Chemistry for Changing Times Thirteenth Edition Lecture Outlines











- Slides: 46

Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chapter 1 Chemistry John Singer, Jackson Community College © 2013 Pearson Education, Inc.

A Science for All Seasons Chemistry is the study of matter and its changes. Everything we do involves chemistry. © 2013 Pearson Education, Inc. Chapter 1 2

Science and Technology Science is the process of seeking an understanding of underlying principles of nature. It involves two facets: technological (or factual) and philosophical (or theoretical). © 2013 Pearson Education, Inc. Chapter 1 3

Science and Technology is the direct application of knowledge to solve problems. Science grew out of natural philosophy or the philosophical speculation about nature. © 2013 Pearson Education, Inc. Chapter 1 4

Baconian Dream and Carsonian Nightmare It was the dream of Francis Bacon (philosopher) that science would solve the world’s problems and enrich human life with new inventions, thereby increasing happiness and prosperity. © 2013 Pearson Education, Inc. Chapter 1 5

Baconian Dream and Carsonian Nightmare Rachel Carson (biologist) published Silent Spring in 1962. She proposed that the use of chemicals to control insects threatened the destruction of all life. © 2013 Pearson Education, Inc. Chapter 1 6

Green and Sustainable Chemistry Green chemistry uses materials and processes that are intended to prevent or reduce pollution at its source. Sustainable chemistry is designed to meet the needs of the present generation without compromising the needs of future generations. © 2013 Pearson Education, Inc. Chapter 1 7

Science has five characteristics. Science is • Testable • Reproducible • Explanatory • Predictive • Tentative © 2013 Pearson Education, Inc. Chapter 1 8

© 2013 Pearson Education, Inc. Chapter 1 9

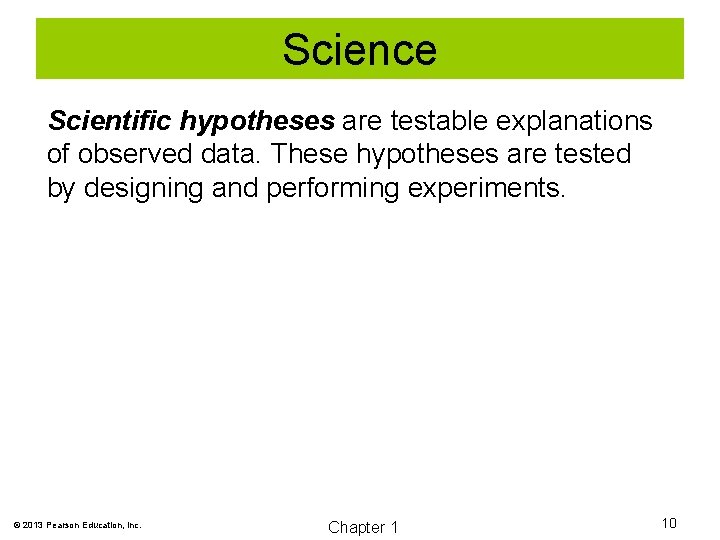
Science Scientific hypotheses are testable explanations of observed data. These hypotheses are tested by designing and performing experiments. © 2013 Pearson Education, Inc. Chapter 1 10

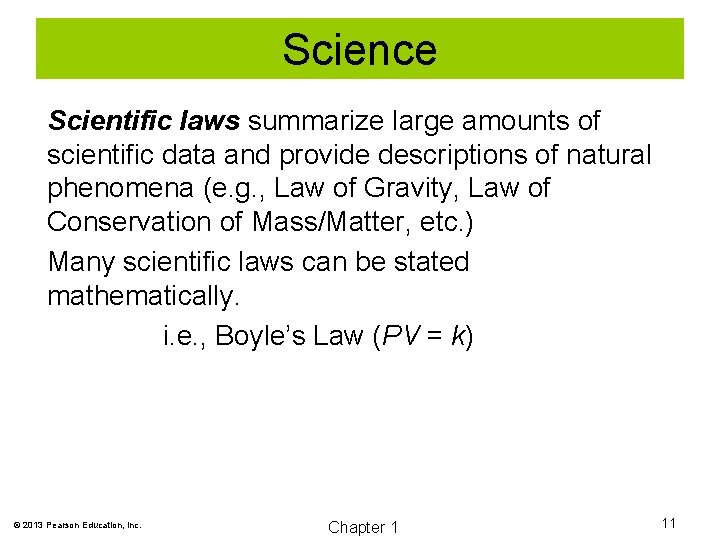
Science Scientific laws summarize large amounts of scientific data and provide descriptions of natural phenomena (e. g. , Law of Gravity, Law of Conservation of Mass/Matter, etc. ) Many scientific laws can be stated mathematically. i. e. , Boyle’s Law (PV = k) © 2013 Pearson Education, Inc. Chapter 1 11

Science A scientific theory is a set of tested hypotheses that explain natural phenomena. Scientific theories are the best current explanation for natural phenomena. Theories are always tentative and may change as observations of nature change. © 2013 Pearson Education, Inc. Chapter 1 12

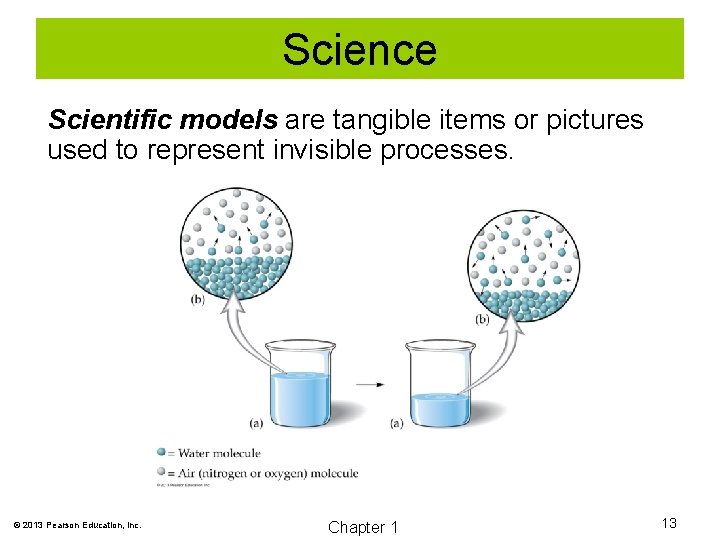
Science Scientific models are tangible items or pictures used to represent invisible processes. © 2013 Pearson Education, Inc. Chapter 1 13

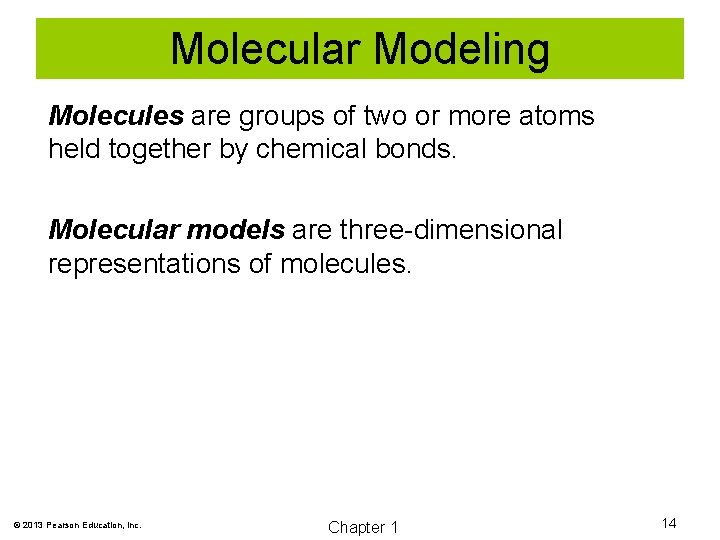
Molecular Modeling Molecules are groups of two or more atoms held together by chemical bonds. Molecular models are three-dimensional representations of molecules. © 2013 Pearson Education, Inc. Chapter 1 14

Limitations of Science is limited to studying that which is observable as well as processes in which variables can be controlled. © 2013 Pearson Education, Inc. Chapter 1 15

Science and Technology: Risks and Benefits Science and technology are interrelated. They involve both risks and benefits. Risk-benefit analysis involves an estimation called the desirability quotient (DQ). DQ = Benefits Risks © 2013 Pearson Education, Inc. Chapter 1 16

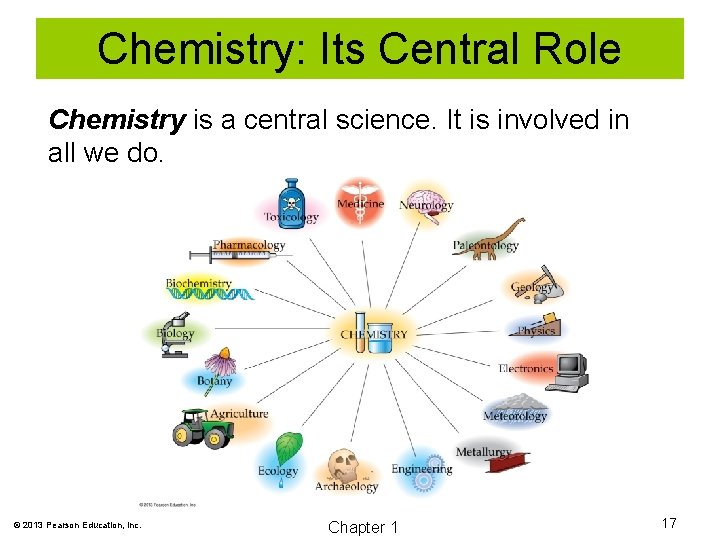
Chemistry: Its Central Role Chemistry is a central science. It is involved in all we do. © 2013 Pearson Education, Inc. Chapter 1 17

Solving Society’s Problems: Scientific Research Applied research involves studying a specific problem in industry or the environment. George Washington Carver’s work with peanuts is an example of applied research. In his research, he developed more than 300 products from peanuts. © 2013 Pearson Education, Inc. Chapter 1 18

Solving Society’s Problems: Scientific Research Basic research involves the search for knowledge for its own sake. The findings of basic research may someday be applied to a specific problem in industry or the environment. Gertrude Ellion’s work with purines and their role in the cell is an example of basic research. © 2013 Pearson Education, Inc. Chapter 1 19

Chemistry: The Study of Matter and Its Changes Chemistry is the study of matter and its changes. Matter is anything that has mass and also volume. © 2013 Pearson Education, Inc. Chapter 1 20

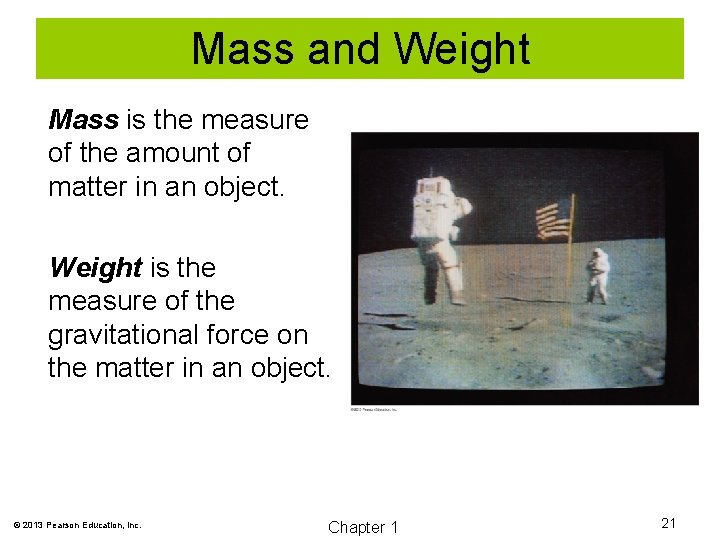
Mass and Weight Mass is the measure of the amount of matter in an object. Weight is the measure of the gravitational force on the matter in an object. © 2013 Pearson Education, Inc. Chapter 1 21

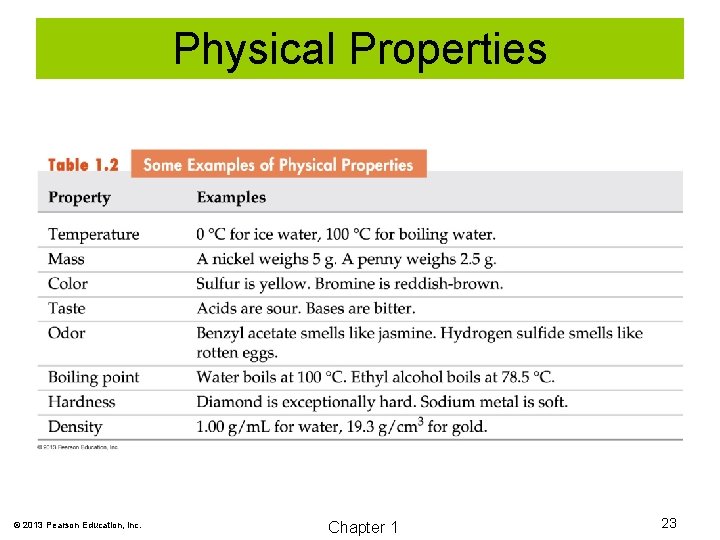
Physical Properties Physical properties are those properties of a substance that can be observed without changing the substance. Examples are • Color • Mass • Weight © 2013 Pearson Education, Inc. Chapter 1 22

Physical Properties © 2013 Pearson Education, Inc. Chapter 1 23

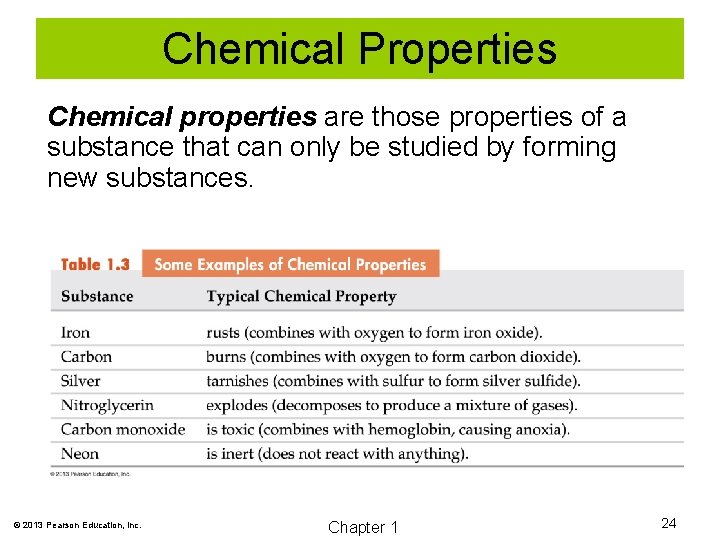
Chemical Properties Chemical properties are those properties of a substance that can only be studied by forming new substances. © 2013 Pearson Education, Inc. Chapter 1 24

Physical Changes Physical changes are changes in which the chemical identity of the substance is not changed. Examples are • Melting • Freezing © 2013 Pearson Education, Inc. Chapter 1 25

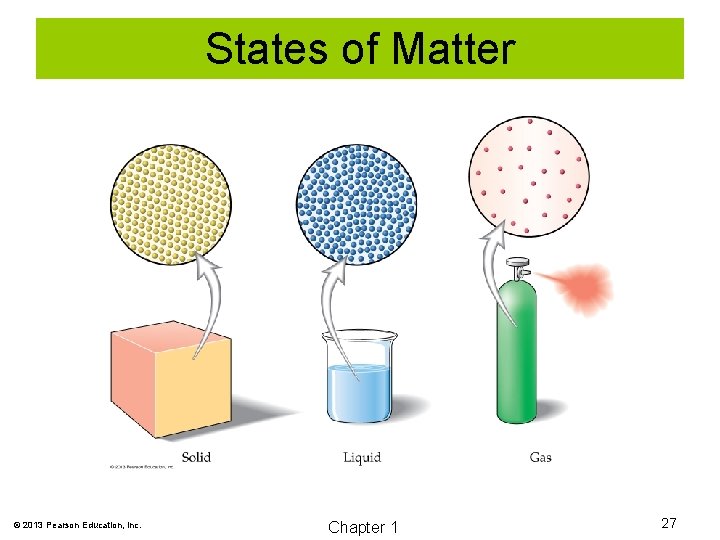
Classification of Matter A solid has a definite shape and volume. A liquid has a definite volume, but has no definite shape. A gas has neither definite volume nor definite shape. © 2013 Pearson Education, Inc. Chapter 1 26

States of Matter © 2013 Pearson Education, Inc. Chapter 1 27

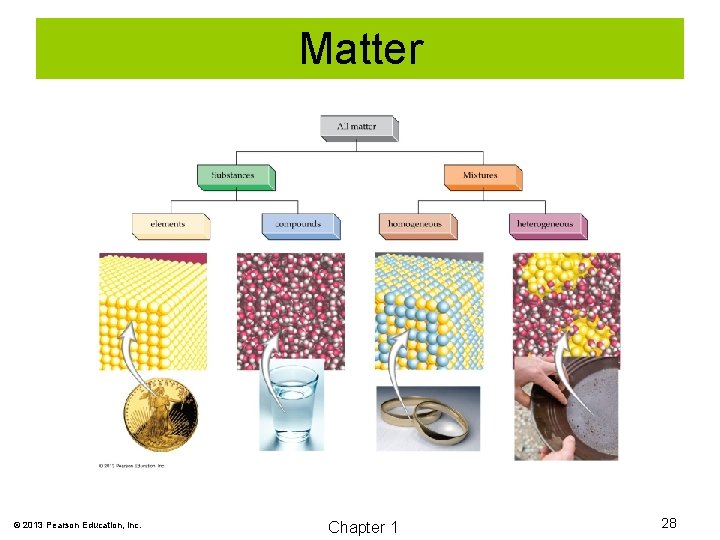
Matter © 2013 Pearson Education, Inc. Chapter 1 28

Elements are composed of one type of atom. Atoms are the smallest particle of an element. Elements are represented by chemical symbols. Examples are Cl, H, and Mg. © 2013 Pearson Education, Inc. Chapter 1 29

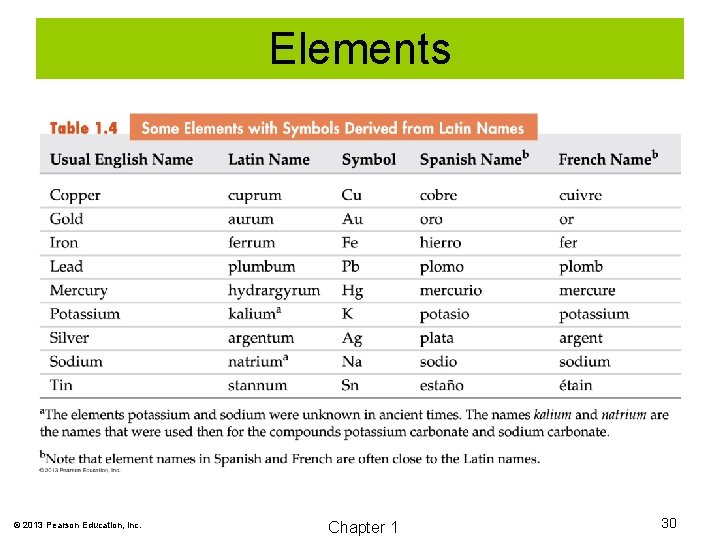
Elements © 2013 Pearson Education, Inc. Chapter 1 30

Compounds are made of two or more elements chemically combined. Many compounds exist as groups of atoms bonded together as a unit. These units are called molecules. © 2013 Pearson Education, Inc. Chapter 1 31

Mixtures A mixture is a physical blend of two or more substances. Homogeneous mixtures are uniform in composition. Heterogeneous mixtures are not uniform in composition. © 2013 Pearson Education, Inc. Chapter 1 32

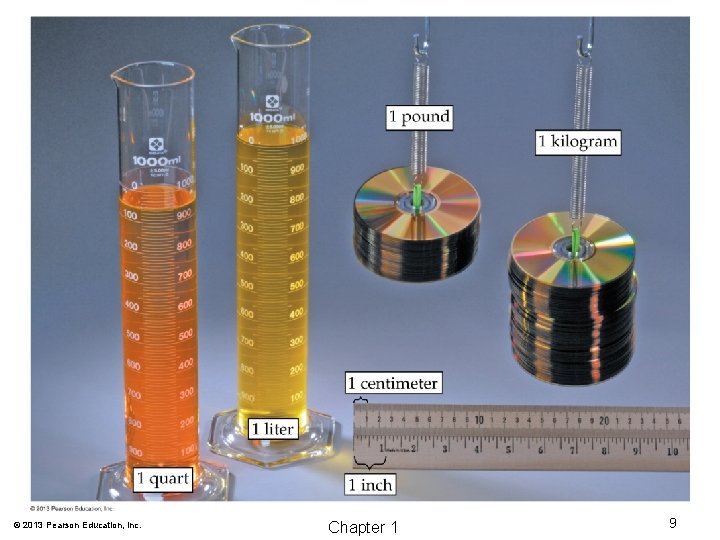
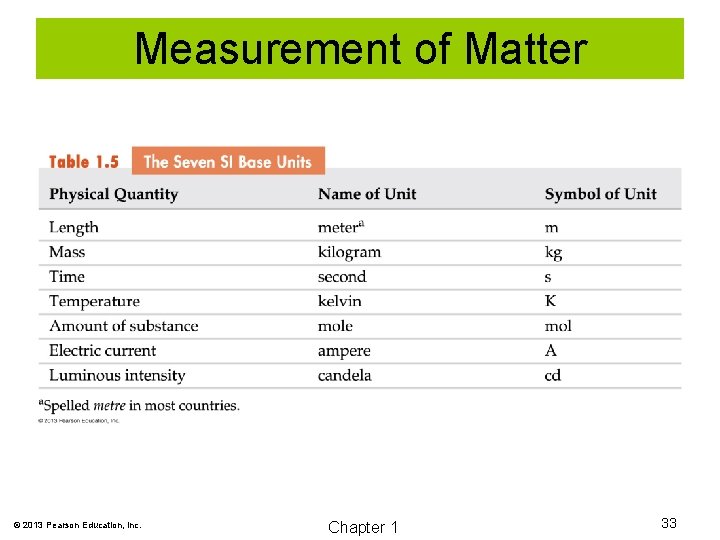
Measurement of Matter © 2013 Pearson Education, Inc. Chapter 1 33

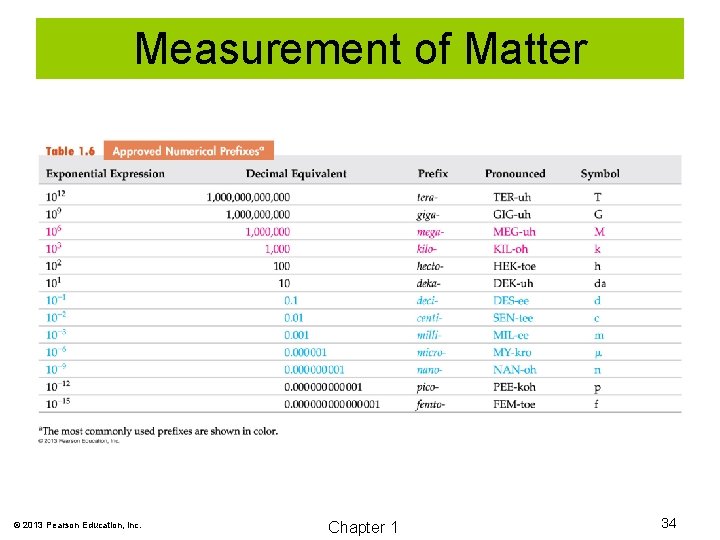
Measurement of Matter © 2013 Pearson Education, Inc. Chapter 1 34

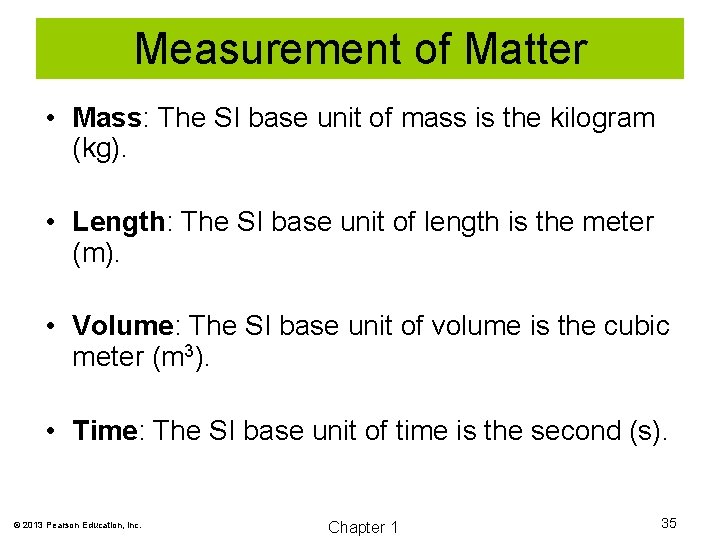
Measurement of Matter • Mass: The SI base unit of mass is the kilogram (kg). • Length: The SI base unit of length is the meter (m). • Volume: The SI base unit of volume is the cubic meter (m 3). • Time: The SI base unit of time is the second (s). © 2013 Pearson Education, Inc. Chapter 1 35

Nanotechnology • Nanotechnology is the study of manipulating matter at the atomic or molecular level. © 2013 Pearson Education, Inc. Chapter 1 36

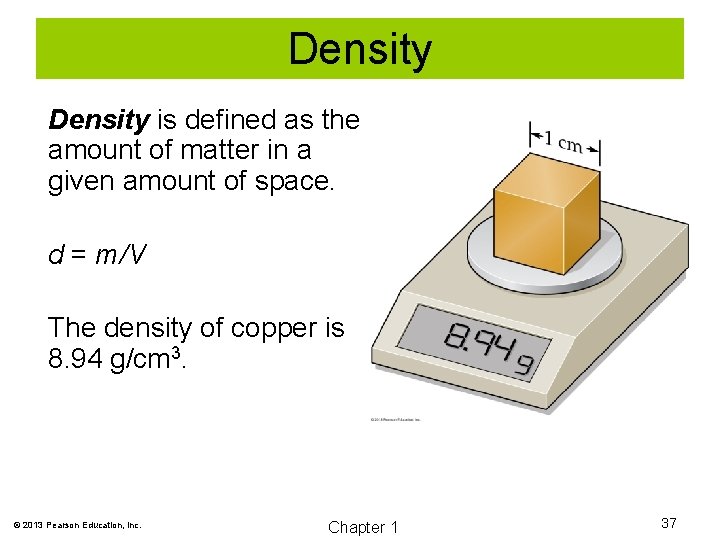
Density is defined as the amount of matter in a given amount of space. d = m/V The density of copper is 8. 94 g/cm 3. © 2013 Pearson Education, Inc. Chapter 1 37

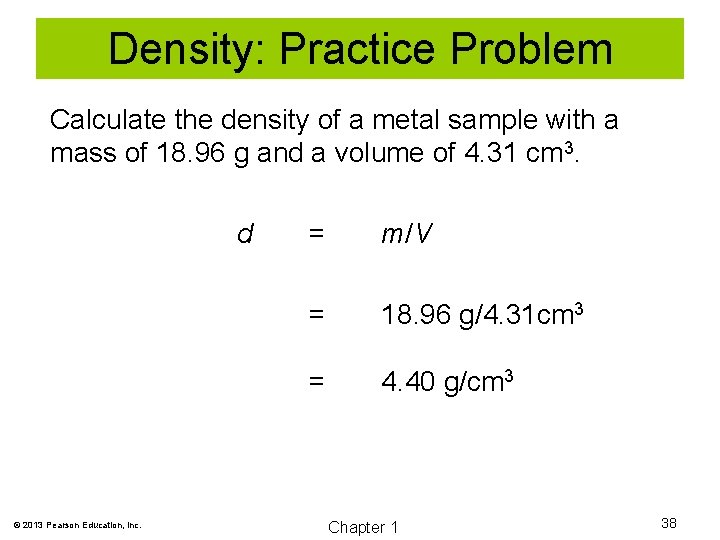
Density: Practice Problem Calculate the density of a metal sample with a mass of 18. 96 g and a volume of 4. 31 cm 3. d © 2013 Pearson Education, Inc. = m/V = 18. 96 g/4. 31 cm 3 = 4. 40 g/cm 3 Chapter 1 38

Energy: Heat and Temperature Energy is the ability to do work or transfer heat. Energy exists in two major forms: • Potential energy is stored energy. • Kinetic energy is energy in motion. © 2013 Pearson Education, Inc. Chapter 1 39

Heat vs. Temperature Heat is energy that is transferred from hotter objects to cooler objects. Temperature is the average kinetic energy of the atoms or molecules that make up an object. © 2013 Pearson Education, Inc. Chapter 1 40

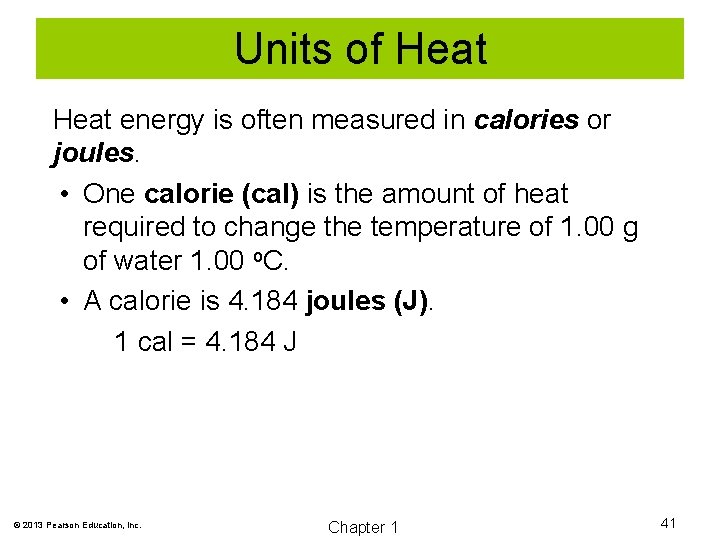
Units of Heat energy is often measured in calories or joules. • One calorie (cal) is the amount of heat required to change the temperature of 1. 00 g of water 1. 00 o. C. • A calorie is 4. 184 joules (J). 1 cal = 4. 184 J © 2013 Pearson Education, Inc. Chapter 1 41

Food Calories A food calorie (Cal, “C” is capitalized) is actually a kilocalorie. 1 Cal = 1 kcal = 1000 cal = 4184 J © 2013 Pearson Education, Inc. Chapter 1 42

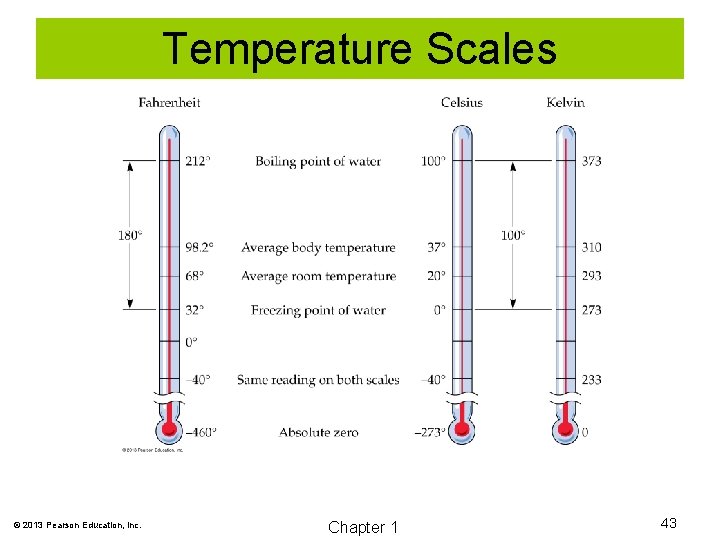
Temperature Scales © 2013 Pearson Education, Inc. Chapter 1 43

Celsius to Kelvin Conversion K = o. C + 273. 15 © 2013 Pearson Education, Inc. Chapter 1 44

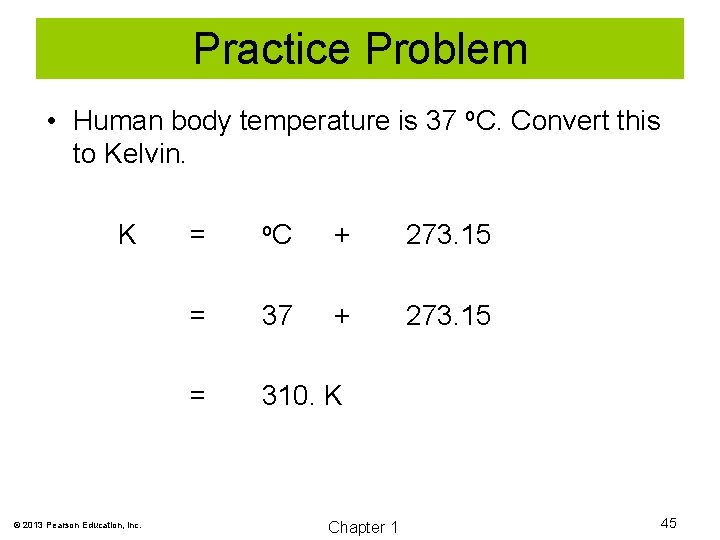
Practice Problem • Human body temperature is 37 o. C. Convert this to Kelvin. K © 2013 Pearson Education, Inc. = o. C + 273. 15 = 37 + 273. 15 = 310. K Chapter 1 45

Critical Thinking and Validity You can test the validity of a claim by using the FLa. Re. S test: • Falsifiability • Logic • Replicability • Sufficiency If a claim passes all four FLa. Re. S tests, then it may be true. Though it can still be proven false. If it fails even one of the tests, it is likely to be false. © 2013 Pearson Education, Inc. Chapter 1 46
 Carsonian nightmare
Carsonian nightmare Changing times essay
Changing times essay Activity 1 changing times
Activity 1 changing times The times they are a changing
The times they are a changing Mat0022
Mat0022 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Parallelism outline
Parallelism outline Teaching outline
Teaching outline Kairos program manual
Kairos program manual Commercial law outline
Commercial law outline Four main components for effective outlines
Four main components for effective outlines A business plan is a document that outlines
A business plan is a document that outlines Pathology outline
Pathology outline Sermon outlines on the shunammite woman
Sermon outlines on the shunammite woman Anime character outline
Anime character outline Two types of outlines
Two types of outlines Haunted house outlines
Haunted house outlines Ksf outlines
Ksf outlines Mucoepidermoid carcinoma pathology outlines
Mucoepidermoid carcinoma pathology outlines Cmmi model outlines
Cmmi model outlines Book of acts outline
Book of acts outline Exegetical sermon outlines
Exegetical sermon outlines The cjis security policy outlines the minimum requirements
The cjis security policy outlines the minimum requirements Mun position paper outline
Mun position paper outline Define:visible
Define:visible Catarrhal appendicitis
Catarrhal appendicitis A clear concise document which outlines preventive
A clear concise document which outlines preventive Using mis 10th edition
Using mis 10th edition Using mis (10th edition)
Using mis (10th edition) Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Rearranged most stable carbocation is
Rearranged most stable carbocation is Pericyclic
Pericyclic Is alkane an organic compound
Is alkane an organic compound Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Chemistry the central science 14th edition
Chemistry the central science 14th edition Reaction of grignard reagent with acid chloride
Reaction of grignard reagent with acid chloride David klein organic chemistry
David klein organic chemistry Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes Organic chemistry third edition david klein
Organic chemistry third edition david klein General chemistry
General chemistry Lesson 81 drop in molecular views
Lesson 81 drop in molecular views Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Democritus atomic model diagram
Democritus atomic model diagram Halohydrin formation
Halohydrin formation