Chapter 16 39

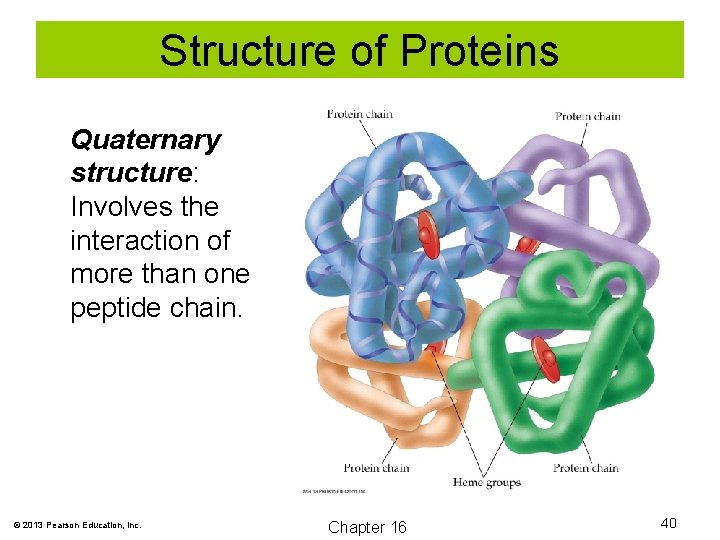
Structure of Proteins Quaternary structure: Involves the interaction of more than one peptide chain. © 2013 Pearson Education, Inc. Chapter 16 40

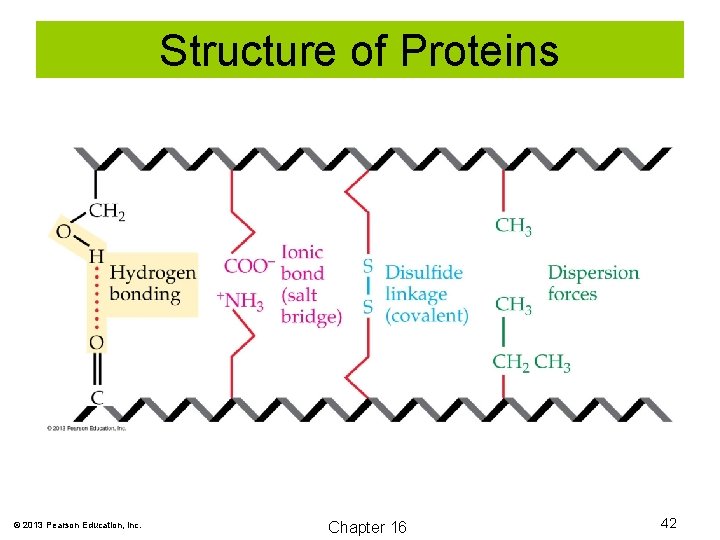
Structure of Proteins Four Ways to Link Protein Chains 1. Hydrogen bond: The secondary structures occur when hydrogen bonds are formed between amide hydrogen (N-H) and carboxyl oxygen (C=O). Tertiary structures also involve hydrogen bonding between side chains of the amino acids. 2. Ionic bonds: Sometimes called salt bridges, these occur between oppositely charged side chains. 3. Disulfide linkages: When two cysteine side chains are oxidized, a (-S-S-) disulfide linkage can form. 4. Dispersion forces: These are attractive forces between two nonpolar side chains. © 2013 Pearson Education, Inc. Chapter 16 41

Structure of Proteins © 2013 Pearson Education, Inc. Chapter 16 42

Enzymes are biological catalysts. Most are proteins. Many are highly specific, only catalyzing a single reaction or related group of reactions. The substrate is the reactant molecule whose reaction the enzyme catalyzes. © 2013 Pearson Education, Inc. Chapter 16 43

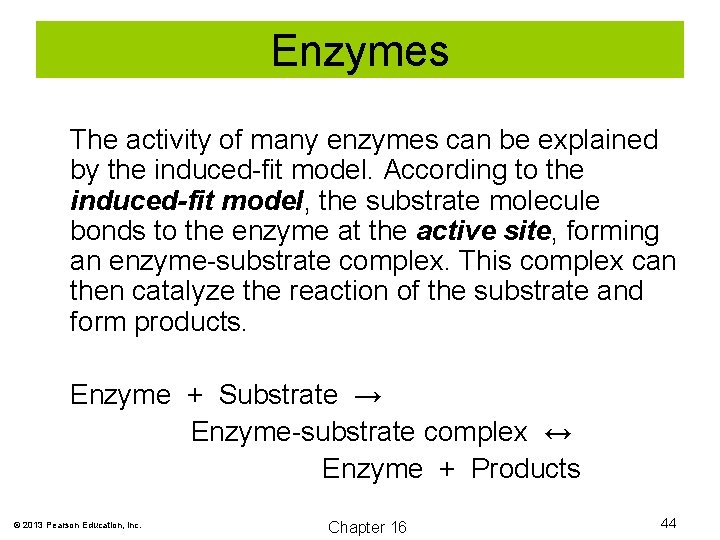
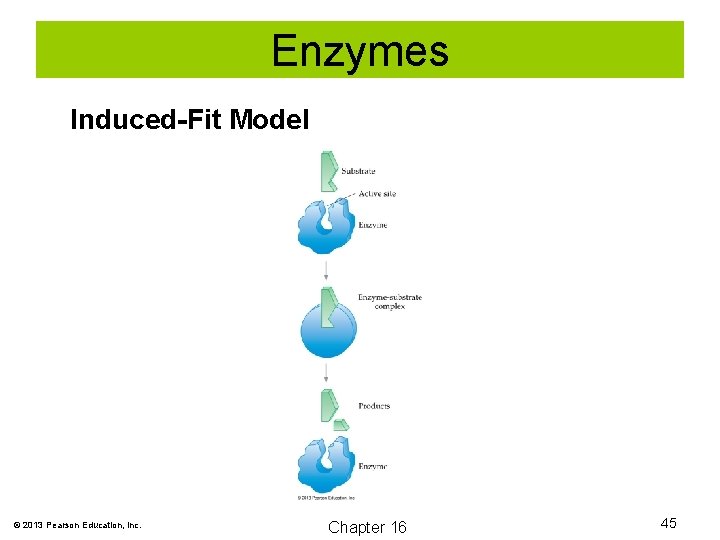
Enzymes The activity of many enzymes can be explained by the induced-fit model. According to the induced-fit model, the substrate molecule bonds to the enzyme at the active site, forming an enzyme-substrate complex. This complex can then catalyze the reaction of the substrate and form products. Enzyme + Substrate → Enzyme-substrate complex ↔ Enzyme + Products © 2013 Pearson Education, Inc. Chapter 16 44

Enzymes Induced-Fit Model © 2013 Pearson Education, Inc. Chapter 16 45

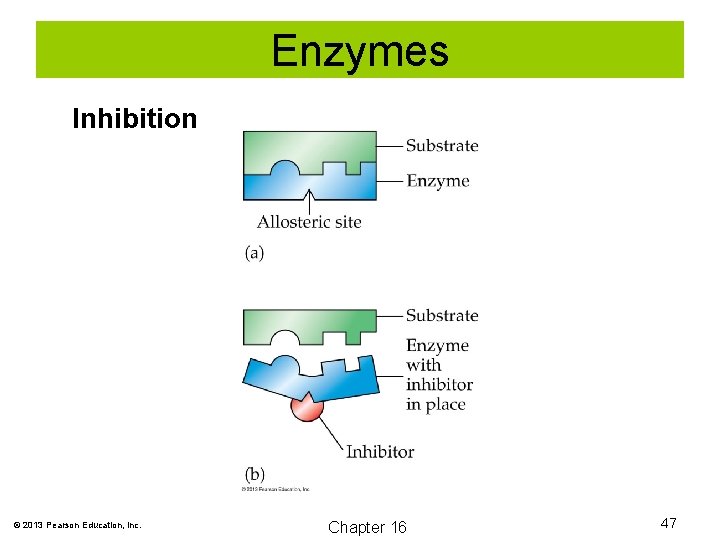
Enzymes Inhibition The action of enzymes can be inhibited. In one mechanism for enzyme inhibition, a molecule bonds to the enzyme protein at a site other than the active site. This changes the shape of the protein and prevents the substrate from bonding at the active site. © 2013 Pearson Education, Inc. Chapter 16 46

Enzymes Inhibition © 2013 Pearson Education, Inc. Chapter 16 47

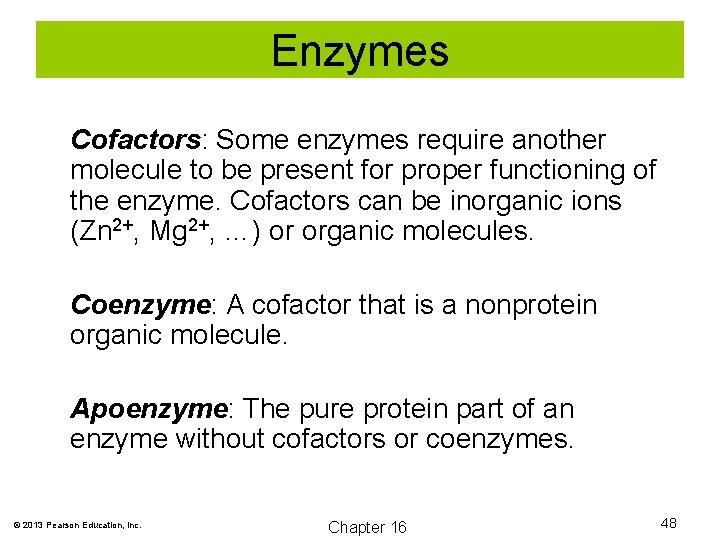
Enzymes Cofactors: Some enzymes require another molecule to be present for proper functioning of the enzyme. Cofactors can be inorganic ions (Zn 2+, Mg 2+, …) or organic molecules. Coenzyme: A cofactor that is a nonprotein organic molecule. Apoenzyme: The pure protein part of an enzyme without cofactors or coenzymes. © 2013 Pearson Education, Inc. Chapter 16 48

Enzymes Diabetic test strips use two enzymes to measure blood sugar. One enzyme catalyzes the oxidation of glucose, producing hydrogen peroxide as a byproduct. The other enzyme catalyzes the breakdown of hydrogen peroxide and oxidizes a dye to produce a color change. Enzymes can be monitored to diagnose liver damage or heart damage. Enzymes can also be used to break up clots after a heart attack or to increase clotting to treat hemophelia. © 2013 Pearson Education, Inc. Chapter 16 49

Enzymes in Industry Enzymes have many industrial applications, including the production of baby foods, beer, sweeteners for soft drinks, animal feeds, and blue jeans. © 2013 Pearson Education, Inc. Chapter 16 50

Enzymes and Green Chemistry Enzymes are continually being investigated for their use in producing specialty chemicals and new drugs. In addition, enzymes can be used to break down complex pollutants. © 2013 Pearson Education, Inc. Chapter 16 51

Enzymes in Everyday Life Enzymes are used in stain removers and meat tenderizers. People who are lactose-intolerant can also take enzymes to reduce the discomfort caused by ingesting dairy foods. Worldwide production of enzymes is worth more than $1 billion per year? © 2013 Pearson Education, Inc. Chapter 16 52

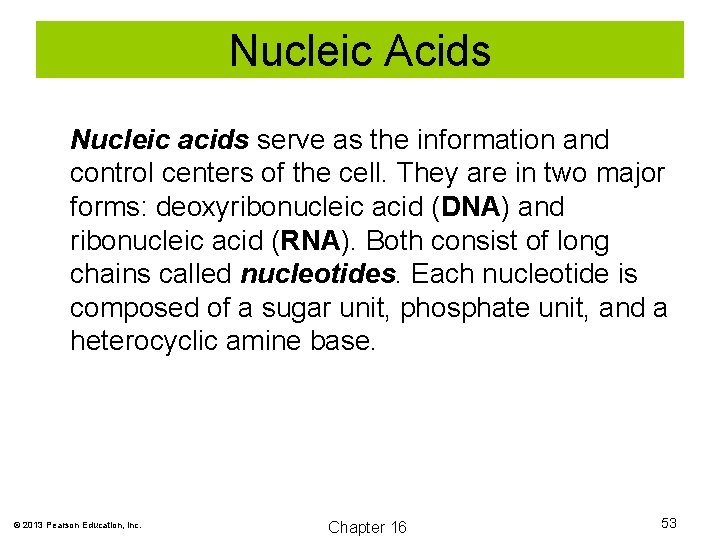
Nucleic Acids Nucleic acids serve as the information and control centers of the cell. They are in two major forms: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Both consist of long chains called nucleotides. Each nucleotide is composed of a sugar unit, phosphate unit, and a heterocyclic amine base. © 2013 Pearson Education, Inc. Chapter 16 53

Nucleic Acids © 2013 Pearson Education, Inc. Chapter 16 54

Nucleic Acids Nucleotides are composed of a sugar, phosphate, and an amine base. © 2013 Pearson Education, Inc. Chapter 16 55

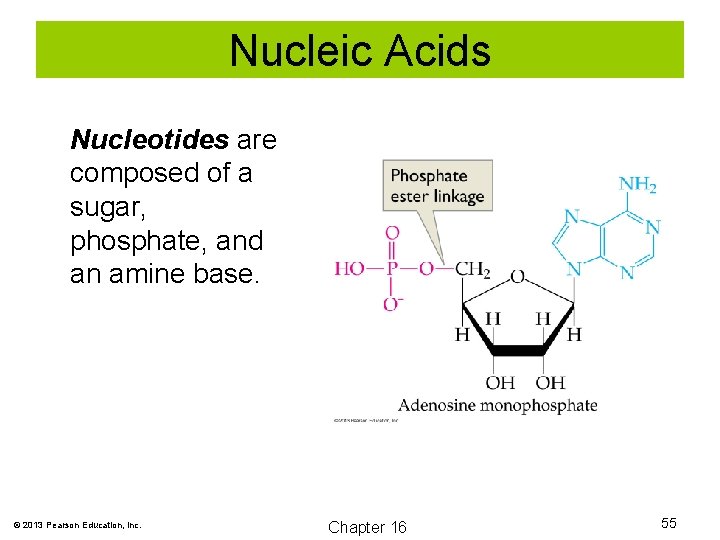
Nucleic Acids DNA consists of a double helix. © 2013 Pearson Education, Inc. Chapter 16 56

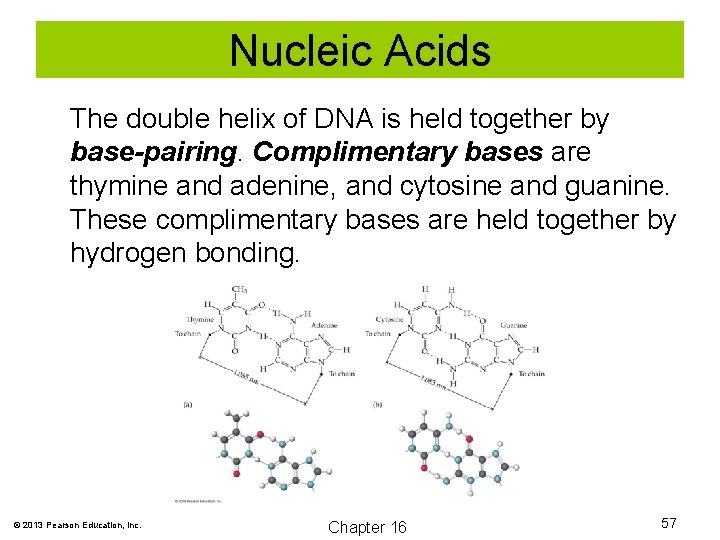
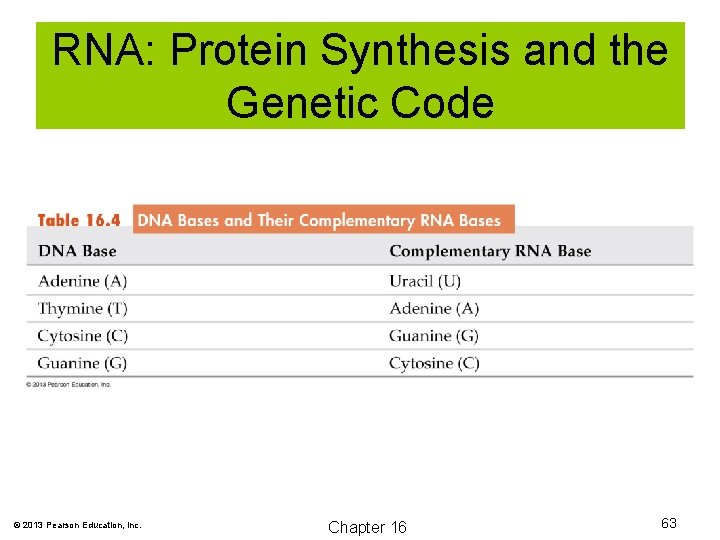
Nucleic Acids The double helix of DNA is held together by base-pairing. Complimentary bases are thymine and adenine, and cytosine and guanine. These complimentary bases are held together by hydrogen bonding. © 2013 Pearson Education, Inc. Chapter 16 57

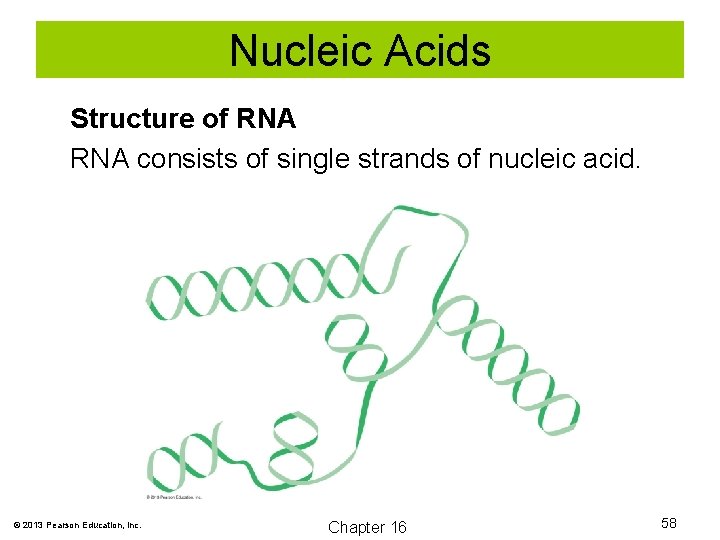
Nucleic Acids Structure of RNA consists of single strands of nucleic acid. © 2013 Pearson Education, Inc. Chapter 16 58

Enzymes Chromosomes: Threadlike strands of DNA that are tightly coiled into X-shaped bodies. Human body cells contain 46 chromosomes. Twentythree come from the egg of the mother, 23 come from the sperm of the father. Gene: Section of a DNA molecule that controls the synthesis of protein. Replication: Copying of DNA during cell division. © 2013 Pearson Education, Inc. Chapter 16 59

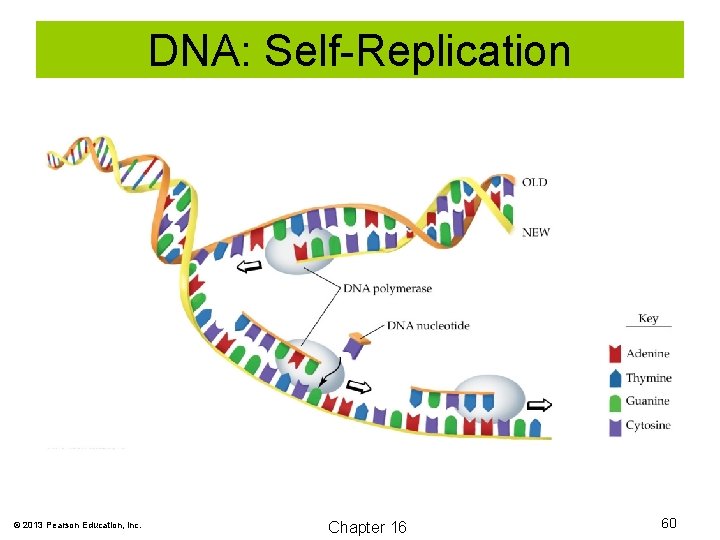
DNA: Self-Replication © 2013 Pearson Education, Inc. Chapter 16 60

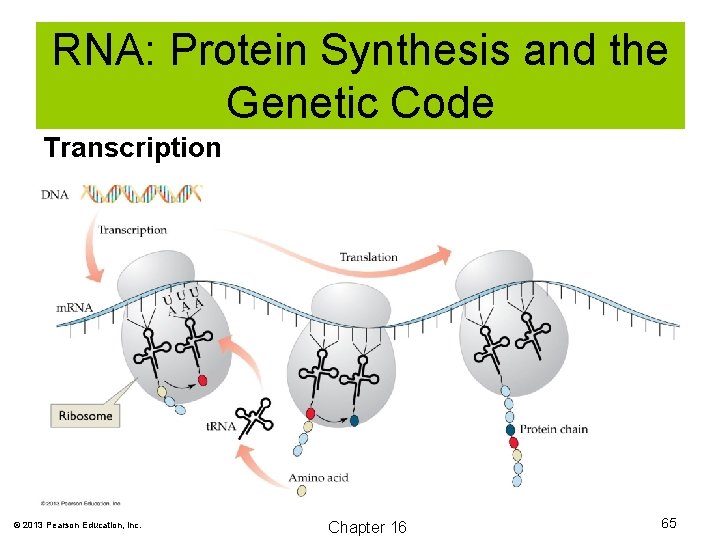
RNA: Protein Synthesis and the Genetic Code The genetic code is carried in a three-base sequence known as a codon. © 2013 Pearson Education, Inc. Chapter 16 61

RNA: Protein Synthesis and the Genetic Code During protein synthesis, the genetic information of DNA is transferred to RNA by a process known as transcription. During transcription, messenger RNA (m. RNA) is synthesized. © 2013 Pearson Education, Inc. Chapter 16 62

RNA: Protein Synthesis and the Genetic Code © 2013 Pearson Education, Inc. Chapter 16 63

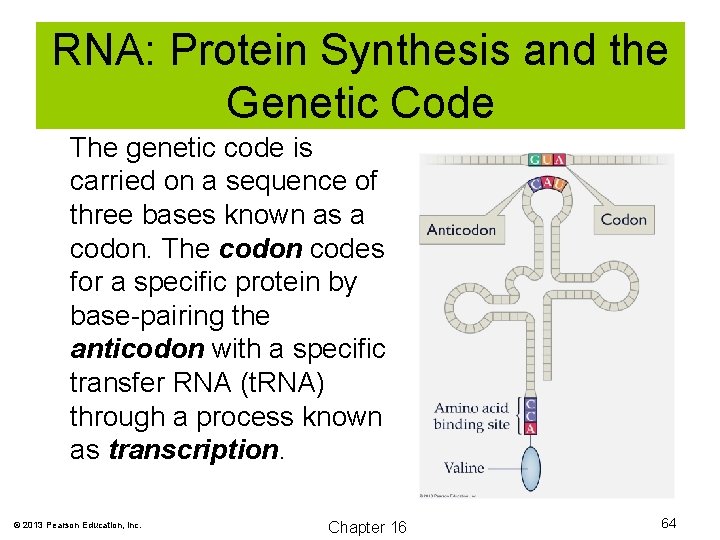
RNA: Protein Synthesis and the v Genetic Code The genetic code is carried on a sequence of three bases known as a codon. The codon codes for a specific protein by base-pairing the anticodon with a specific transfer RNA (t. RNA) through a process known as transcription. © 2013 Pearson Education, Inc. Chapter 16 64

RNA: Protein Synthesis and the Genetic Code Transcription © 2013 Pearson Education, Inc. Chapter 16 65

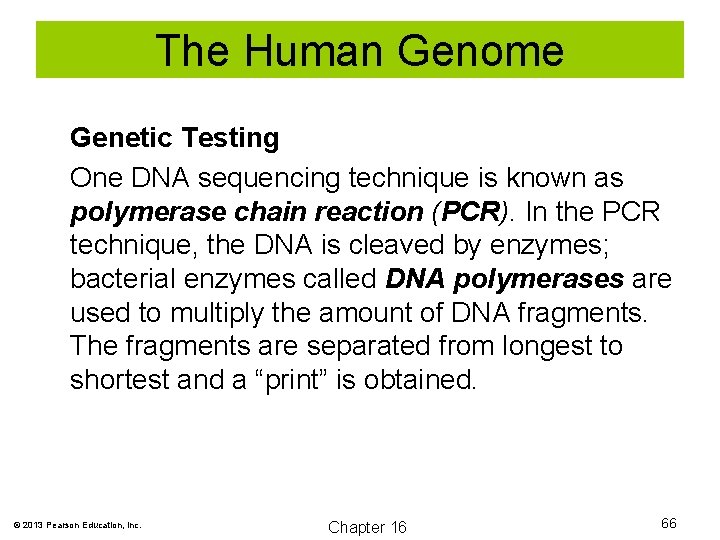
The Human Genome Genetic Testing One DNA sequencing technique is known as polymerase chain reaction (PCR). In the PCR technique, the DNA is cleaved by enzymes; bacterial enzymes called DNA polymerases are used to multiply the amount of DNA fragments. The fragments are separated from longest to shortest and a “print” is obtained. © 2013 Pearson Education, Inc. Chapter 16 66

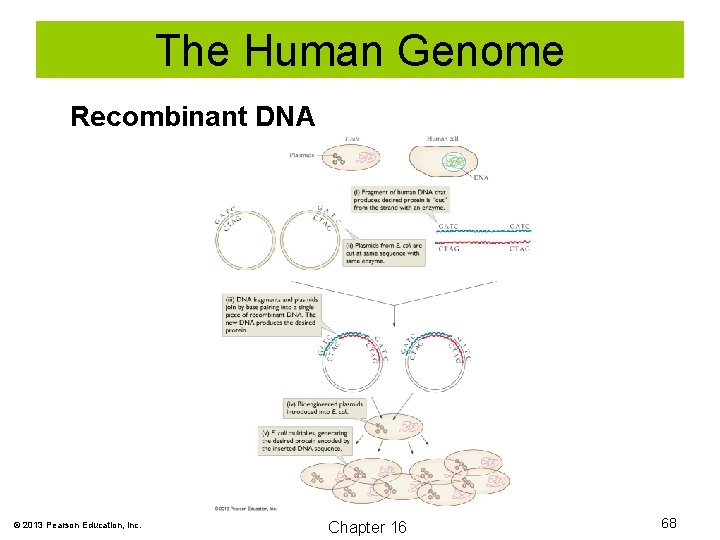
The Human Genome Recombinant DNA is DNA that is produced artificially and contains DNA from two different sources. In one technique, restriction enzymes are used to cleave the DNA. The DNA fragments can then be inserted into bacterial plasmids and the plasmid inserted into a host organism. There it replicates, producing exact copies of itself. © 2013 Pearson Education, Inc. Chapter 16 67

The Human Genome Recombinant DNA © 2013 Pearson Education, Inc. Chapter 16 68

The Human Genome Gene therapy involves introducing a functioning gene into a person’s cells to correct the action of a defective gene. Viruses are used to carry the DNA into cells. Gene therapy is still experimental. © 2013 Pearson Education, Inc. Chapter 16 69

The Human Genome Controversy and Promise in Genetic Engineering Cloning of animals and plants holds much promise for food production and treatment of disease. There is also much controversy and concern. © 2013 Pearson Education, Inc. Chapter 16 70
 Chemistry for changing times
Chemistry for changing times Changing times essay
Changing times essay Activity 1 changing times
Activity 1 changing times The times they are a-changin'
The times they are a-changin' Factors of 168
Factors of 168 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad What is an outline? *
What is an outline? * Lesson outline for teaching
Lesson outline for teaching Prison ministry training manual download
Prison ministry training manual download Foley v classique coaches
Foley v classique coaches Four main components for effective outlines
Four main components for effective outlines A business plan is a document that outlines
A business plan is a document that outlines Pyelonephritis
Pyelonephritis Elijah and the shunammite woman
Elijah and the shunammite woman Anime outline character
Anime outline character Two types of outlines
Two types of outlines A haunted house by virginia woolf characters
A haunted house by virginia woolf characters Ksf outlines
Ksf outlines Mucoepidermoid carcinoma histology
Mucoepidermoid carcinoma histology Cmmi model outlines
Cmmi model outlines Acts outline
Acts outline Exegetical sermon outlines
Exegetical sermon outlines Level 3 cjis security test
Level 3 cjis security test Mun position paper outline
Mun position paper outline Visible outlines
Visible outlines Acute suppurative appendicitis
Acute suppurative appendicitis A clear concise document which outlines preventive
A clear concise document which outlines preventive Using mis 10th edition
Using mis 10th edition Report
Report Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Rearranged most stable carbocation is
Rearranged most stable carbocation is Pericyclic
Pericyclic Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Chemistry central science 14th edition
Chemistry central science 14th edition Organic chemistry third edition david klein
Organic chemistry third edition david klein David klein organic chemistry
David klein organic chemistry Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes Organic chemistry third edition david klein
Organic chemistry third edition david klein General chemistry 11th edition
General chemistry 11th edition Living by chemistry 2nd edition answers
Living by chemistry 2nd edition answers Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Democritus atomic model diagram
Democritus atomic model diagram Halohydrin
Halohydrin Thermodynamic control
Thermodynamic control Organic chemistry second edition david klein
Organic chemistry second edition david klein Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Kontinuitetshantering
Kontinuitetshantering Novell typiska drag
Novell typiska drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok
Tidbok Sura för anatom
Sura för anatom Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Tes debattartikel
Tes debattartikel Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Lufttryck formel
Lufttryck formel Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Kyssande vind
Kyssande vind Presentera för publik crossboss
Presentera för publik crossboss