Chemistry for Changing Times Thirteenth Edition Lecture Outlines




















- Slides: 57

Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chapter 13 Air John Singer, Jackson Community College © 2013 Pearson Education, Inc.

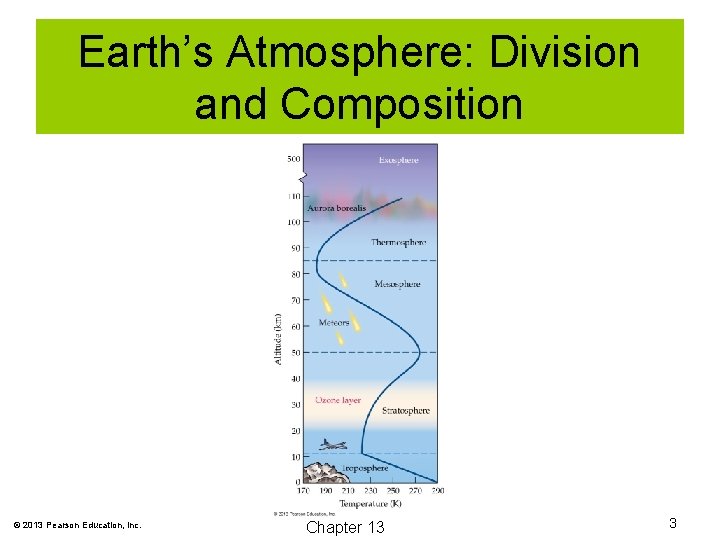
Earth’s Atmosphere: Division and Composition The Earth’s atmosphere is divided into four regions: 1. Troposphere: This layer is nearest Earth and contains nearly all living things. The temperature decreases as altitude increases in the troposphere. 2. Stratosphere: This layer lies above the troposphere and contains the protective ozone layer. In this layer, temperature increases with increasing altitude. 3. Mesosphere: This layer lies above the stratosphere. 4. Thermosphere: This layer lies above the mesosphere. © 2013 Pearson Education, Inc. Chapter 13 2

Earth’s Atmosphere: Division and Composition © 2013 Pearson Education, Inc. Chapter 13 3

Earth’s Atmosphere: Division and Composition © 2013 Pearson Education, Inc. Chapter 13 4

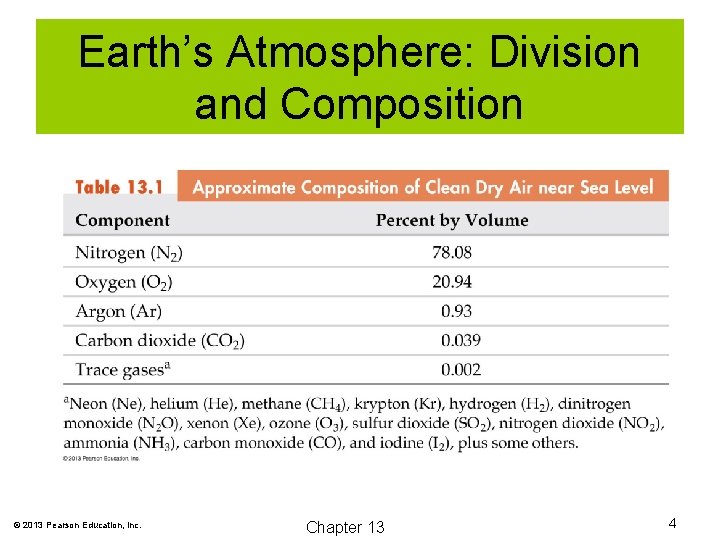
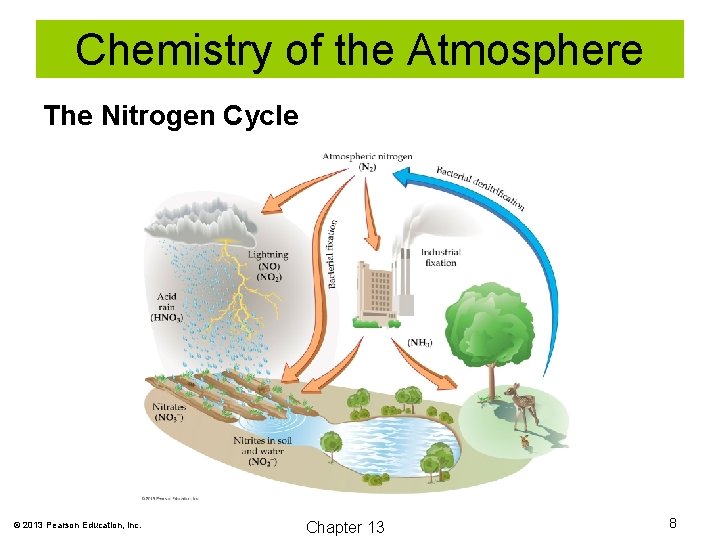
Chemistry of the Atmosphere Nitrogen accounts for about 78% of all gases in the atmosphere. All animals and most plants cannot use the nitrogen available in the atmosphere as N 2 molecules. Organisms must first “fix” or use “fixed” nitrogen. Fixed means that the nitrogen atoms are combined with another element. © 2013 Pearson Education, Inc. Chapter 13 5

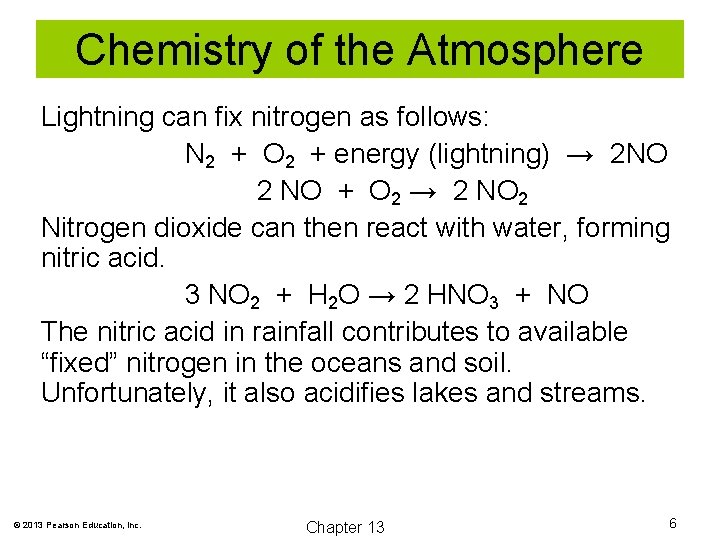
Chemistry of the Atmosphere Lightning can fix nitrogen as follows: N 2 + O 2 + energy (lightning) → 2 NO 2 NO + O 2 → 2 NO 2 Nitrogen dioxide can then react with water, forming nitric acid. 3 NO 2 + H 2 O → 2 HNO 3 + NO The nitric acid in rainfall contributes to available “fixed” nitrogen in the oceans and soil. Unfortunately, it also acidifies lakes and streams. © 2013 Pearson Education, Inc. Chapter 13 6

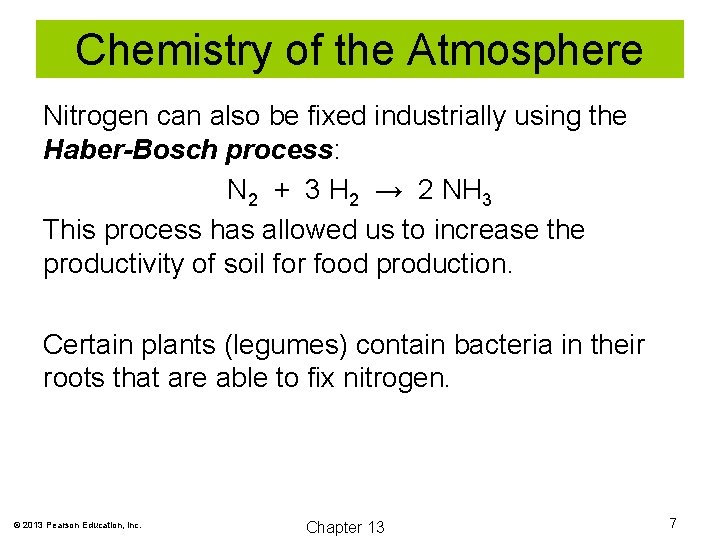
Chemistry of the Atmosphere Nitrogen can also be fixed industrially using the Haber-Bosch process: N 2 + 3 H 2 → 2 NH 3 This process has allowed us to increase the productivity of soil for food production. Certain plants (legumes) contain bacteria in their roots that are able to fix nitrogen. © 2013 Pearson Education, Inc. Chapter 13 7

Chemistry of the Atmosphere The Nitrogen Cycle © 2013 Pearson Education, Inc. Chapter 13 8

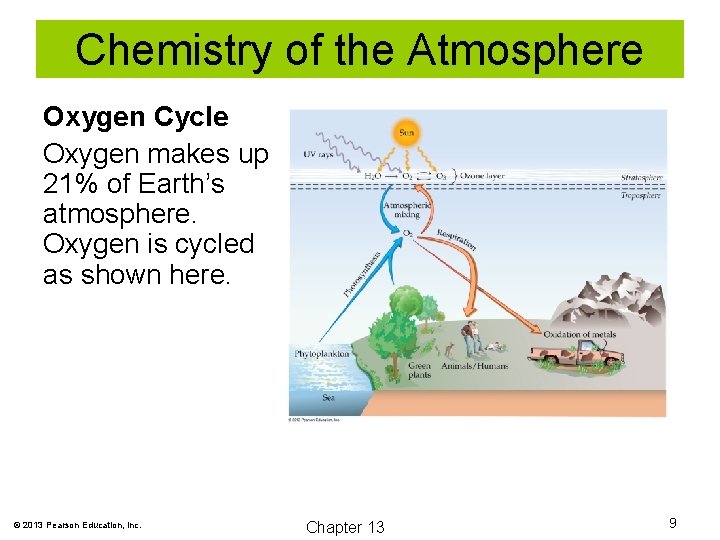
Chemistry of the Atmosphere Oxygen Cycle Oxygen makes up 21% of Earth’s atmosphere. Oxygen is cycled as shown here. © 2013 Pearson Education, Inc. Chapter 13 9

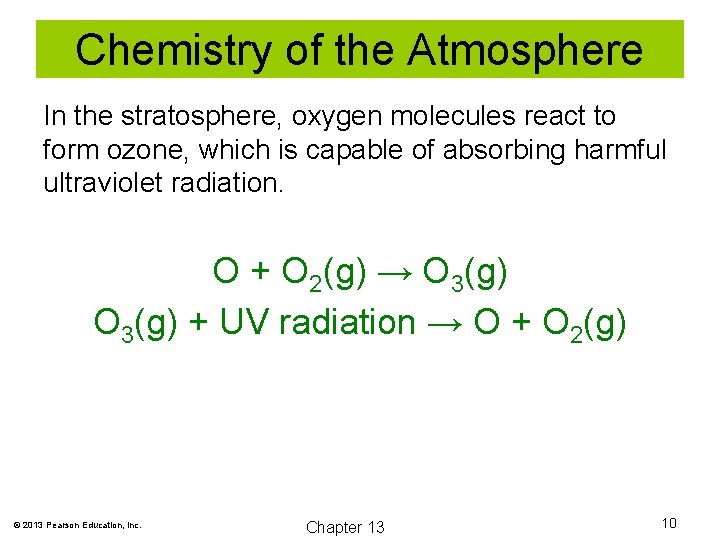
Chemistry of the Atmosphere In the stratosphere, oxygen molecules react to form ozone, which is capable of absorbing harmful ultraviolet radiation. O + O 2(g) → O 3(g) + UV radiation → O + O 2(g) © 2013 Pearson Education, Inc. Chapter 13 10

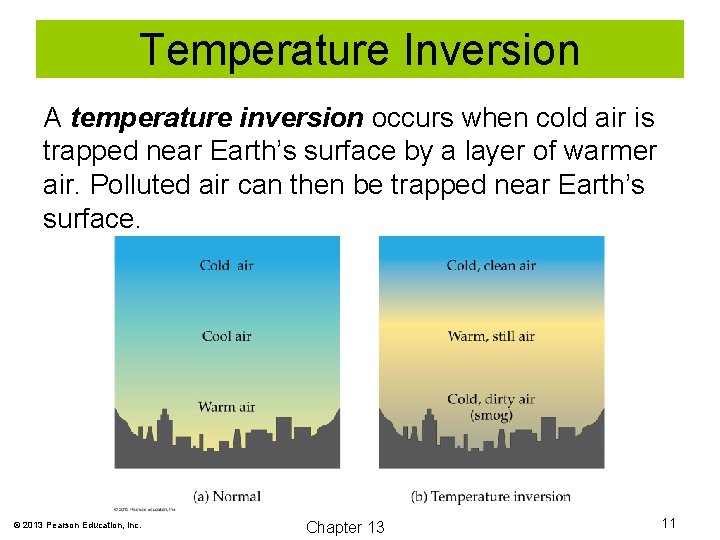
Temperature Inversion A temperature inversion occurs when cold air is trapped near Earth’s surface by a layer of warmer air. Polluted air can then be trapped near Earth’s surface. © 2013 Pearson Education, Inc. Chapter 13 11

Pollution Through the Ages Wildfires, windblown dust, and volcanic action can all contribute to air pollution. © 2013 Pearson Education, Inc. Chapter 13 12

A Closed Ecosystem? Earth is a closed ecosystem. What you see is what you get. © 2013 Pearson Education, Inc. Chapter 13 13

The Air Our Ancestors Breathed Air pollution has always been with us. Humans have always altered their environment. Clearing of land use of fire have always impacted the atmosphere. © 2013 Pearson Education, Inc. Chapter 13 14

Pollution Goes Global A pollutant is any substance in the wrong place at the wrong time. With increased urbanization and globalization, air pollution has become a global concern. © 2013 Pearson Education, Inc. Chapter 13 15

Industrial Smog The term smog is a contraction of smoke and fog. Air that has been polluted by industrial activity is called industrial smog. © 2013 Pearson Education, Inc. Chapter 13 16

Air Pollution in China As China industrializes its economy, its people are experiencing tremendous air pollution. © 2013 Pearson Education, Inc. Chapter 13 17

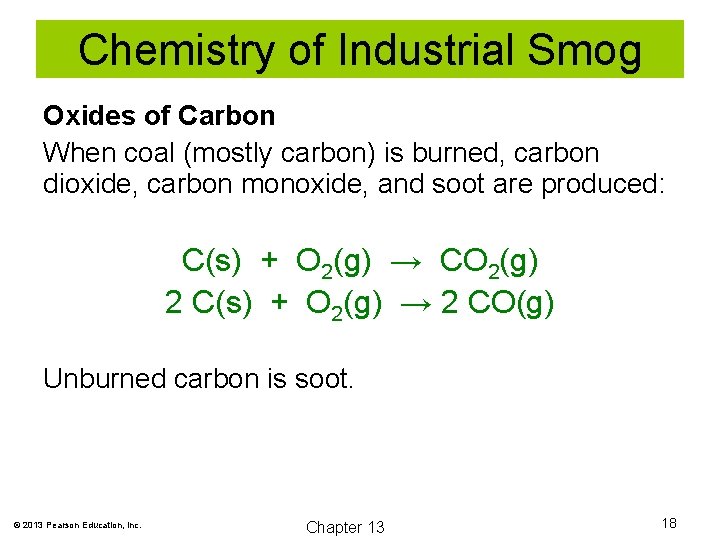
Chemistry of Industrial Smog Oxides of Carbon When coal (mostly carbon) is burned, carbon dioxide, carbon monoxide, and soot are produced: C(s) + O 2(g) → CO 2(g) 2 C(s) + O 2(g) → 2 CO(g) Unburned carbon is soot. © 2013 Pearson Education, Inc. Chapter 13 18

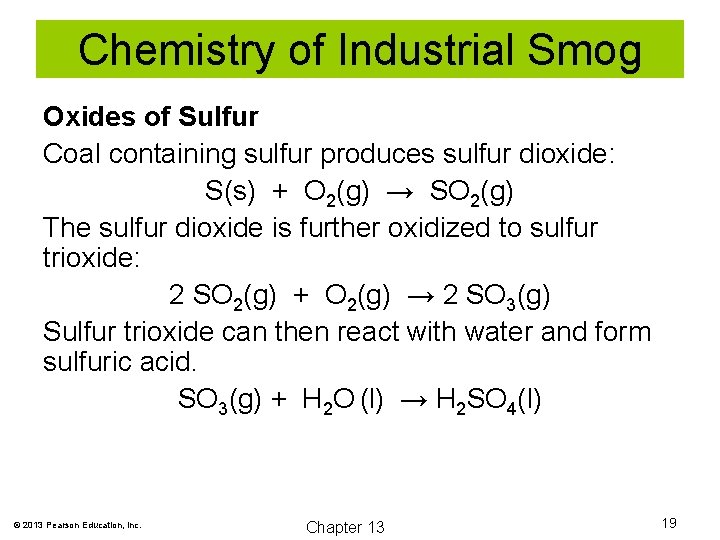
Chemistry of Industrial Smog Oxides of Sulfur Coal containing sulfur produces sulfur dioxide: S(s) + O 2(g) → SO 2(g) The sulfur dioxide is further oxidized to sulfur trioxide: 2 SO 2(g) + O 2(g) → 2 SO 3(g) Sulfur trioxide can then react with water and form sulfuric acid. SO 3(g) + H 2 O (l) → H 2 SO 4(l) © 2013 Pearson Education, Inc. Chapter 13 19

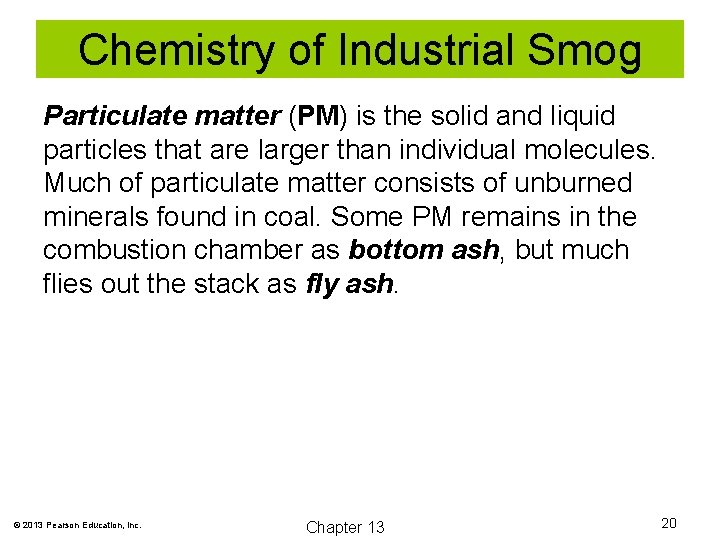
Chemistry of Industrial Smog Particulate matter (PM) is the solid and liquid particles that are larger than individual molecules. Much of particulate matter consists of unburned minerals found in coal. Some PM remains in the combustion chamber as bottom ash, but much flies out the stack as fly ash. © 2013 Pearson Education, Inc. Chapter 13 20

Chemistry of Industrial Smog The United States Environmental Protection Agency estimates that as many as 40, 000 premature deaths occur each year due to PM. © 2013 Pearson Education, Inc. Chapter 13 21

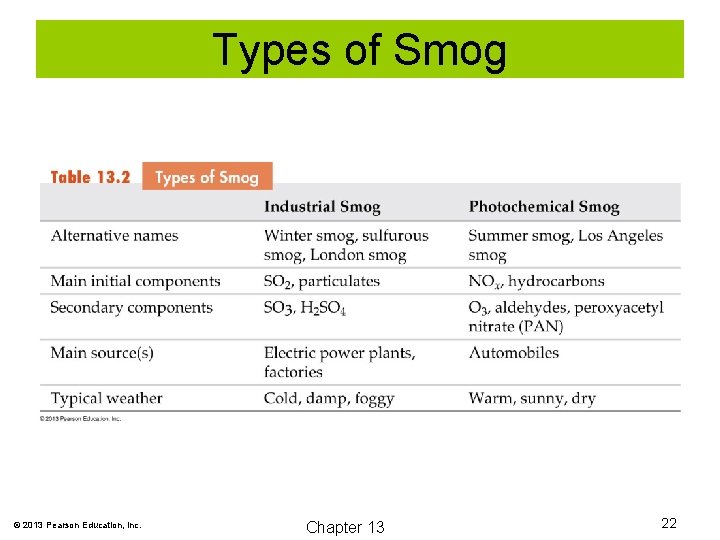
Types of Smog © 2013 Pearson Education, Inc. Chapter 13 22

Heath and Environmental Effects of Industrial Smog Health Sulfuric acid and smaller particulates act synergistically to harm health. The alveoli of the lungs lose resiliency and this lung damage can lead to pulmonary emphysema characterized by shortness of breath. Environmental Acidic precipitation and smaller particulates can damage plants, including farm crops. © 2013 Pearson Education, Inc. Chapter 13 23

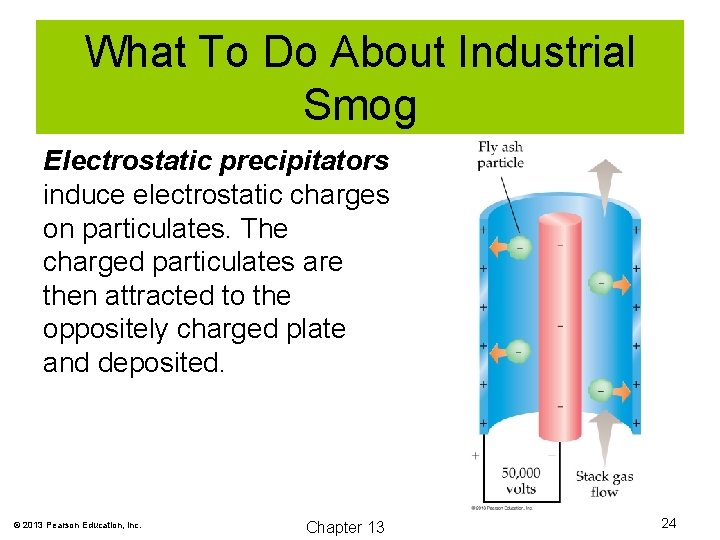
What To Do About Industrial Smog Electrostatic precipitators induce electrostatic charges on particulates. The charged particulates are then attracted to the oppositely charged plate and deposited. © 2013 Pearson Education, Inc. Chapter 13 24

What To Do About Industrial Smog Bag filtration works like a giant vacuum cleaner. Flue gases are passed through a series of filters in a bag house, which removes particulates. A cyclone separator works by cycling stack gases in a spiral motion. Heavier particulates hit the outer walls of the separator and deposit out of the gas stream. © 2013 Pearson Education, Inc. Chapter 13 25

What To Do About Industrial Smog Wet scrubbers remove particulates by passing the stack gases through water. Sulfur dioxide can be reduced by either removing sulfur from coal before combustion or by adding limestone (Ca. CO 3) to the coal. Ca. CO 3(s) + heat → Ca. O(s) + CO 2(g) Ca. O(s) + SO 2(g) → Ca. SO 3(s) © 2013 Pearson Education, Inc. Chapter 13 26

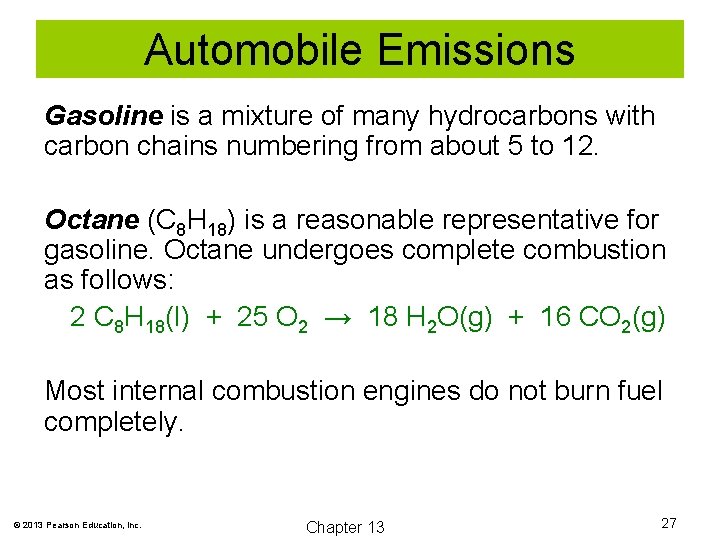
Automobile Emissions Gasoline is a mixture of many hydrocarbons with carbon chains numbering from about 5 to 12. Octane (C 8 H 18) is a reasonable representative for gasoline. Octane undergoes complete combustion as follows: 2 C 8 H 18(l) + 25 O 2 → 18 H 2 O(g) + 16 CO 2(g) Most internal combustion engines do not burn fuel completely. © 2013 Pearson Education, Inc. Chapter 13 27

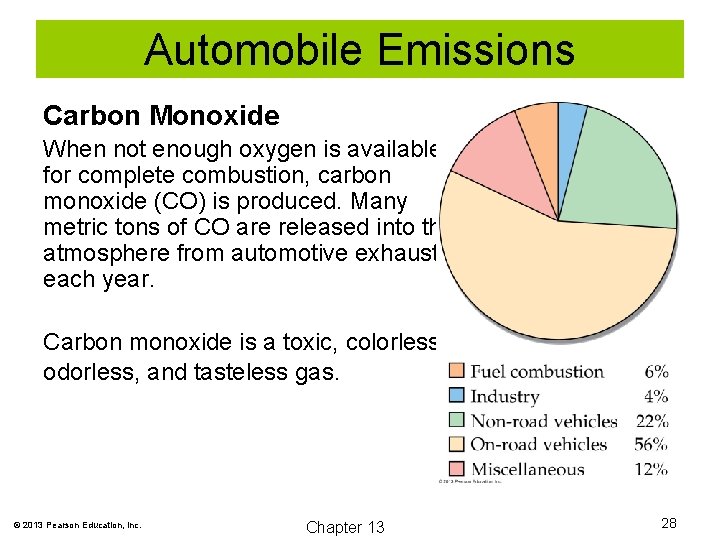
Automobile Emissions Carbon Monoxide When not enough oxygen is available for complete combustion, carbon monoxide (CO) is produced. Many metric tons of CO are released into the atmosphere from automotive exhaust each year. Carbon monoxide is a toxic, colorless, odorless, and tasteless gas. © 2013 Pearson Education, Inc. Chapter 13 28

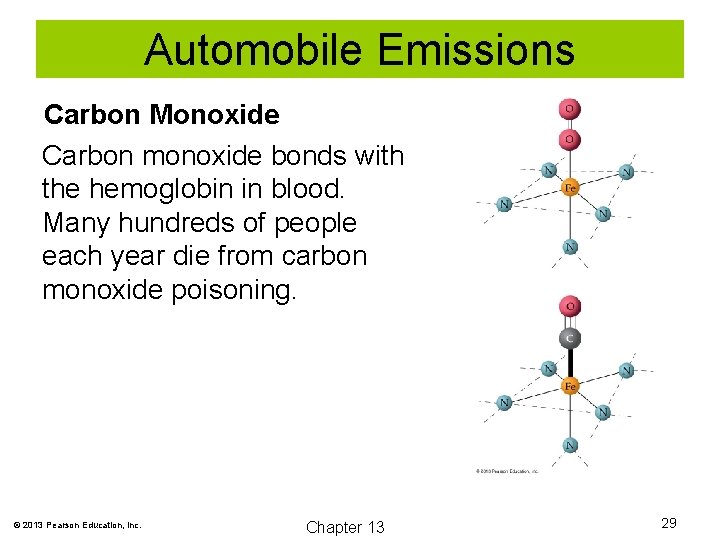
Automobile Emissions Carbon Monoxide Carbon monoxide bonds with the hemoglobin in blood. Many hundreds of people each year die from carbon monoxide poisoning. © 2013 Pearson Education, Inc. Chapter 13 29

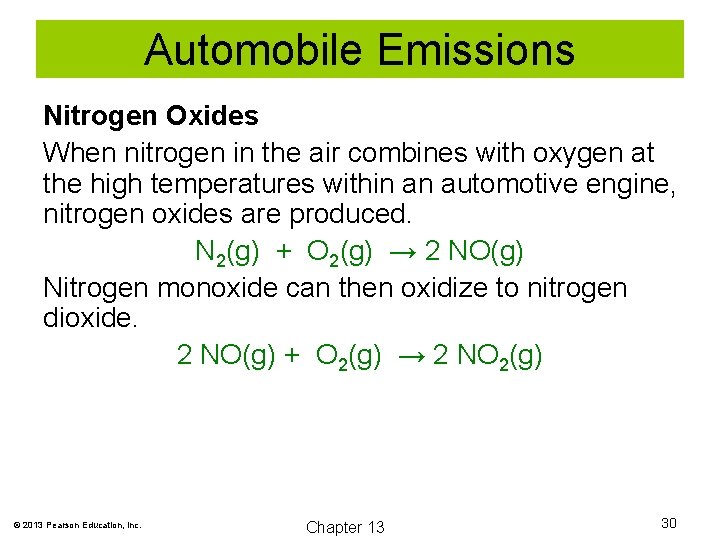
Automobile Emissions Nitrogen Oxides When nitrogen in the air combines with oxygen at the high temperatures within an automotive engine, nitrogen oxides are produced. N 2(g) + O 2(g) → 2 NO(g) Nitrogen monoxide can then oxidize to nitrogen dioxide. 2 NO(g) + O 2(g) → 2 NO 2(g) © 2013 Pearson Education, Inc. Chapter 13 30

Nitrogen Oxides Nitrogen oxides: Together, nitrogen monoxide and nitrogen dioxide are known as NOx. These oxides react with water in the atmosphere to form nitrous and nitric acids. They lead to smog formation and are components of acid rain. Breathing high concentrations of NOx can lead to lung damage with serious complications. © 2013 Pearson Education, Inc. Chapter 13 31

Automobile Emissions Volatile organic compounds (VOCs) are major contributors to smog formation. They are produced by the evaporation of gasoline, unburned fuel in exhaust, paints, and consumer products. Most VOCs are hydrocarbons. Some are released from natural sources. Alkenes in VOCS can react with oxygen or ozone to form aldehydes. © 2013 Pearson Education, Inc. Chapter 13 32

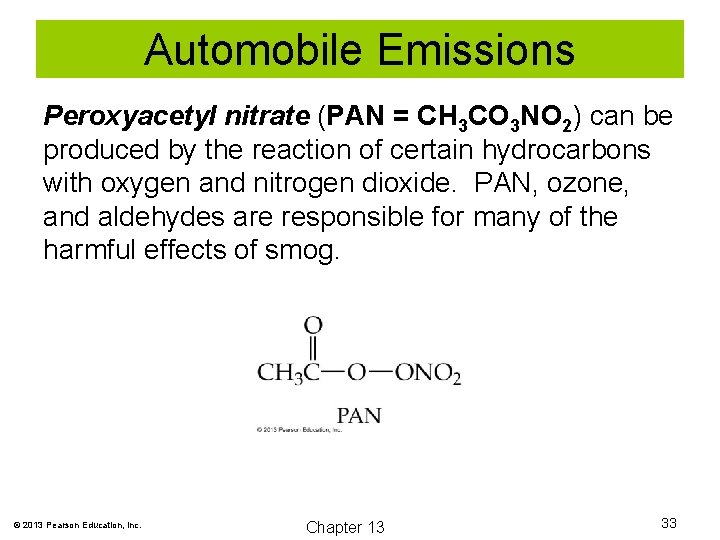
Automobile Emissions Peroxyacetyl nitrate (PAN = CH 3 CO 3 NO 2) can be produced by the reaction of certain hydrocarbons with oxygen and nitrogen dioxide. PAN, ozone, and aldehydes are responsible for many of the harmful effects of smog. © 2013 Pearson Education, Inc. Chapter 13 33

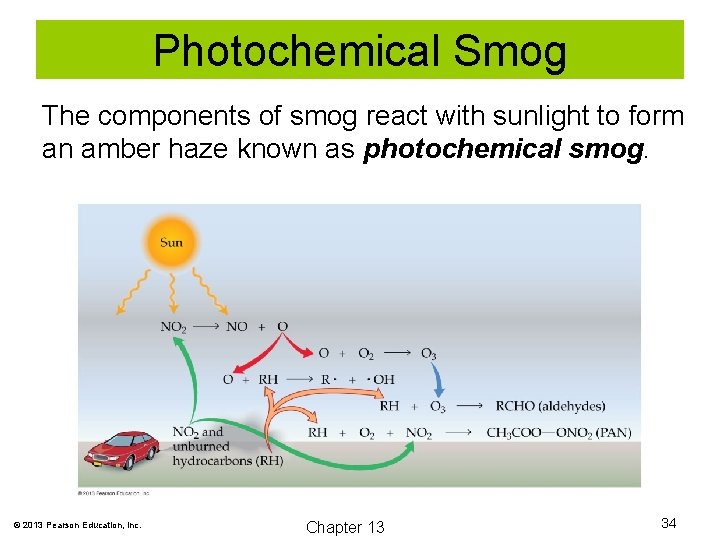
Photochemical Smog The components of smog react with sunlight to form an amber haze known as photochemical smog. © 2013 Pearson Education, Inc. Chapter 13 34

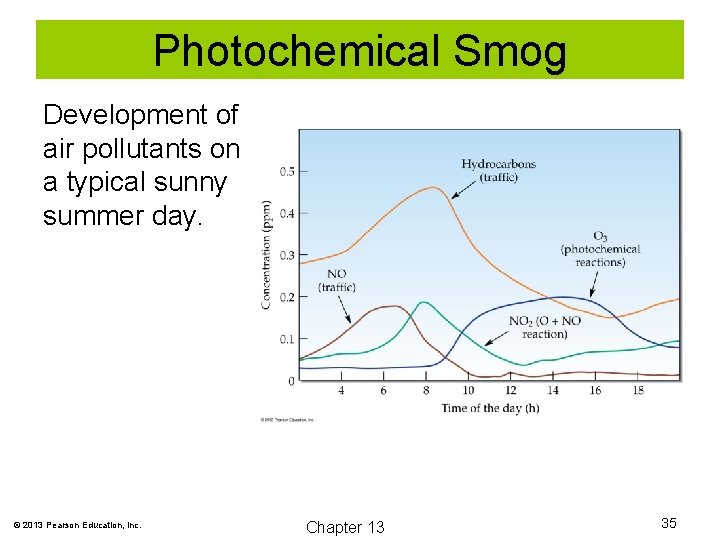
Photochemical Smog Development of air pollutants on a typical sunny summer day. © 2013 Pearson Education, Inc. Chapter 13 35

Solutions to Photochemical Smog To reduce photochemical smog, the quantities of pollutants entering the atmosphere must be reduced. Improved design of gasoline storage and dispensing systems reduces the emissions of hydrocarbon VOCs. Catalytic converters reduce hydrocarbon and carbon monoxide emissions from automobiles. © 2013 Pearson Education, Inc. Chapter 13 36

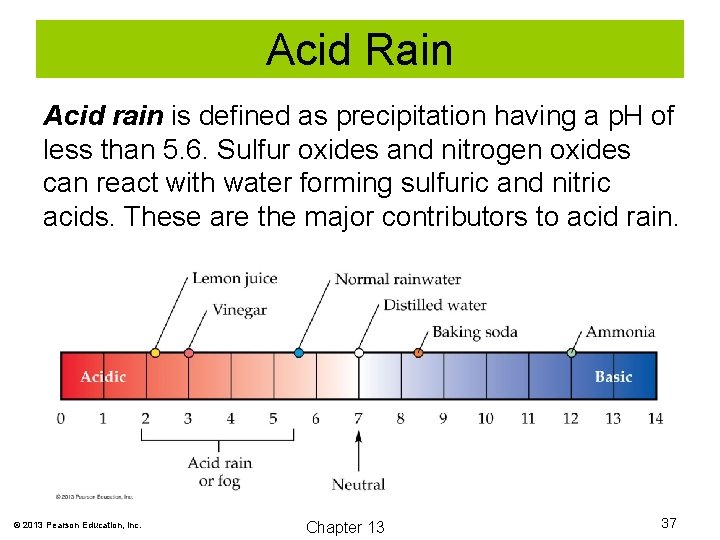
Acid Rain Acid rain is defined as precipitation having a p. H of less than 5. 6. Sulfur oxides and nitrogen oxides can react with water forming sulfuric and nitric acids. These are the major contributors to acid rain. © 2013 Pearson Education, Inc. Chapter 13 37

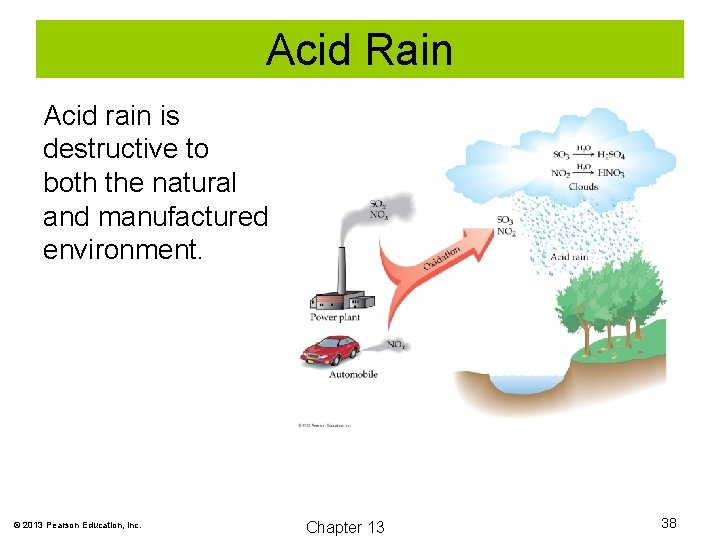
Acid Rain Acid rain is destructive to both the natural and manufactured environment. © 2013 Pearson Education, Inc. Chapter 13 38

Indoor Air Pollution Indoor air pollution is a major health concern. The EPA estimates that pollutant levels of indoor air ranges from 2 to 100 times higher than the levels of outdoor air. © 2013 Pearson Education, Inc. Chapter 13 39

Indoor Air Pollution CO and NOx are released by gas kitchen stoves, cigarette smoke, and free-standing unvented kerosene heaters. Mold will grow wherever there is moisture. Mold spores can exacerbate asthma, bronchitis, and other lung diseases. Ozone is released from copy machines, electronic air cleaners, and other devices. Ozone is a respiratory tract irritant. © 2013 Pearson Education, Inc. Chapter 13 40

Indoor Air Pollution Cigarettes and Secondhand Smoke Cigarette smoke has been shown to contain at least 40 different carcinogens. The EPA considers secondhand smoke to be a Class A carcinogen. Regular exposure to smoke and secondhand smoke has been shown to increase the risk of heart disease, lung cancer, miscarriage, and sudden infant death syndrome (SIDS). © 2013 Pearson Education, Inc. Chapter 13 41

Indoor Air Pollution Radon is a radioactive noble gas. It is colorless, odorless, and tasteless. Radon is released naturally from rock and soil. Radon decays by alpha emission. Polonium-218 is a daughter isotope of radon. It deposits in lung tissue and continues to emit radiation. © 2013 Pearson Education, Inc. Chapter 13 42

Ozone: The Double-Edged Sword Ozone (O 3) is an allotrope of oxygen (O 2). Ozone in the troposphere is a hazardous, toxic substance. It contributes to smog and indoor air pollution. Ozone in the stratosphere shields life on Earth from harmful ultraviolet radiation. © 2013 Pearson Education, Inc. Chapter 13 43

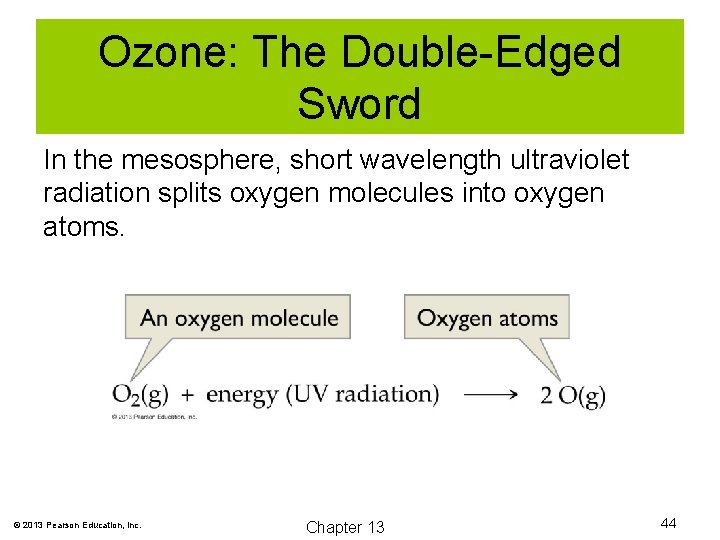
Ozone: The Double-Edged Sword In the mesosphere, short wavelength ultraviolet radiation splits oxygen molecules into oxygen atoms. © 2013 Pearson Education, Inc. Chapter 13 44

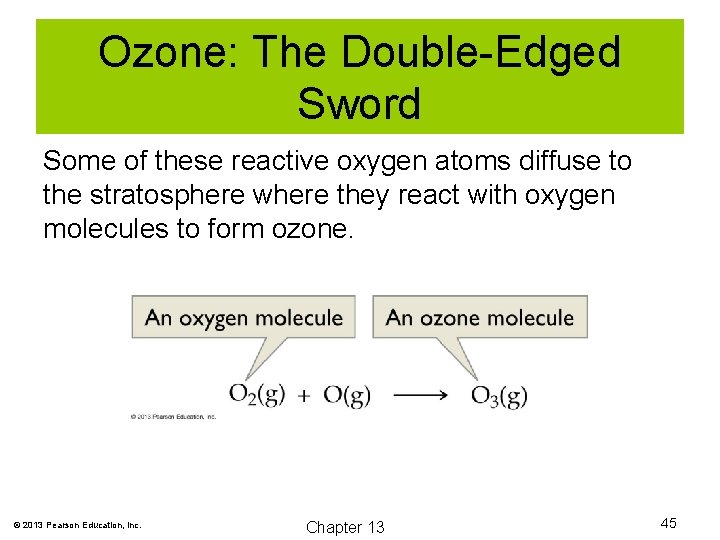
Ozone: The Double-Edged Sword Some of these reactive oxygen atoms diffuse to the stratosphere where they react with oxygen molecules to form ozone. © 2013 Pearson Education, Inc. Chapter 13 45

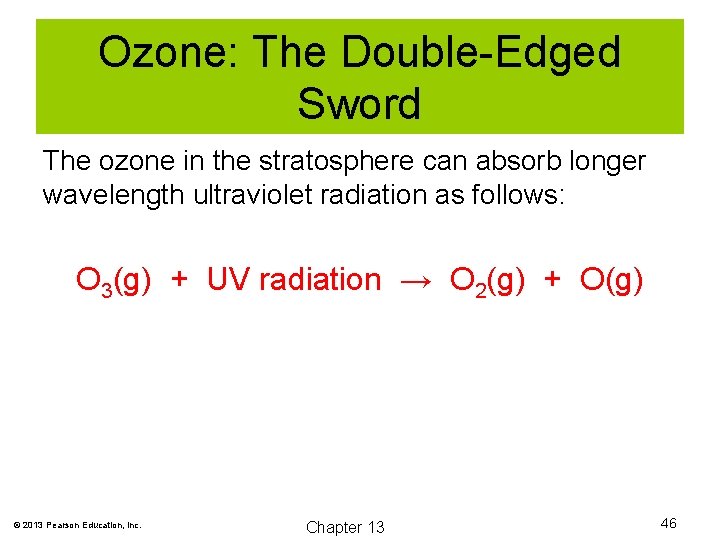
Ozone: The Double-Edged Sword The ozone in the stratosphere can absorb longer wavelength ultraviolet radiation as follows: O 3(g) + UV radiation → O 2(g) + O(g) © 2013 Pearson Education, Inc. Chapter 13 46

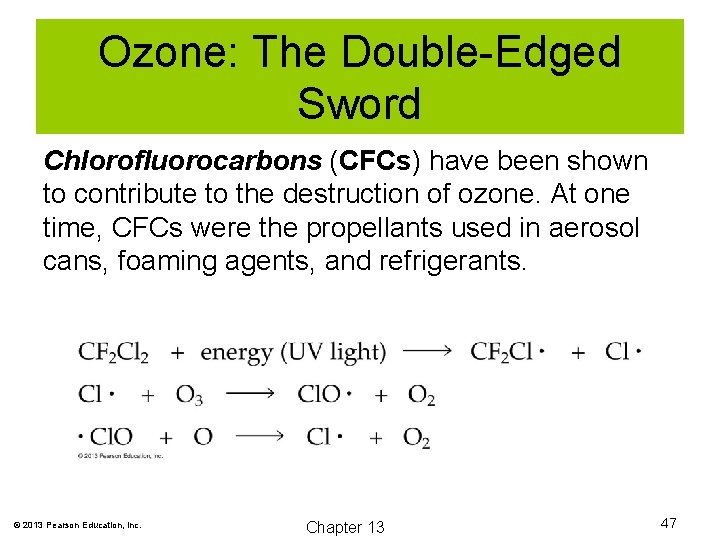
Ozone: The Double-Edged Sword Chlorofluorocarbons (CFCs) have been shown to contribute to the destruction of ozone. At one time, CFCs were the propellants used in aerosol cans, foaming agents, and refrigerants. © 2013 Pearson Education, Inc. Chapter 13 47

Ozone: The Double-Edged Sword Many countries have banned the use of CFCs. Effective substitutes have been developed. © 2013 Pearson Education, Inc. Chapter 13 48

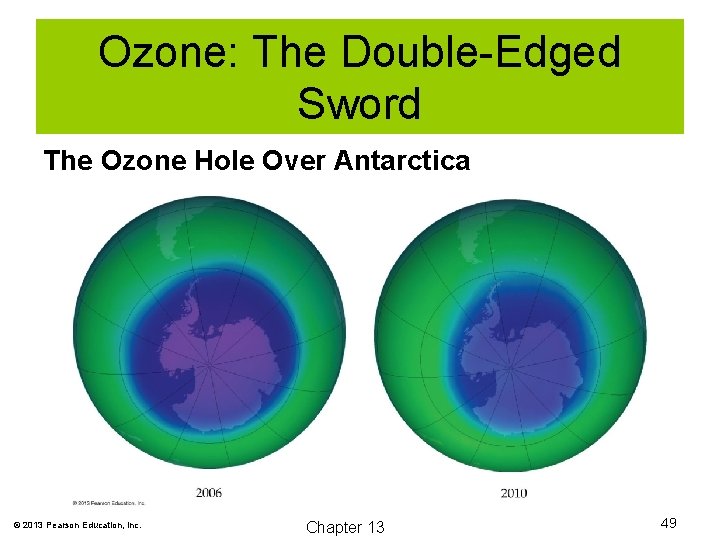
Ozone: The Double-Edged Sword The Ozone Hole Over Antarctica © 2013 Pearson Education, Inc. Chapter 13 49

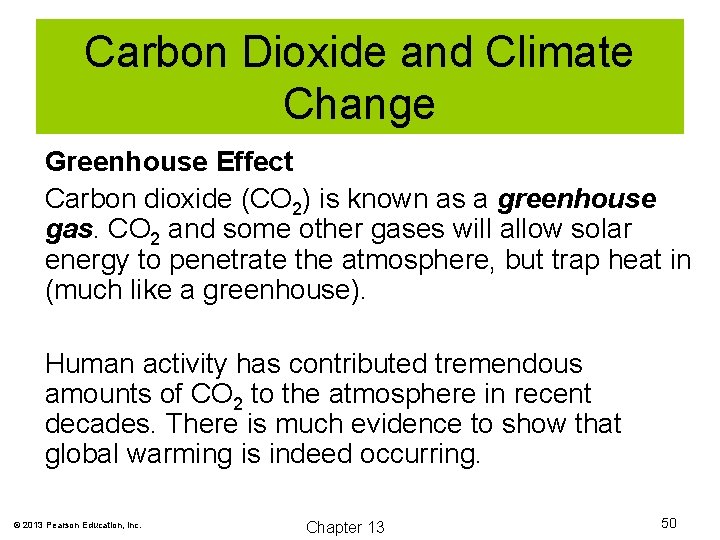
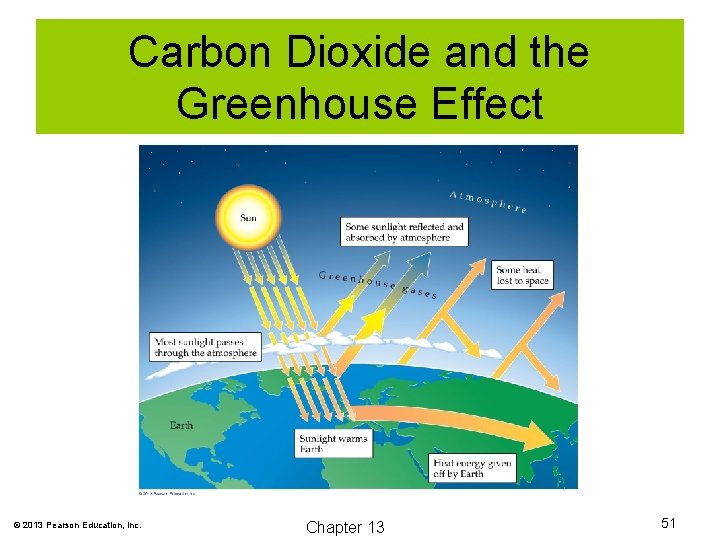
Carbon Dioxide and Climate Change Greenhouse Effect Carbon dioxide (CO 2) is known as a greenhouse gas. CO 2 and some other gases will allow solar energy to penetrate the atmosphere, but trap heat in (much like a greenhouse). Human activity has contributed tremendous amounts of CO 2 to the atmosphere in recent decades. There is much evidence to show that global warming is indeed occurring. © 2013 Pearson Education, Inc. Chapter 13 50

Carbon Dioxide and the Greenhouse Effect © 2013 Pearson Education, Inc. Chapter 13 51

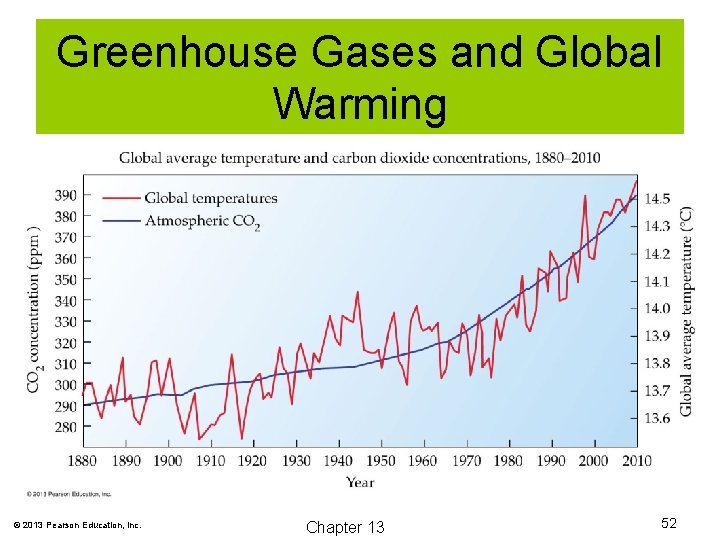
Greenhouse Gases and Global Warming © 2013 Pearson Education, Inc. Chapter 13 52

Climate Change and Weather As Earth’s climate changes, impacts on food production, flooding, and increases in infectious diseases are predicted. © 2013 Pearson Education, Inc. Chapter 13 53

Mitigation of Global Warming Reducing the output of greenhouse gases has no easy fix. Combinations of emerging technologies, such as solar, nuclear, and wind, along with carbon sequestration, are potential answers. © 2013 Pearson Education, Inc. Chapter 13 54

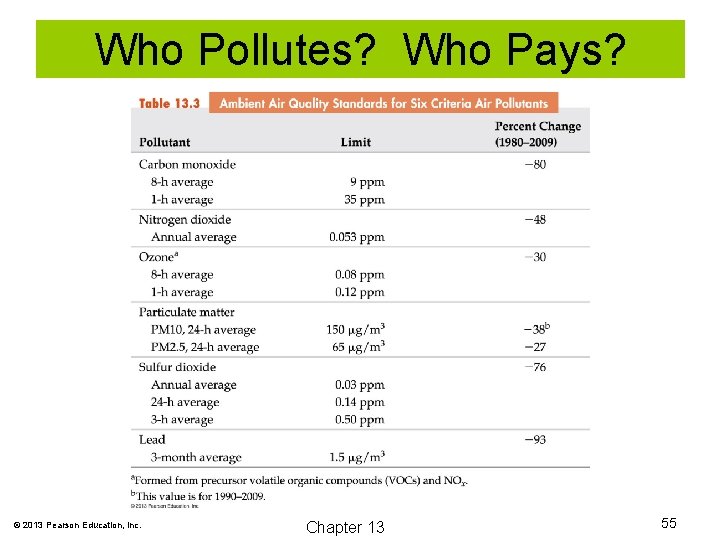
Who Pollutes? Who Pays? © 2013 Pearson Education, Inc. Chapter 13 55

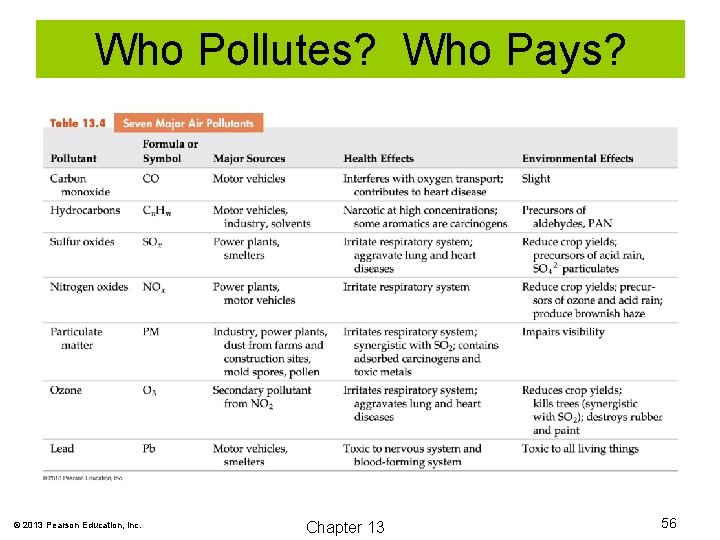
Who Pollutes? Who Pays? © 2013 Pearson Education, Inc. Chapter 13 56

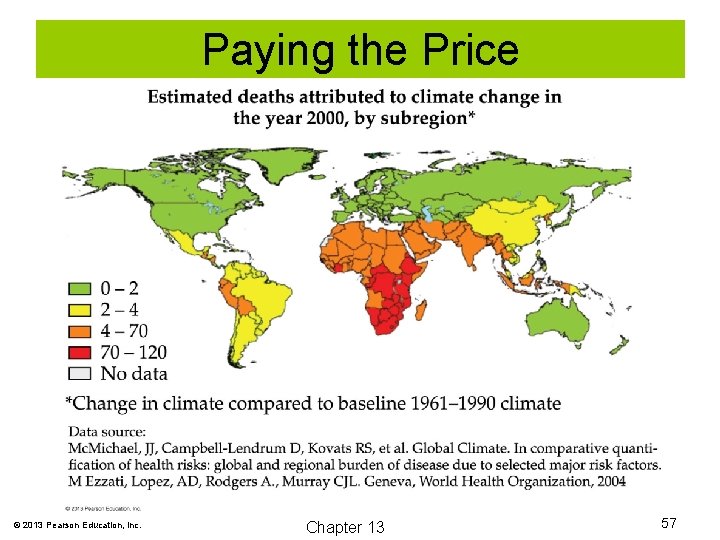
Paying the Price © 2013 Pearson Education, Inc. Chapter 13 57