Chemistry for Changing Times Thirteenth Edition Lecture Outlines














- Slides: 56

Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chapter 15 Energy John Singer, Jackson Community College © 2013 Pearson Education, Inc.

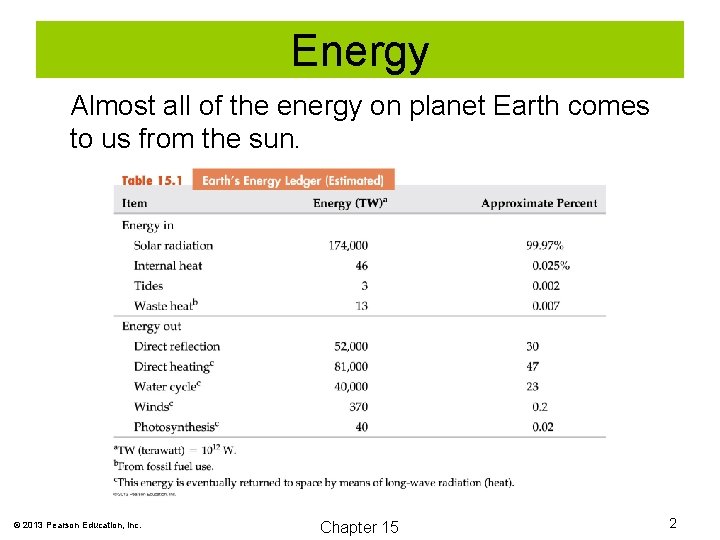
Energy Almost all of the energy on planet Earth comes to us from the sun. © 2013 Pearson Education, Inc. Chapter 15 2

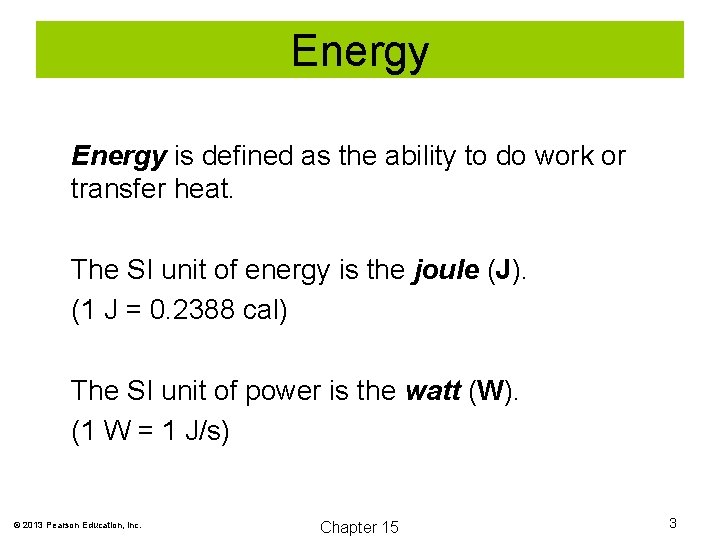
Energy is defined as the ability to do work or transfer heat. The SI unit of energy is the joule (J). (1 J = 0. 2388 cal) The SI unit of power is the watt (W). (1 W = 1 J/s) © 2013 Pearson Education, Inc. Chapter 15 3

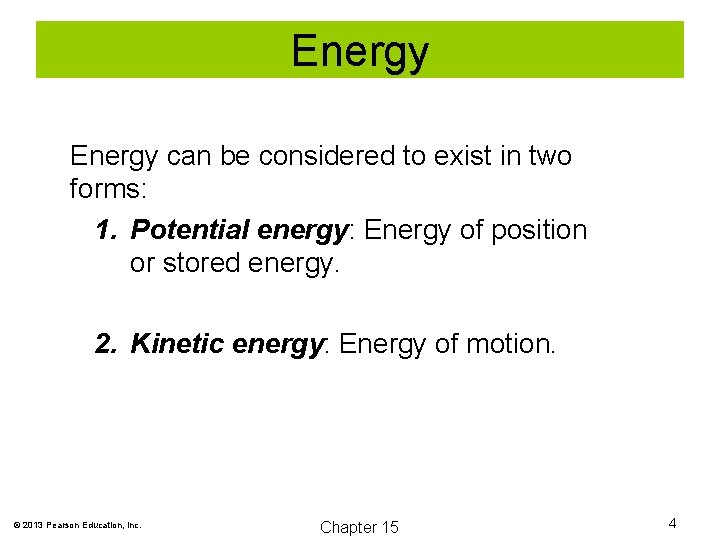
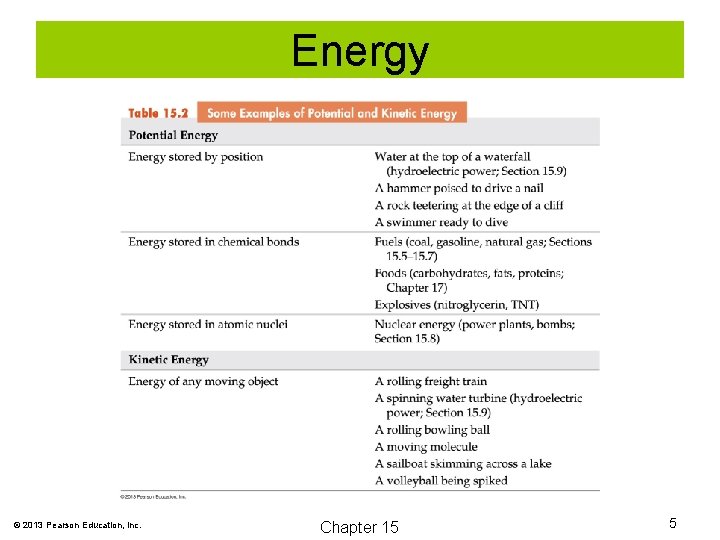
Energy can be considered to exist in two forms: 1. Potential energy: Energy of position or stored energy. 2. Kinetic energy: Energy of motion. © 2013 Pearson Education, Inc. Chapter 15 4

Energy © 2013 Pearson Education, Inc. Chapter 15 5

Energy and the Life Support System The biosphere is the thin film of air, water, and soil where life exists. Only a small amount of the energy entering the biosphere is used to support life. 30% of solar radiation is reflected back to space. 23% of solar radiation powers the water cycle. <0. 02% is used by green plants to power photosynthesis. Photosynthesis produces oxygen and stores energy for all animals on the planet. © 2013 Pearson Education, Inc. Chapter 15 6

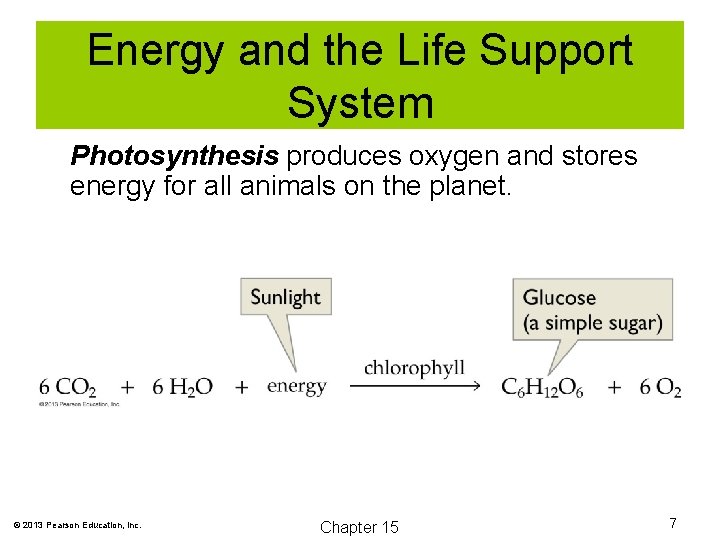
Energy and the Life Support System Photosynthesis produces oxygen and stores energy for all animals on the planet. © 2013 Pearson Education, Inc. Chapter 15 7

Energy and Chemical Reactions Factors that affect the rate of a chemical reaction include • Temperature: Increasing temperature increases reaction rates. • Concentration of reactants: Reaction rates are dependent on reactant concentration. As concentration increases, rate increases. • Presence of catalysts: Catalysts increase the rate of reactions by lowering activation energy. © 2013 Pearson Education, Inc. Chapter 15 8

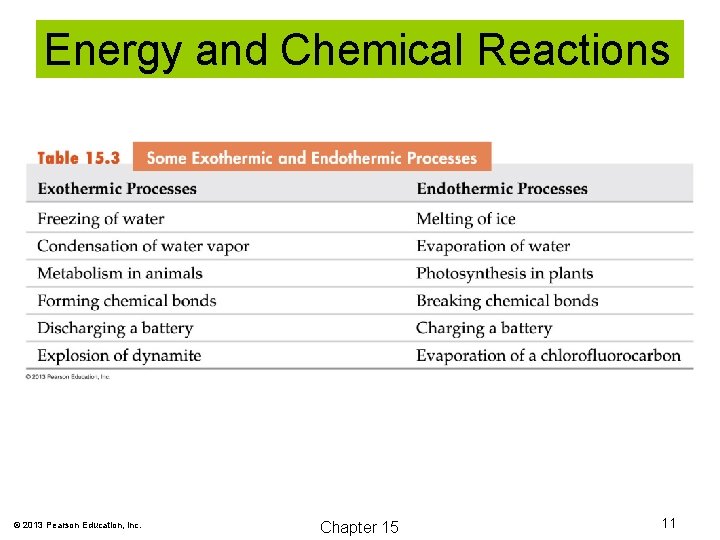
Energy and Chemical Reactions Exothermic reactions release heat energy to the surroundings. © 2013 Pearson Education, Inc. Chapter 15 9

Energy and Chemical Reactions Endothermic reactions absorb heat energy from the surroundings. © 2013 Pearson Education, Inc. Chapter 15 10

Energy and Chemical Reactions © 2013 Pearson Education, Inc. Chapter 15 11

The First Law of Thermodynamics Energy can neither be created nor destroyed. This is also known as the law of conservation of energy. © 2013 Pearson Education, Inc. Chapter 15 12

The Second Law of Thermodynamics Energy flows spontaneously from hot objects to cooler objects. © 2013 Pearson Education, Inc. Chapter 15 13

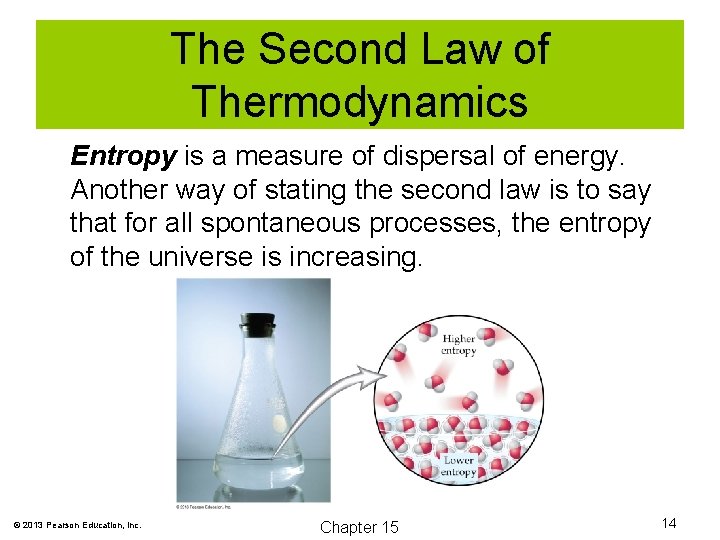
The Second Law of Thermodynamics Entropy is a measure of dispersal of energy. Another way of stating the second law is to say that for all spontaneous processes, the entropy of the universe is increasing. © 2013 Pearson Education, Inc. Chapter 15 14

People Power: Early Uses of Energy Early humans obtained energy by hunting animals and gathering plants. Plant materials were the first fuels. Even today, wood and dried dung are the principal fuels used by one-third of the people on Earth. © 2013 Pearson Education, Inc. Chapter 15 15

People Power: Early Uses of Energy Waterwheels and windmills convert the kinetic energy of moving water and wind into mechanical energy. © 2013 Pearson Education, Inc. Chapter 15 16

Fossil Fuels Fossil fuels, including coal, oil, and natural gas, provide more than 90% of the energy consumed in a modern society. Fuels are substances that, when burned, release significant amounts of energy. Fuels are a reduced form of matter. Combustion is an oxidation process that is exothermic. © 2013 Pearson Education, Inc. Chapter 15 17

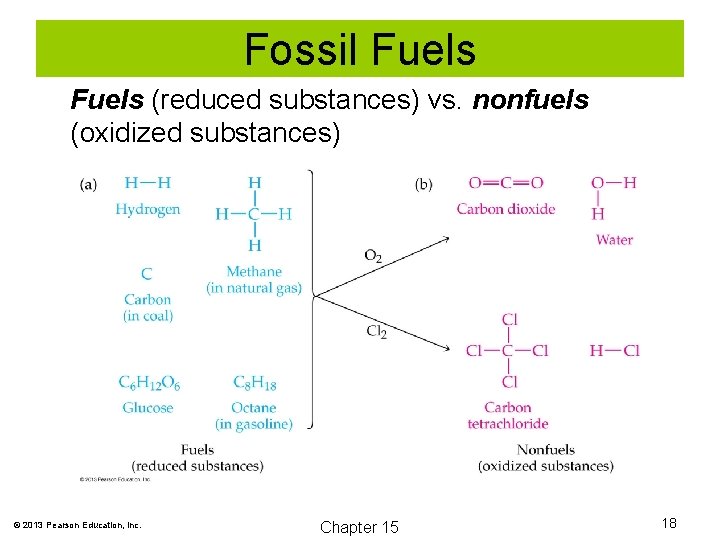
Fossil Fuels (reduced substances) vs. nonfuels (oxidized substances) © 2013 Pearson Education, Inc. Chapter 15 18

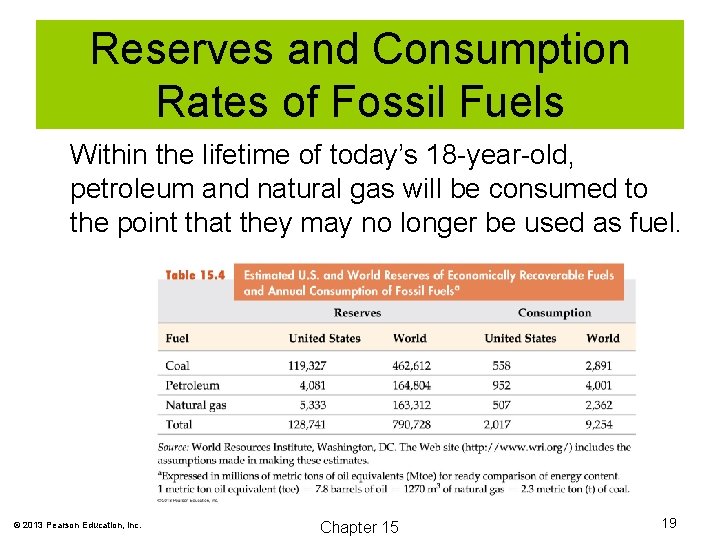
Reserves and Consumption Rates of Fossil Fuels Within the lifetime of today’s 18 -year-old, petroleum and natural gas will be consumed to the point that they may no longer be used as fuel. © 2013 Pearson Education, Inc. Chapter 15 19

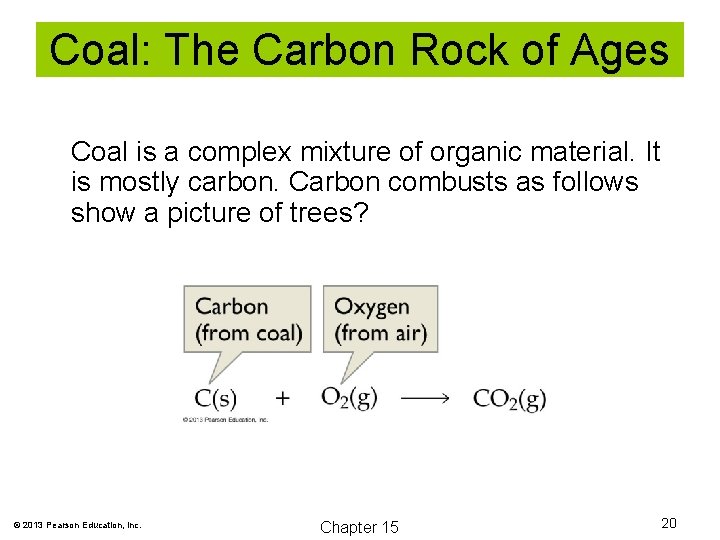
Coal: The Carbon Rock of Ages Coal is a complex mixture of organic material. It is mostly carbon. Carbon combusts as follows show a picture of trees? © 2013 Pearson Education, Inc. Chapter 15 20

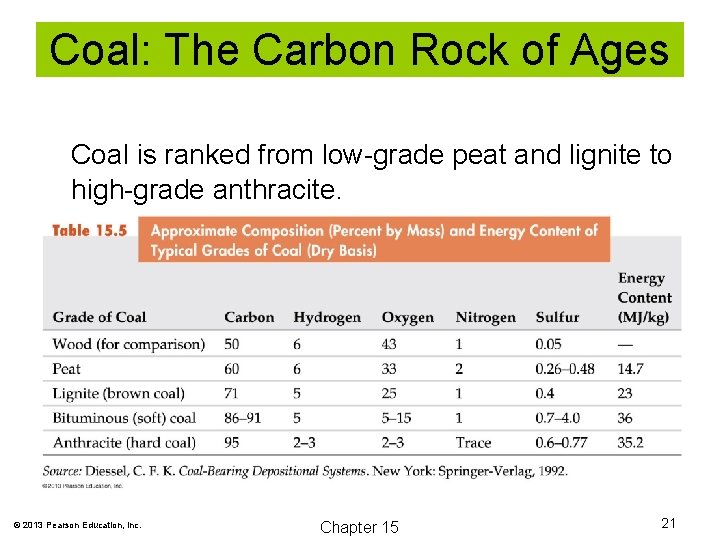
Coal: The Carbon Rock of Ages Coal is ranked from low-grade peat and lignite to high-grade anthracite. © 2013 Pearson Education, Inc. Chapter 15 21

Coal: The Carbon Rock of Ages Coal is abundant and by far the most plentiful fossil fuel. It is, however, hazardous to obtain and inconvenient to use due to its solid nature. © 2013 Pearson Education, Inc. Chapter 15 22

Coal: The Carbon Rock of Ages Much coal contains sulfur, which when combusted, produces sulfur dioxide (SO 2), which contributes to acid rain. © 2013 Pearson Education, Inc. Chapter 15 23

Coal: The Carbon Rock of Ages Coal is also a source of other chemical substances. When coal is heated in the absence of air, the volatile compounds are driven off, which leaves coke. Coke is used to produce iron and steel. The volatile materials can be condensed into coal tar, which can be used as a source of organic compounds for medical and industrial purposes. © 2013 Pearson Education, Inc. Chapter 15 24

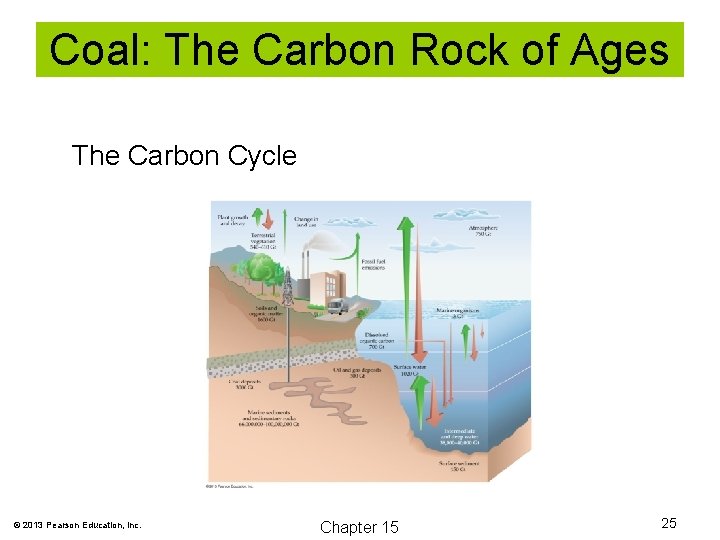
Coal: The Carbon Rock of Ages The Carbon Cycle © 2013 Pearson Education, Inc. Chapter 15 25

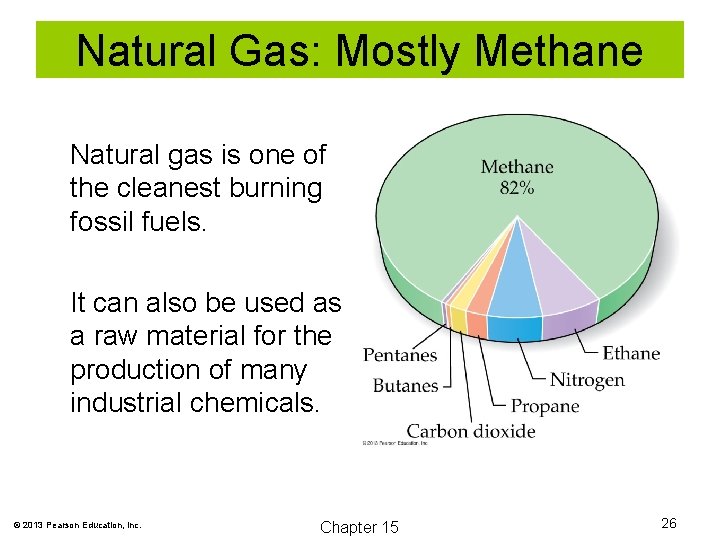
Natural Gas: Mostly Methane Natural gas is one of the cleanest burning fossil fuels. It can also be used as a raw material for the production of many industrial chemicals. © 2013 Pearson Education, Inc. Chapter 15 26

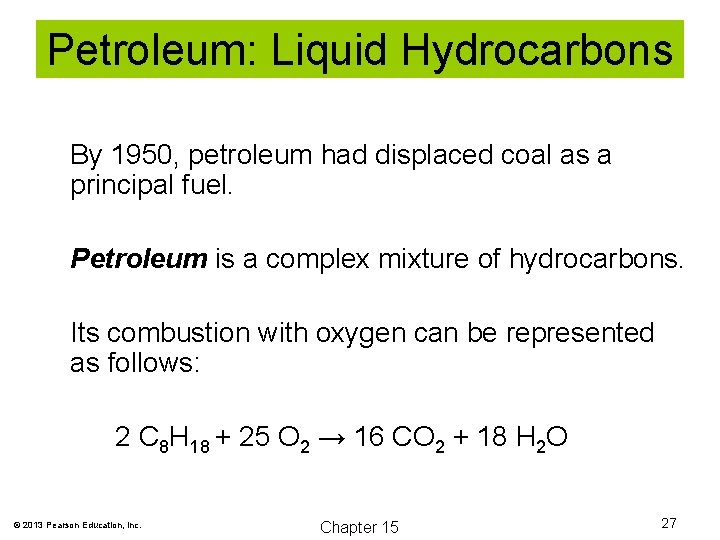
Petroleum: Liquid Hydrocarbons By 1950, petroleum had displaced coal as a principal fuel. Petroleum is a complex mixture of hydrocarbons. Its combustion with oxygen can be represented as follows: 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O © 2013 Pearson Education, Inc. Chapter 15 27

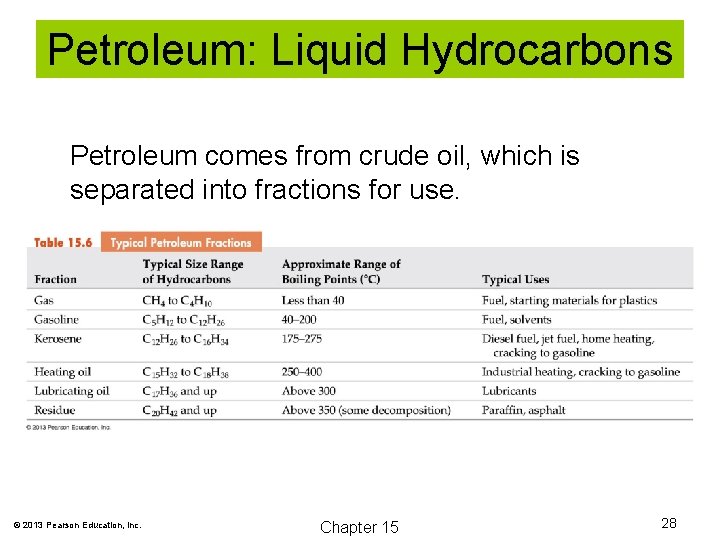
Petroleum: Liquid Hydrocarbons Petroleum comes from crude oil, which is separated into fractions for use. © 2013 Pearson Education, Inc. Chapter 15 28

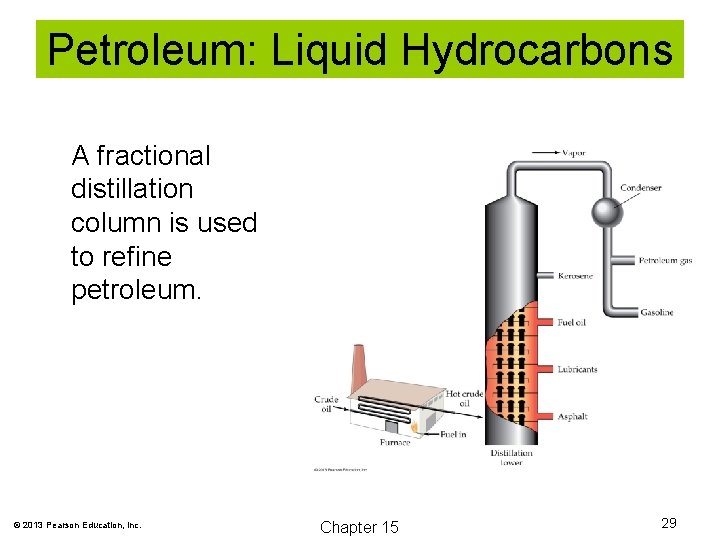
Petroleum: Liquid Hydrocarbons A fractional distillation column is used to refine petroleum. © 2013 Pearson Education, Inc. Chapter 15 29

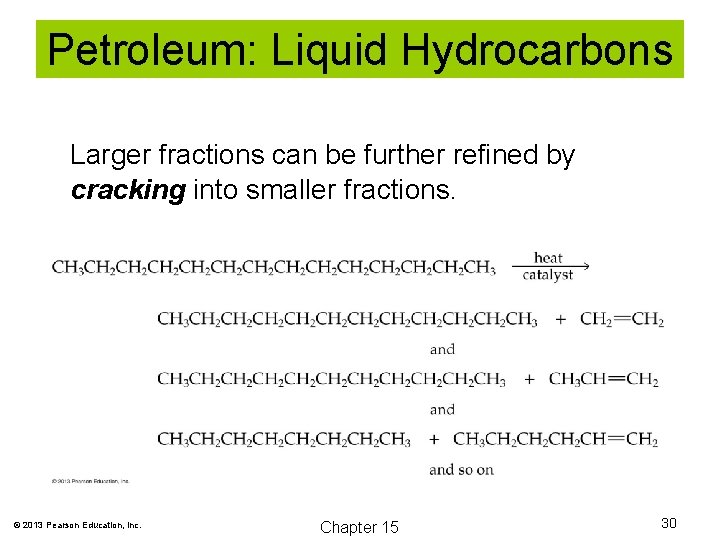
Petroleum: Liquid Hydrocarbons Larger fractions can be further refined by cracking into smaller fractions. © 2013 Pearson Education, Inc. Chapter 15 30

Petroleum: Liquid Hydrocarbons Gasoline is a lighter fraction from crude oil. It is actually a mixture of more than 150 different compounds. The gasoline fraction that comes from a distillation column is called straight-run gasoline and does not perform well in a modern, high-compression automobile engine. © 2013 Pearson Education, Inc. Chapter 15 31

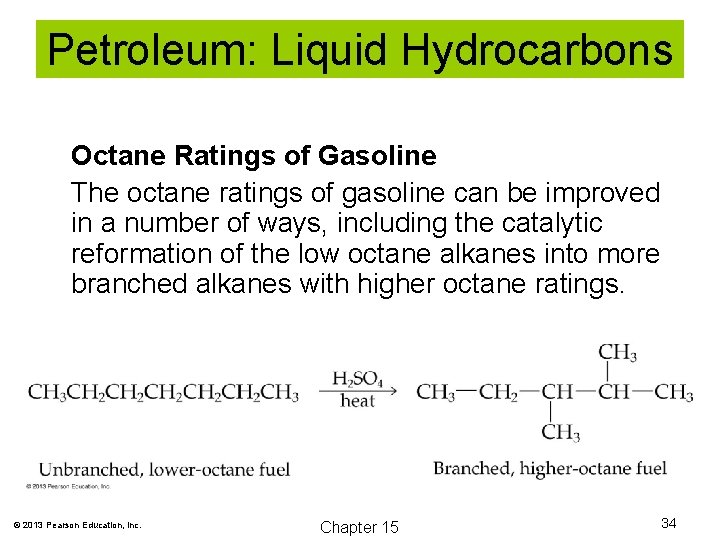
Petroleum: Liquid Hydrocarbons Octane Ratings of Gasoline The octane rating of gasoline is a measure of the fuel’s ability to resist knocking. Knocking can occurs when the fuel combusts before the spark plug fires. © 2013 Pearson Education, Inc. Chapter 15 32

Petroleum: Liquid Hydrocarbons Octane Ratings of Gasoline Isooctane is very resistant to knocking and is assigned an octane rating of 100. Heptane is given an octane rating of zero. Gasoline with an octane rating of 90 performs the same as a mixture of 90% isooctane and 10% heptane. © 2013 Pearson Education, Inc. Chapter 15 33

Petroleum: Liquid Hydrocarbons Octane Ratings of Gasoline The octane ratings of gasoline can be improved in a number of ways, including the catalytic reformation of the low octane alkanes into more branched alkanes with higher octane ratings. © 2013 Pearson Education, Inc. Chapter 15 34

Petroleum: Liquid Hydrocarbons Alternative Fuels Automobile engines can be made to run on nearly any liquid or gaseous fuel. Diesel fuels are mainly C 9 -C 12 hydrocarbons. Biodiesel is made from animal or vegetable fats and oils. Ethanol can be used. E-85 is a mixture of 85% ethanol and 15% gasoline. © 2013 Pearson Education, Inc. Chapter 15 35

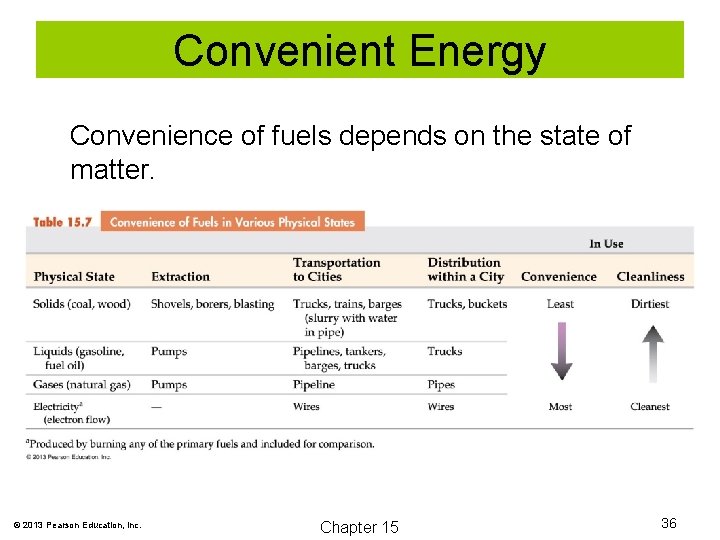
Convenient Energy Convenience of fuels depends on the state of matter. © 2013 Pearson Education, Inc. Chapter 15 36

Convenient Energy: Electricity Coal Gasification and Liquification Solid coal can be made more convenient by gasification and liquification. Electricity is perhaps the most convenient fuel of all. © 2013 Pearson Education, Inc. Chapter 15 37

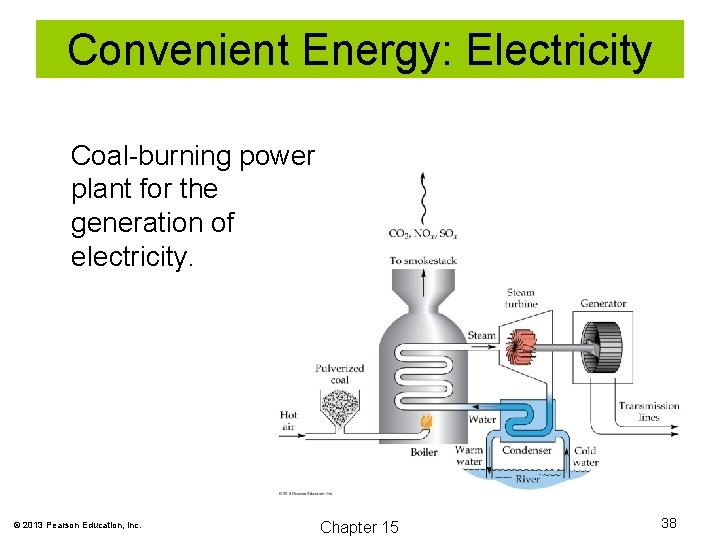
Convenient Energy: Electricity Coal-burning power plant for the generation of electricity. © 2013 Pearson Education, Inc. Chapter 15 38

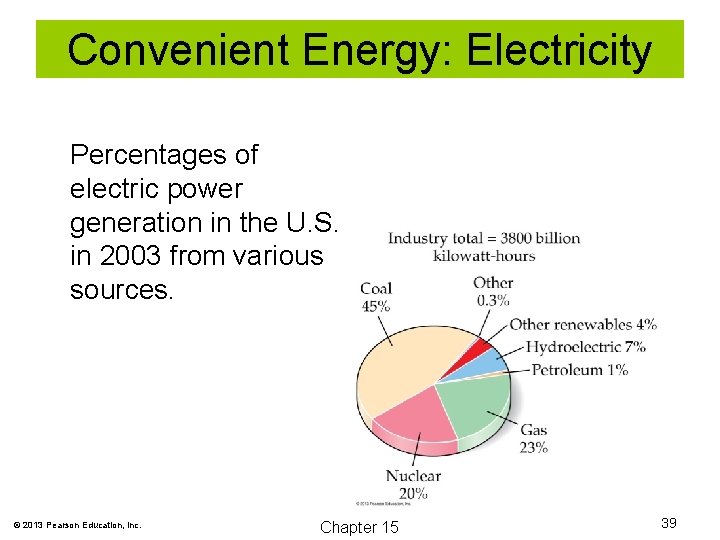
Convenient Energy: Electricity Percentages of electric power generation in the U. S. in 2003 from various sources. © 2013 Pearson Education, Inc. Chapter 15 39

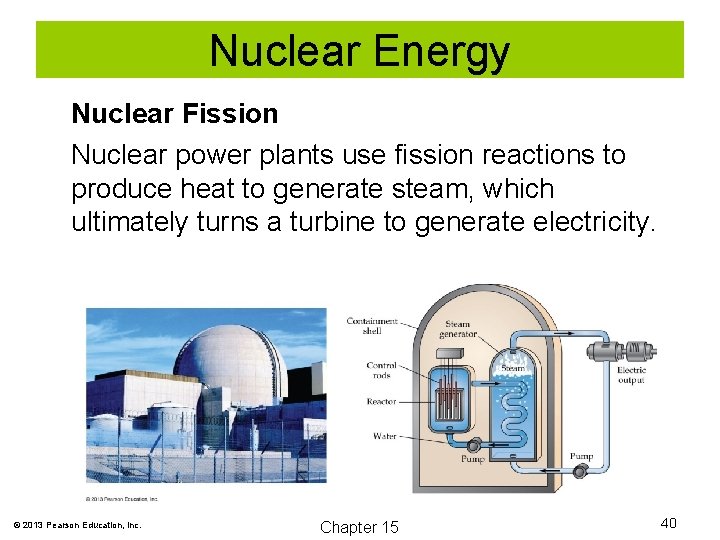
Nuclear Energy Nuclear Fission Nuclear power plants use fission reactions to produce heat to generate steam, which ultimately turns a turbine to generate electricity. © 2013 Pearson Education, Inc. Chapter 15 40

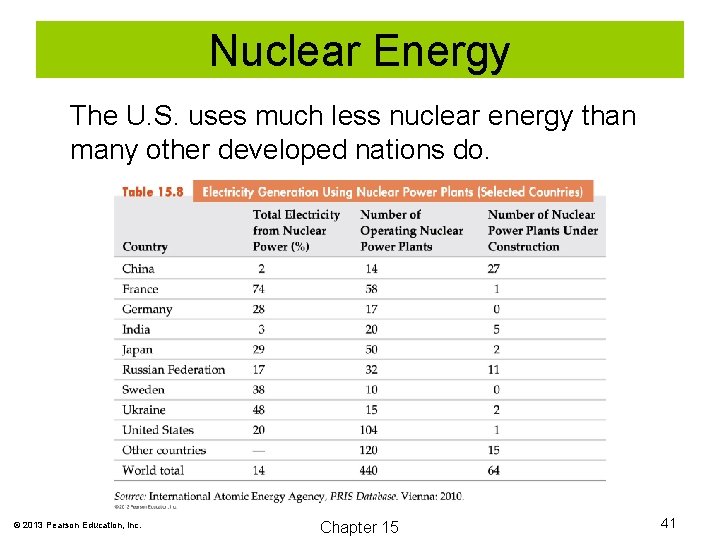
Nuclear Energy The U. S. uses much less nuclear energy than many other developed nations do. © 2013 Pearson Education, Inc. Chapter 15 41

Nuclear Energy Nuclear power plants produce minimal air pollution. However, many elaborate and expensive safety precautions must be employed. Also, fission products (nuclear waste) must be dealt with. © 2013 Pearson Education, Inc. Chapter 15 42

Nuclear Fusion: The Sun in a Magnetic Bottle Controlled fusion would present many advantages over fission reactors. For instance radioactive wastes would be minimized. However, technical difficulties must be overcome for fusion reactors to be a reality. © 2013 Pearson Education, Inc. Chapter 15 43

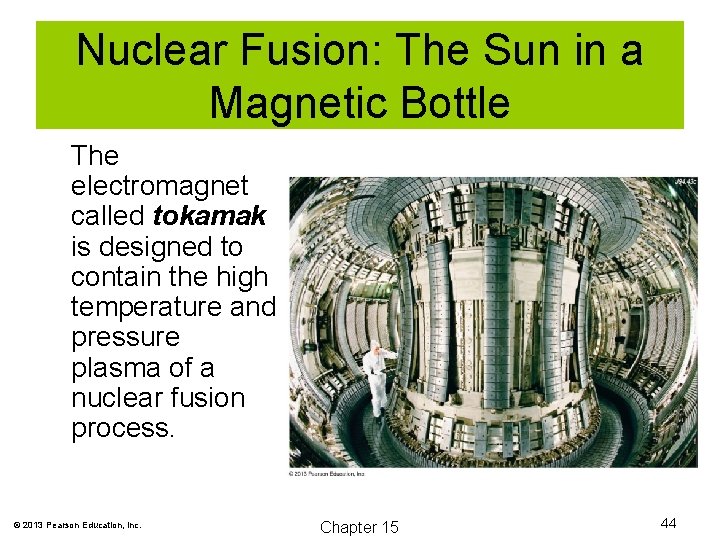
Nuclear Fusion: The Sun in a Magnetic Bottle The electromagnet called tokamak is designed to contain the high temperature and pressure plasma of a nuclear fusion process. © 2013 Pearson Education, Inc. Chapter 15 44

Renewable Energy Sources Solar Energy It has been noted that nearly all of the energy available on Earth comes from the sun. Energy from the sun is diffuse and must be concentrated to make it useful. © 2013 Pearson Education, Inc. Chapter 15 45

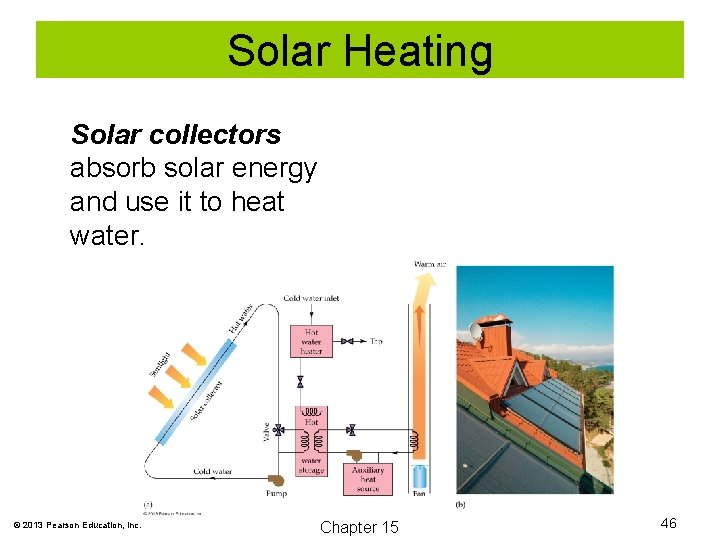
Solar Heating Solar collectors absorb solar energy and use it to heat water. © 2013 Pearson Education, Inc. Chapter 15 46

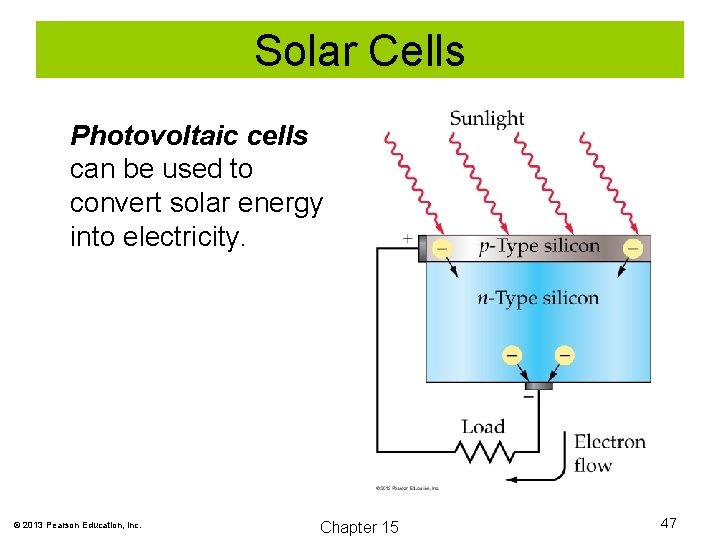
Solar Cells Photovoltaic cells can be used to convert solar energy into electricity. © 2013 Pearson Education, Inc. Chapter 15 47

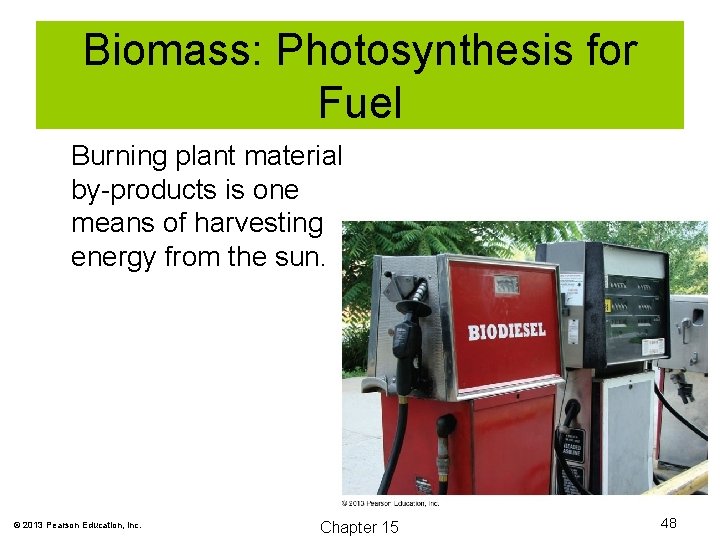
Biomass: Photosynthesis for Fuel Burning plant material by-products is one means of harvesting energy from the sun. © 2013 Pearson Education, Inc. Chapter 15 48

Biomass: Photosynthesis for Fuel Combustion of agricultural waste, fermentation of plants to ethanol or methane, and the fermentation of human and animal wastes to methane have all been used—and are still being used—as sources of energy. © 2013 Pearson Education, Inc. Chapter 15 49

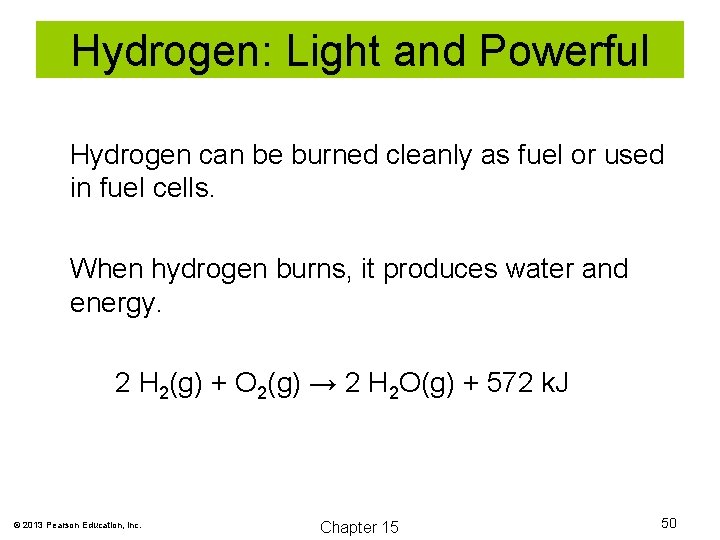
Hydrogen: Light and Powerful Hydrogen can be burned cleanly as fuel or used in fuel cells. When hydrogen burns, it produces water and energy. 2 H 2(g) + O 2(g) → 2 H 2 O(g) + 572 k. J © 2013 Pearson Education, Inc. Chapter 15 50

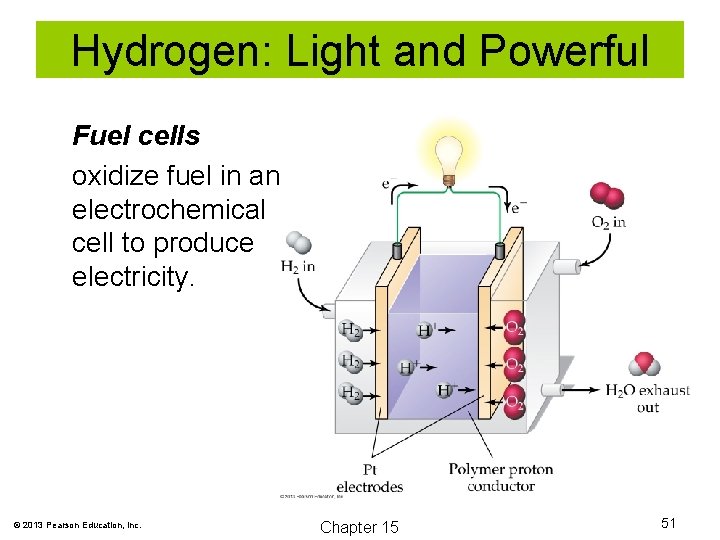
Hydrogen: Light and Powerful Fuel cells oxidize fuel in an electrochemical cell to produce electricity. © 2013 Pearson Education, Inc. Chapter 15 51

Other Renewable Energy Sources Wind and Water Both wind (windmills) and water (dams and hydroelectric plants) have been used to turn turbines and produce electricity. © 2013 Pearson Education, Inc. Chapter 15 52

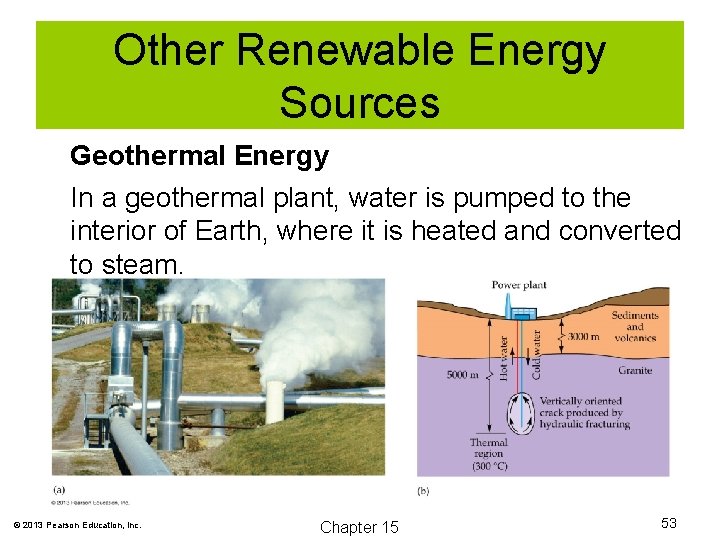
Other Renewable Energy Sources Geothermal Energy In a geothermal plant, water is pumped to the interior of Earth, where it is heated and converted to steam. © 2013 Pearson Education, Inc. Chapter 15 53

Other Renewable Energy Sources Oceans of Energy Ocean thermal energy has been shown to be workable since 1932. Also, tides and wave action can be converted to useful energy with appropriate technology. © 2013 Pearson Education, Inc. Chapter 15 54

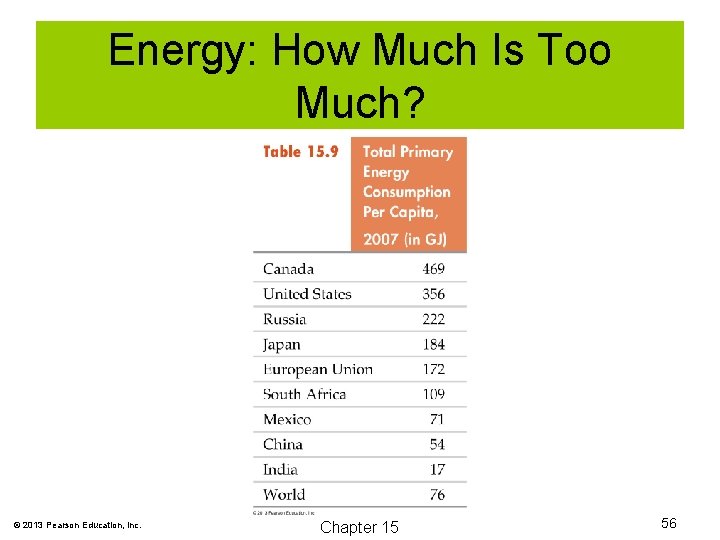
Energy: How Much Is Too Much? The demand for energy is ever increasing and our useful sources are dwindling. These competing concerns will cause many changes in our future. © 2013 Pearson Education, Inc. Chapter 15 55

Energy: How Much Is Too Much? © 2013 Pearson Education, Inc. Chapter 15 56
 Chemistry for changing times
Chemistry for changing times Changing times essay
Changing times essay Activity 1 changing times
Activity 1 changing times The times they are a changing
The times they are a changing Mat0022
Mat0022 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad What is an outlining
What is an outlining Teaching outline
Teaching outline Prison ministry training manual download
Prison ministry training manual download Commercial law outlines
Commercial law outlines Four main components for effective outlines
Four main components for effective outlines A business plan is a document that outlines
A business plan is a document that outlines Pathology outline
Pathology outline 2 kings 4 8 17
2 kings 4 8 17 Anime outlines
Anime outlines Two types of outlines
Two types of outlines A haunted house virginia woolf analysis
A haunted house virginia woolf analysis Ksf outlines
Ksf outlines Mucoepidermoid carcinoma histology
Mucoepidermoid carcinoma histology High level outline
High level outline Acts 9 outline
Acts 9 outline Exegetical outline
Exegetical outline Cjis security & awareness certification
Cjis security & awareness certification Model un position paper outlines
Model un position paper outlines Define visible
Define visible Crohn’s disease
Crohn’s disease A clear concise document which outlines preventive
A clear concise document which outlines preventive Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Transition state energy diagram
Transition state energy diagram Pericyclic
Pericyclic Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Organic chemistry third edition david klein
Organic chemistry third edition david klein David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes Organic chemistry third edition david klein
Organic chemistry third edition david klein General chemistry
General chemistry Lesson 81 drop in molecular views answer key
Lesson 81 drop in molecular views answer key Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Halohydrin formation
Halohydrin formation Organic chemistry
Organic chemistry Klein
Klein Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Formuö
Formuö Typiska drag för en novell
Typiska drag för en novell Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Ekologiskt fotavtryck
Ekologiskt fotavtryck Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering