Chemistry for Changing Times Thirteenth Edition Lecture Outlines




- Slides: 41

Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chapter 4 Chemical Bonds John Singer, Jackson Community College © 2013 Pearson Education, Inc.

The Ties that Bind Carbon exists commonly as soot. When soot is subjected to high temperature and pressure, it can form diamond. This process can be explained by understanding the chemical bonds that hold the atoms together. © 2013 Pearson Education, Inc. Chapter 4 2

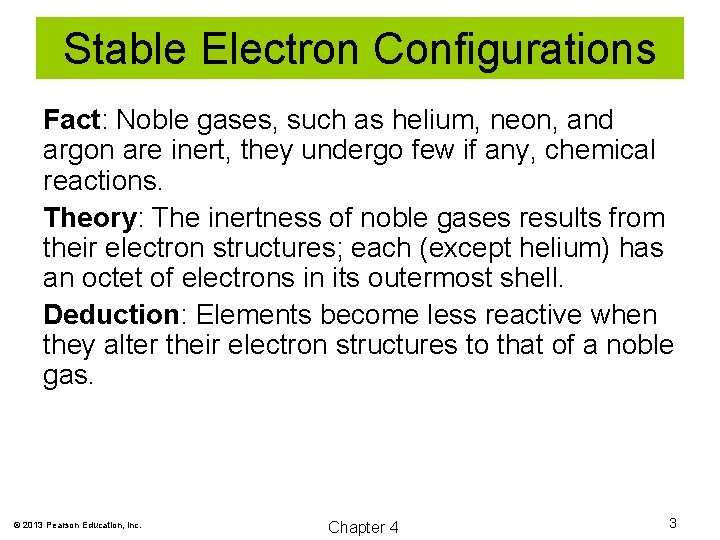
Stable Electron Configurations Fact: Noble gases, such as helium, neon, and argon are inert, they undergo few if any, chemical reactions. Theory: The inertness of noble gases results from their electron structures; each (except helium) has an octet of electrons in its outermost shell. Deduction: Elements become less reactive when they alter their electron structures to that of a noble gas. © 2013 Pearson Education, Inc. Chapter 4 3

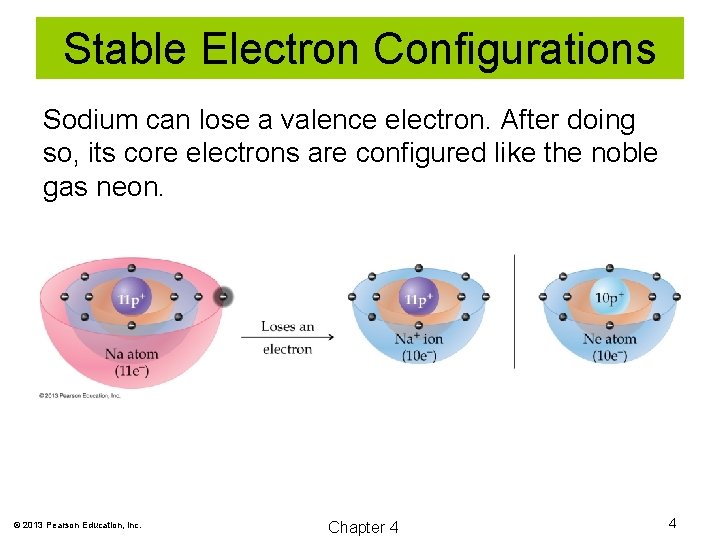
Stable Electron Configurations Sodium can lose a valence electron. After doing so, its core electrons are configured like the noble gas neon. © 2013 Pearson Education, Inc. Chapter 4 4

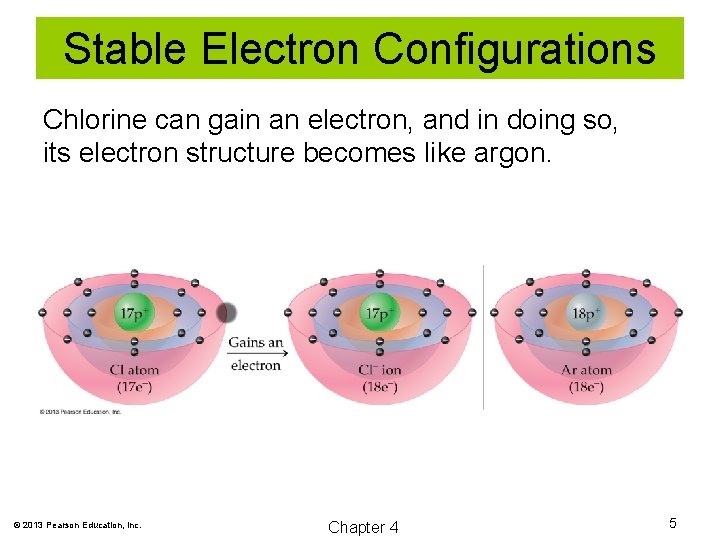
Stable Electron Configurations Chlorine can gain an electron, and in doing so, its electron structure becomes like argon. © 2013 Pearson Education, Inc. Chapter 4 5

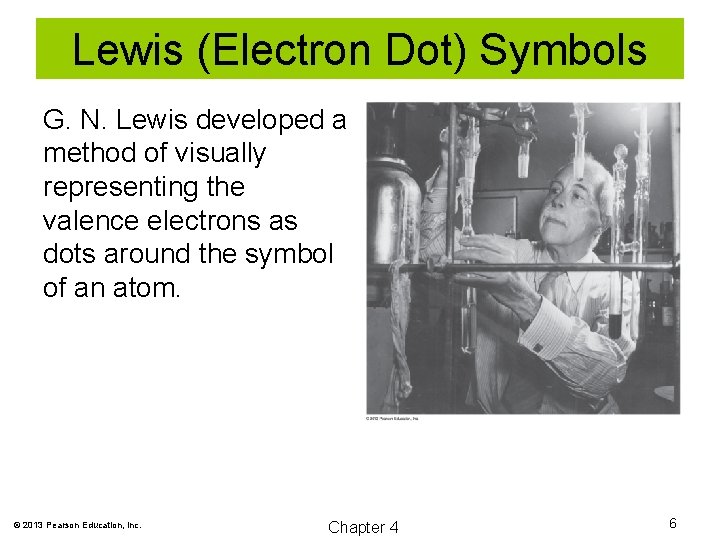
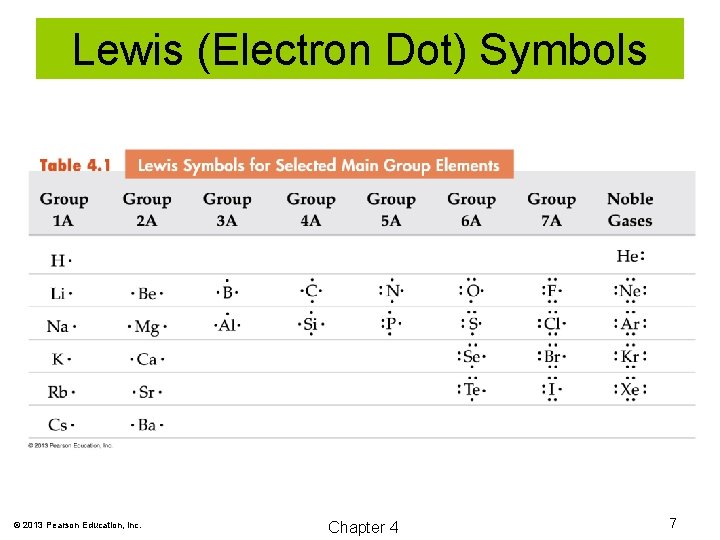
Lewis (Electron Dot) Symbols G. N. Lewis developed a method of visually representing the valence electrons as dots around the symbol of an atom. © 2013 Pearson Education, Inc. Chapter 4 6

Lewis (Electron Dot) Symbols © 2013 Pearson Education, Inc. Chapter 4 7

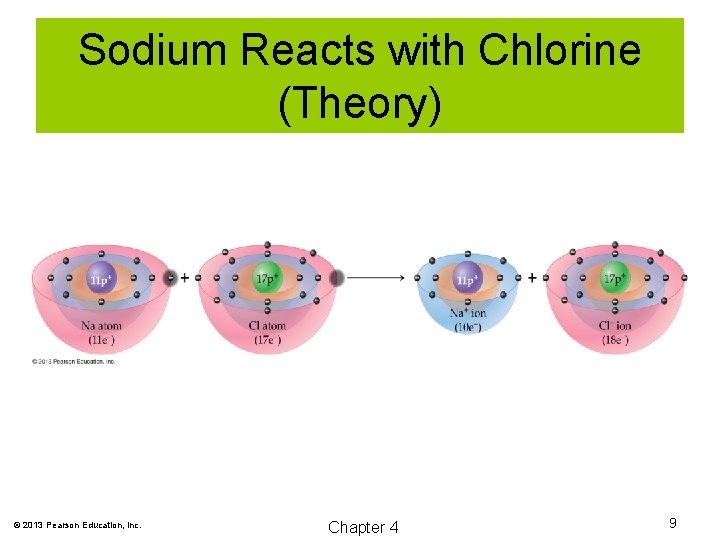
Sodium Reacts with Chlorine (Fact) © 2013 Pearson Education, Inc. Chapter 4 8

Sodium Reacts with Chlorine (Theory) © 2013 Pearson Education, Inc. Chapter 4 9

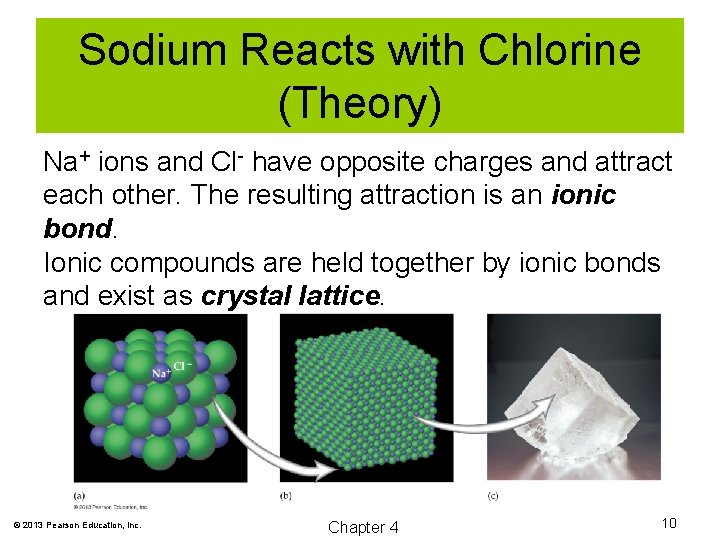
Sodium Reacts with Chlorine (Theory) Na+ ions and Cl- have opposite charges and attract each other. The resulting attraction is an ionic bond. Ionic compounds are held together by ionic bonds and exist as crystal lattice. © 2013 Pearson Education, Inc. Chapter 4 10

Atoms and Ions: Distinctively Different © 2013 Pearson Education, Inc. Chapter 4 11

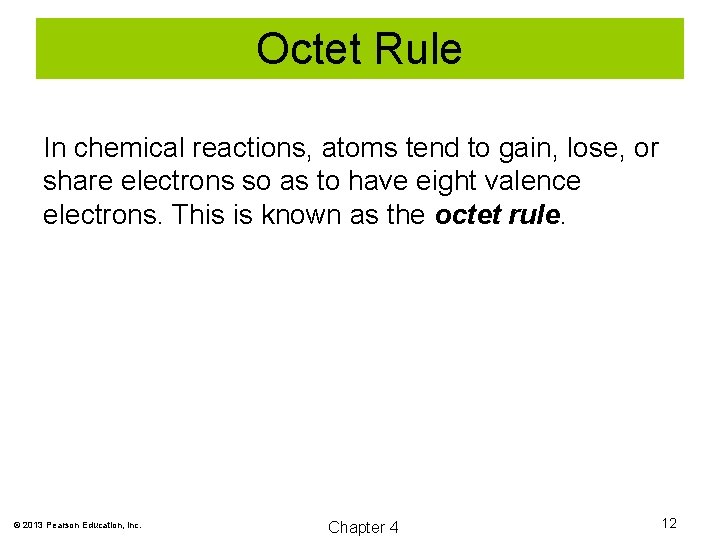
Octet Rule In chemical reactions, atoms tend to gain, lose, or share electrons so as to have eight valence electrons. This is known as the octet rule. © 2013 Pearson Education, Inc. Chapter 4 12

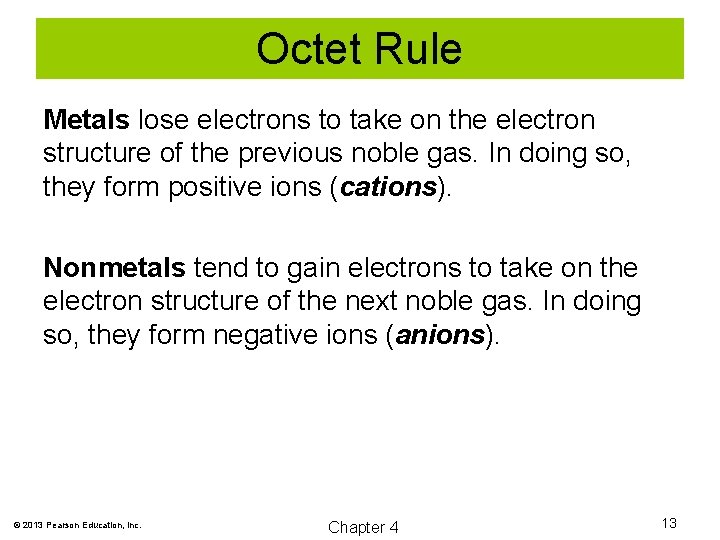
Octet Rule Metals lose electrons to take on the electron structure of the previous noble gas. In doing so, they form positive ions (cations). Nonmetals tend to gain electrons to take on the electron structure of the next noble gas. In doing so, they form negative ions (anions). © 2013 Pearson Education, Inc. Chapter 4 13

Octet Rule © 2013 Pearson Education, Inc. Chapter 4 14

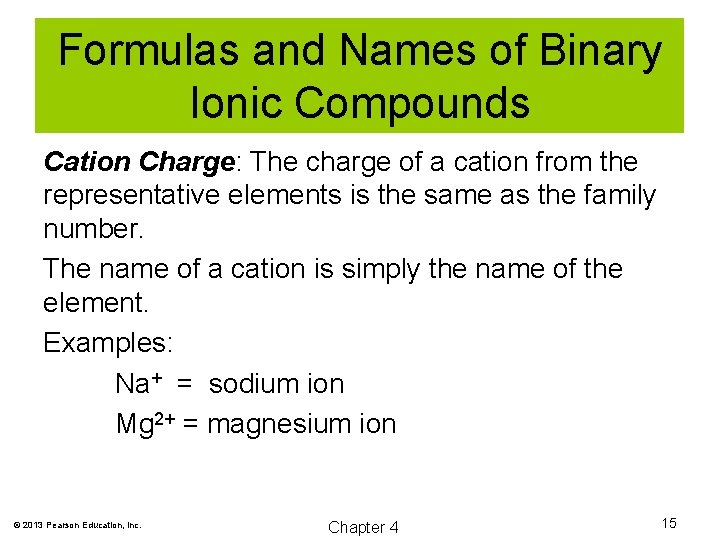
Formulas and Names of Binary Ionic Compounds Cation Charge: The charge of a cation from the representative elements is the same as the family number. The name of a cation is simply the name of the element. Examples: Na+ = sodium ion Mg 2+ = magnesium ion © 2013 Pearson Education, Inc. Chapter 4 15

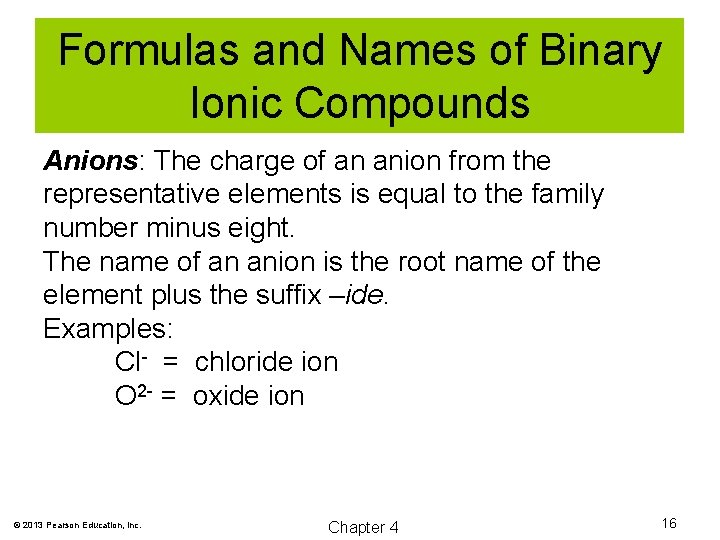
Formulas and Names of Binary Ionic Compounds Anions: The charge of an anion from the representative elements is equal to the family number minus eight. The name of an anion is the root name of the element plus the suffix –ide. Examples: Cl- = chloride ion O 2 - = oxide ion © 2013 Pearson Education, Inc. Chapter 4 16

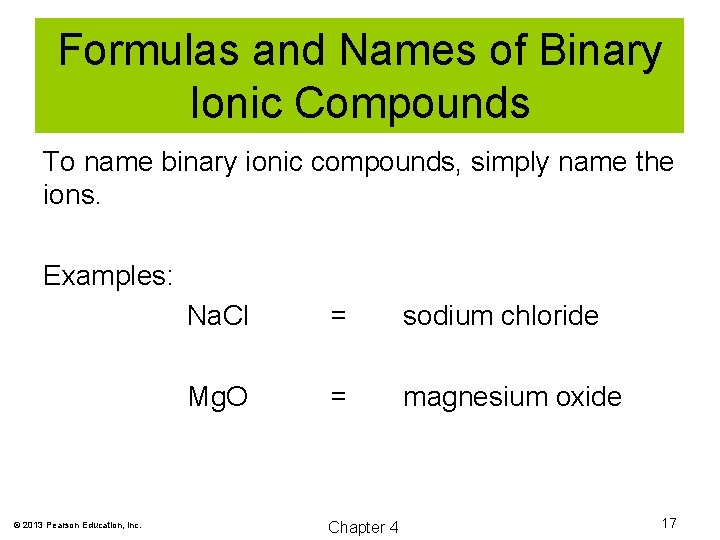
Formulas and Names of Binary Ionic Compounds To name binary ionic compounds, simply name the ions. Examples: © 2013 Pearson Education, Inc. Na. Cl = sodium chloride Mg. O = magnesium oxide Chapter 4 17

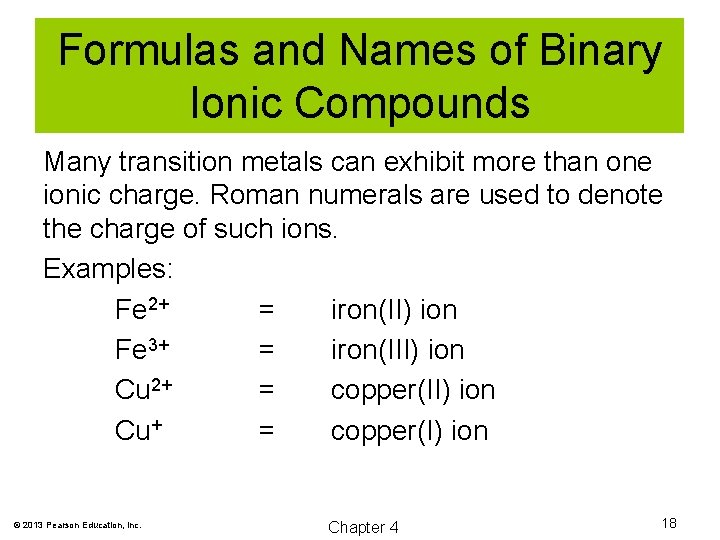
Formulas and Names of Binary Ionic Compounds Many transition metals can exhibit more than one ionic charge. Roman numerals are used to denote the charge of such ions. Examples: Fe 2+ = iron(II) ion Fe 3+ = iron(III) ion Cu 2+ = copper(II) ion Cu+ = copper(I) ion © 2013 Pearson Education, Inc. Chapter 4 18

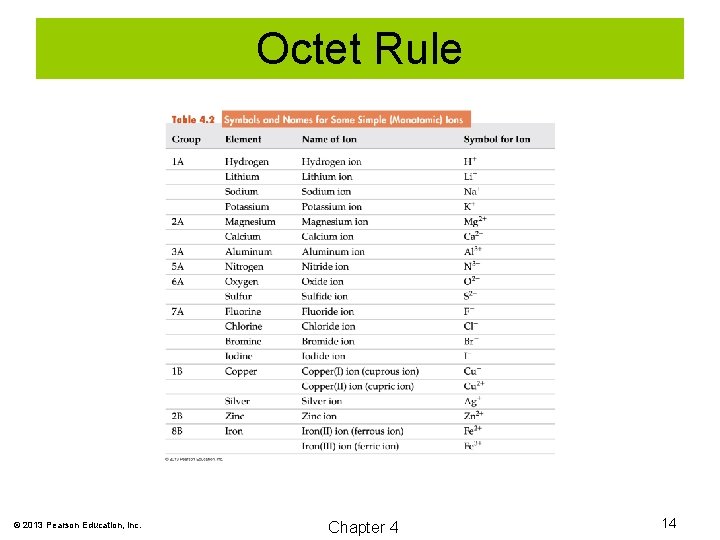
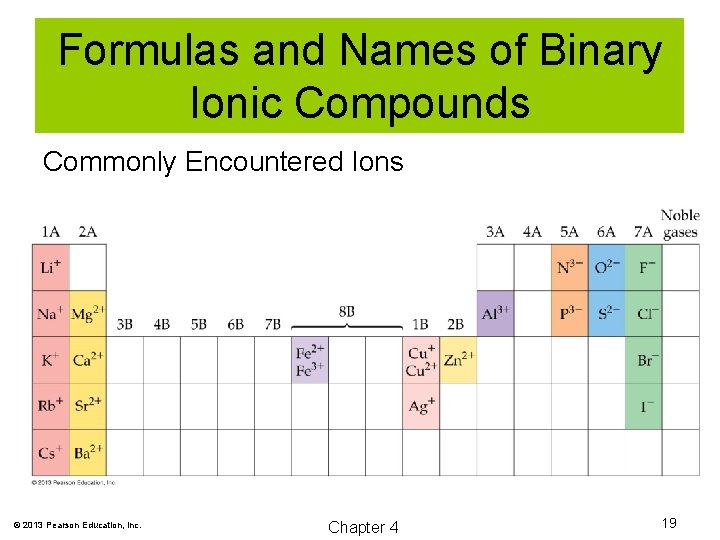
Formulas and Names of Binary Ionic Compounds Commonly Encountered Ions © 2013 Pearson Education, Inc. Chapter 4 19

Covalent Bonds Many nonmetallic elements react by sharing electrons rather than by gaining or losing electrons. When two atoms share a pair of electrons, a covalent bond is formed. Atoms can share one, two, or three pairs of electrons, forming single, double, and triple bonds. © 2013 Pearson Education, Inc. Chapter 4 20

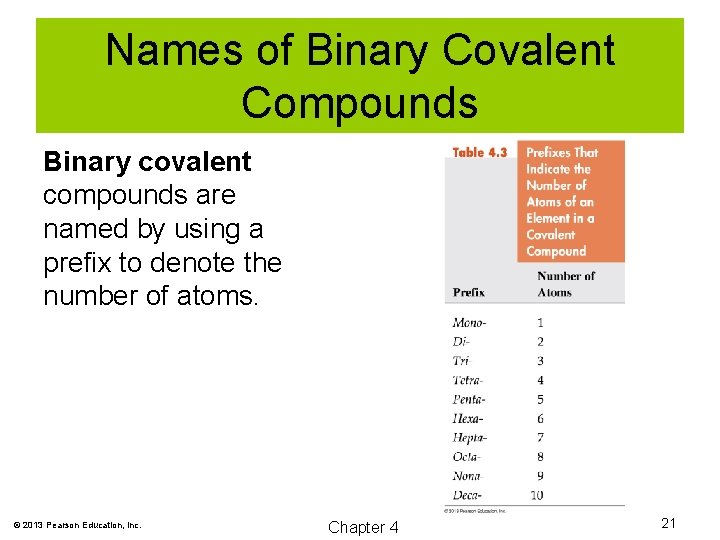
Names of Binary Covalent Compounds Binary covalent compounds are named by using a prefix to denote the number of atoms. © 2013 Pearson Education, Inc. Chapter 4 21

Names of Binary Covalent Compounds Binary covalent compounds have two names: 1. First name = prefix + name of 1 st element (Note: If the first element has only one atom, the prefix mono- is dropped. ) 2. Second name = prefix + root name of second element + suffix –ide. © 2013 Pearson Education, Inc. Chapter 4 22

Names of Binary Covalent Compounds Examples: SBr 4 sulfur tetrabromide P 2 O 3 diphosphorus trioxide © 2013 Pearson Education, Inc. Chapter 4 23

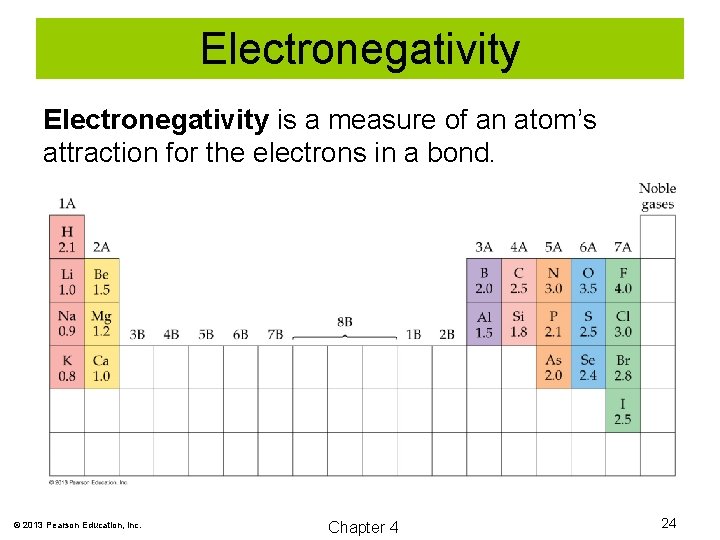
Electronegativity is a measure of an atom’s attraction for the electrons in a bond. © 2013 Pearson Education, Inc. Chapter 4 24

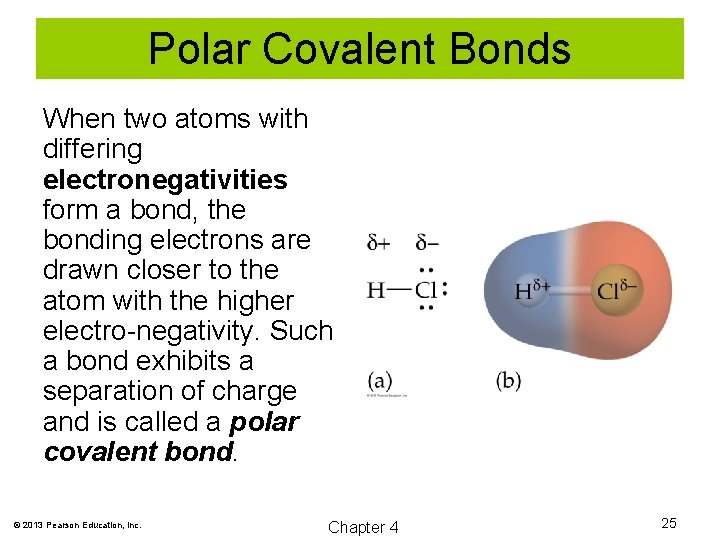
Polar Covalent Bonds When two atoms with differing electronegativities form a bond, the bonding electrons are drawn closer to the atom with the higher electro-negativity. Such a bond exhibits a separation of charge and is called a polar covalent bond. © 2013 Pearson Education, Inc. Chapter 4 25

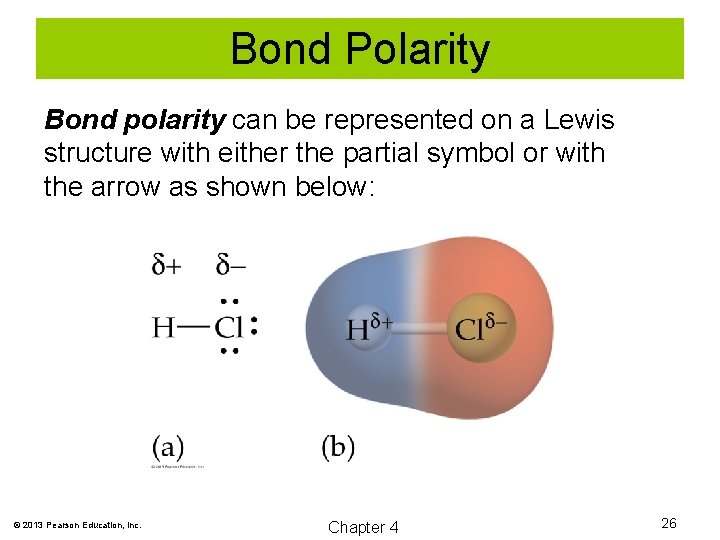
Bond Polarity Bond polarity can be represented on a Lewis structure with either the partial symbol or with the arrow as shown below: © 2013 Pearson Education, Inc. Chapter 4 26

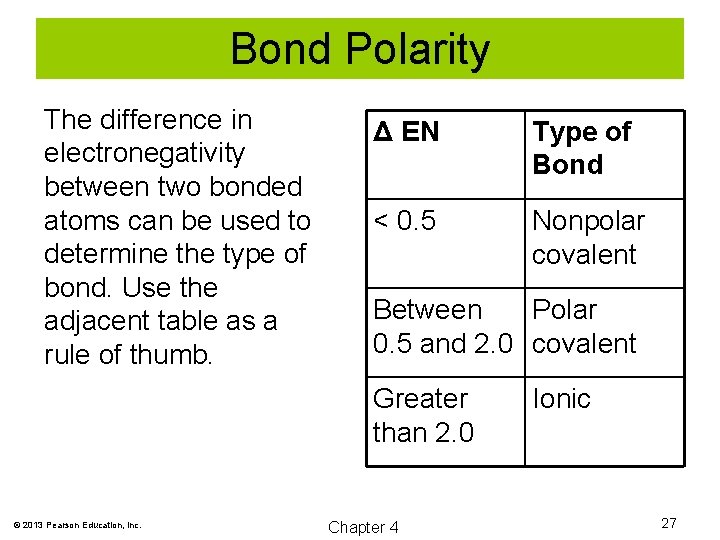
Bond Polarity The difference in electronegativity between two bonded atoms can be used to determine the type of bond. Use the adjacent table as a rule of thumb. Δ EN Type of Bond < 0. 5 Nonpolar covalent Between Polar 0. 5 and 2. 0 covalent Greater than 2. 0 © 2013 Pearson Education, Inc. Chapter 4 Ionic 27

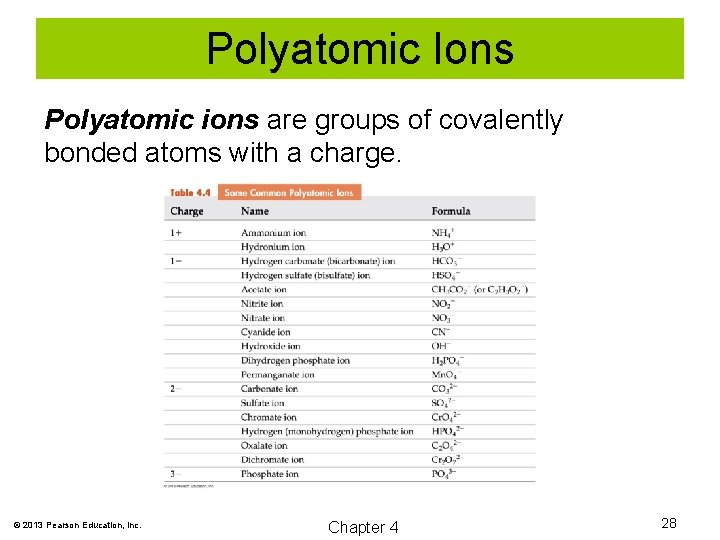
Polyatomic Ions Polyatomic ions are groups of covalently bonded atoms with a charge. © 2013 Pearson Education, Inc. Chapter 4 28

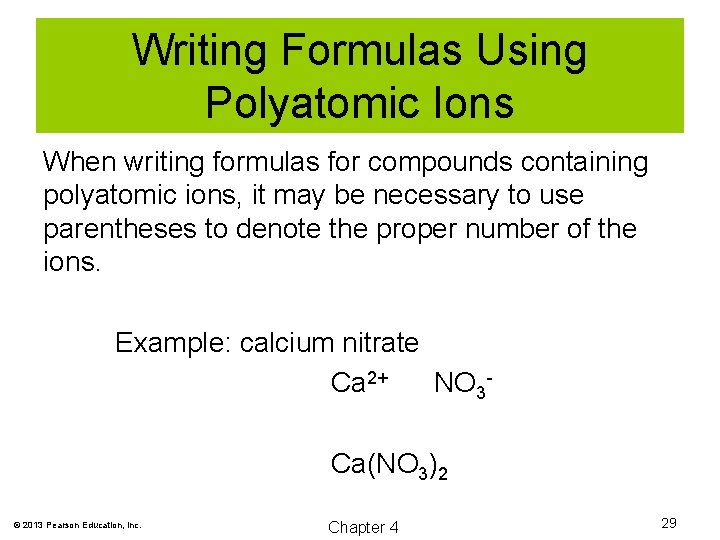
Writing Formulas Using Polyatomic Ions When writing formulas for compounds containing polyatomic ions, it may be necessary to use parentheses to denote the proper number of the ions. Example: calcium nitrate Ca 2+ NO 3 Ca(NO 3)2 © 2013 Pearson Education, Inc. Chapter 4 29

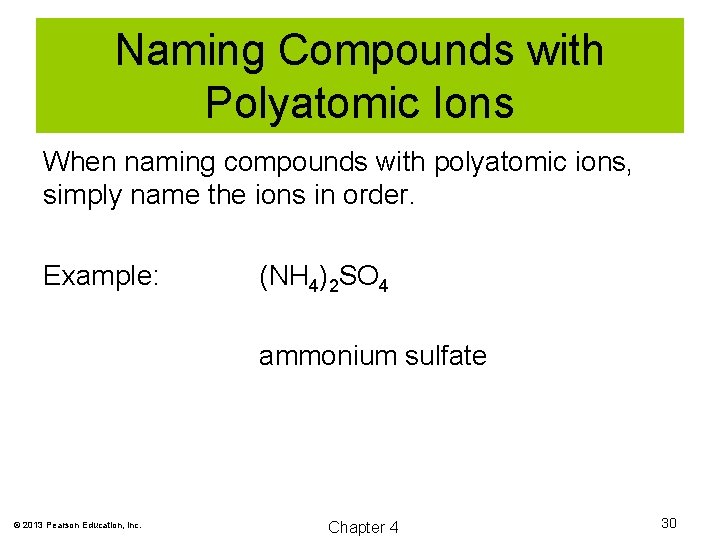
Naming Compounds with Polyatomic Ions When naming compounds with polyatomic ions, simply name the ions in order. Example: (NH 4)2 SO 4 ammonium sulfate © 2013 Pearson Education, Inc. Chapter 4 30

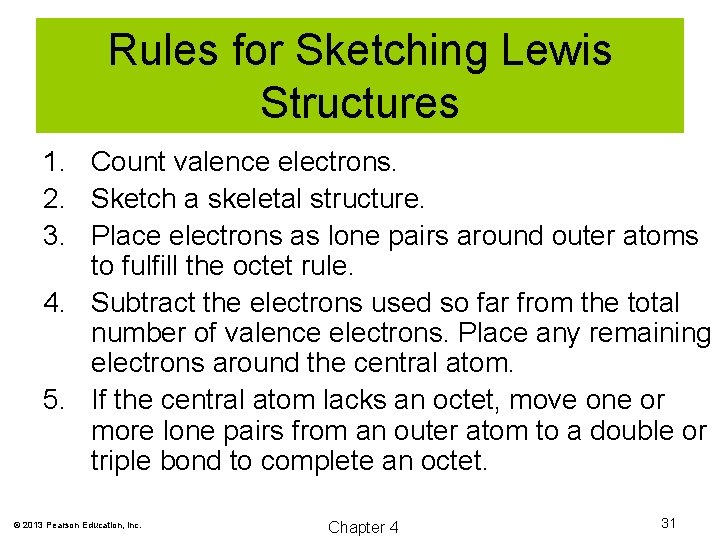
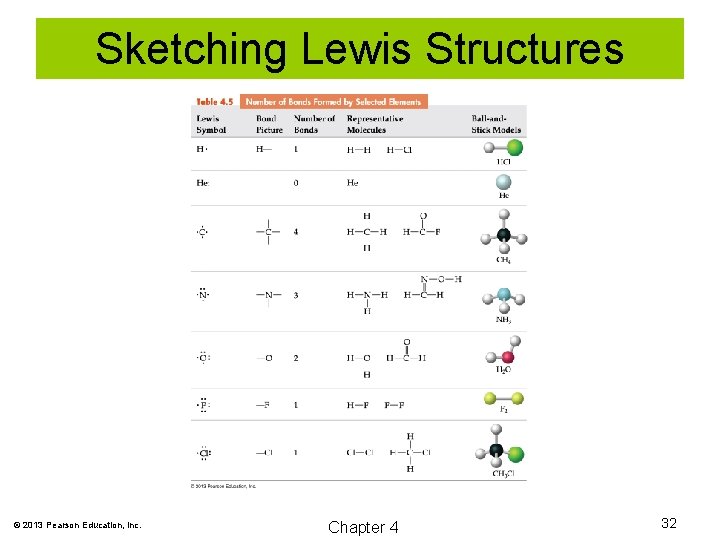
Rules for Sketching Lewis Structures 1. Count valence electrons. 2. Sketch a skeletal structure. 3. Place electrons as lone pairs around outer atoms to fulfill the octet rule. 4. Subtract the electrons used so far from the total number of valence electrons. Place any remaining electrons around the central atom. 5. If the central atom lacks an octet, move one or more lone pairs from an outer atom to a double or triple bond to complete an octet. © 2013 Pearson Education, Inc. Chapter 4 31

Sketching Lewis Structures © 2013 Pearson Education, Inc. Chapter 4 32

Odd Electron Molecules: Free Radicals An atom or molecule with an unpaired electron is known as a free radical. Examples include: NO © 2013 Pearson Education, Inc. NO 2 Cl. O 2 Chapter 4 33

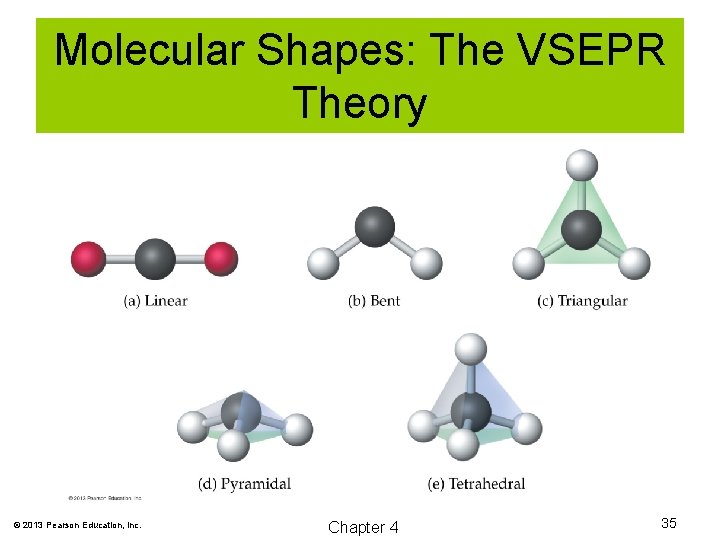
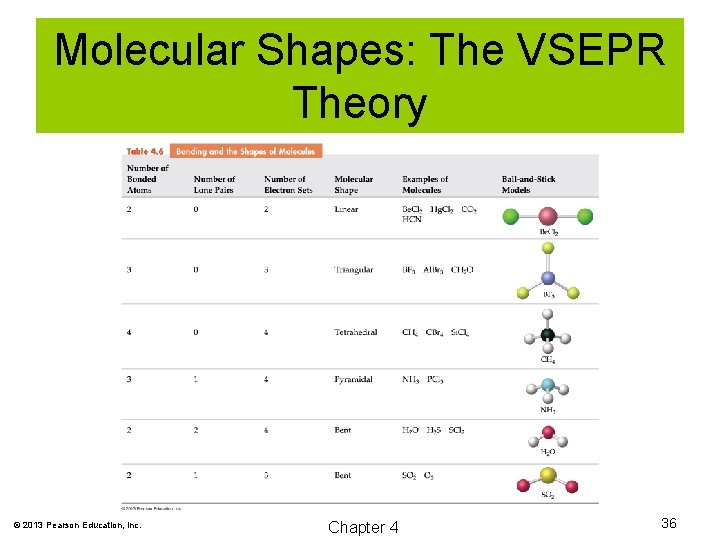
Molecular Shapes: The VSEPR Theory The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts the shape of molecules and polyatomic ions based on repulsions of electron pairs on central atoms. © 2013 Pearson Education, Inc. Chapter 4 34

Molecular Shapes: The VSEPR Theory © 2013 Pearson Education, Inc. Chapter 4 35

Molecular Shapes: The VSEPR Theory © 2013 Pearson Education, Inc. Chapter 4 36

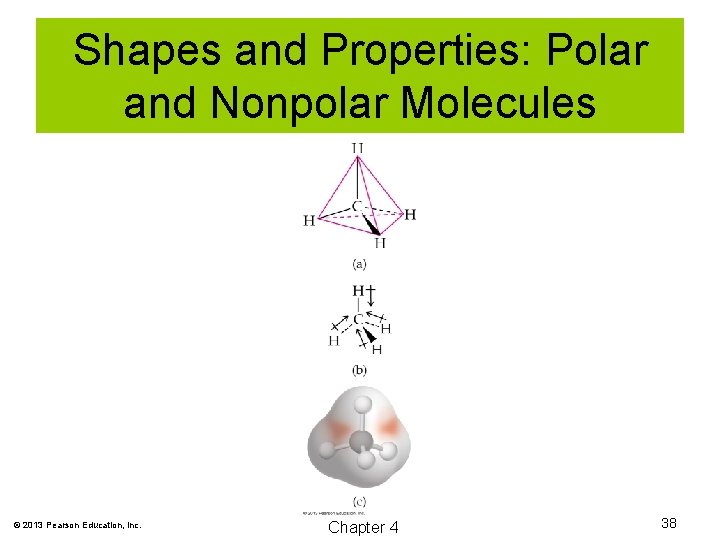
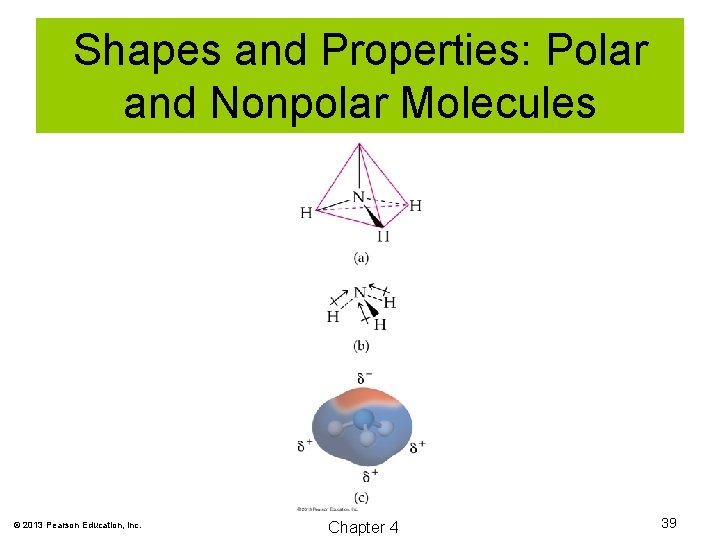
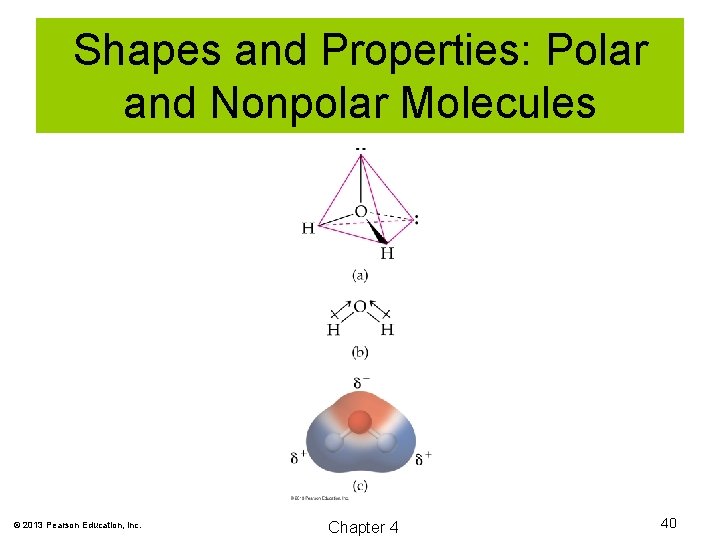
Shapes and Properties: Polar and Nonpolar Molecules In order for a molecule to be polar, two conditions must be met: 1. It must have polar bonds. 2. The bonds must be arranged such that a separation of charge exists. © 2013 Pearson Education, Inc. Chapter 4 37

Shapes and Properties: Polar and Nonpolar Molecules © 2013 Pearson Education, Inc. Chapter 4 38

Shapes and Properties: Polar and Nonpolar Molecules © 2013 Pearson Education, Inc. Chapter 4 39

Shapes and Properties: Polar and Nonpolar Molecules © 2013 Pearson Education, Inc. Chapter 4 40

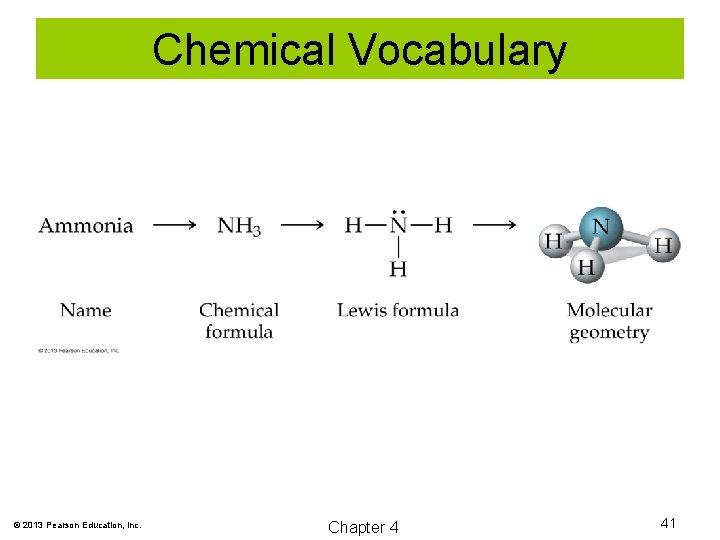
Chemical Vocabulary © 2013 Pearson Education, Inc. Chapter 4 41
 Chemistry for changing times
Chemistry for changing times Changing times essay
Changing times essay Activity 1 changing times
Activity 1 changing times The times they are a changing
The times they are a changing 15 times 15 times 20
15 times 15 times 20 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad What is outline
What is outline Tujuan garis besar adalah
Tujuan garis besar adalah Kairos talk outlines
Kairos talk outlines Commercial law outlines
Commercial law outlines Four main components for effective outlines
Four main components for effective outlines A business plan is a document that outlines
A business plan is a document that outlines Pathology outline
Pathology outline Sermon outlines on the shunammite woman
Sermon outlines on the shunammite woman Anime out line
Anime out line Two types of outlines
Two types of outlines A haunted house virginia woolf characters
A haunted house virginia woolf characters Ksf outlines
Ksf outlines Mucoepidermoid carcinoma histology
Mucoepidermoid carcinoma histology High level outline
High level outline Outline book of acts
Outline book of acts Exegetical sermon outlines
Exegetical sermon outlines Cjis level 4 certification
Cjis level 4 certification Model un position paper outlines
Model un position paper outlines Define:visible
Define:visible Catarrhal appendicitis
Catarrhal appendicitis Disaster management in libraries and information centres
Disaster management in libraries and information centres Using mis 10th edition
Using mis 10th edition Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Lightning elves
Lightning elves Rearranged most stable carbocation is
Rearranged most stable carbocation is Pericyclic
Pericyclic Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes Organic chemistry third edition david klein
Organic chemistry third edition david klein