Chemistry for Changing Times Thirteenth Edition Lecture Outlines





- Slides: 17

Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chapter 2 Atoms John Singer, Jackson Community College © 2013 Pearson Education, Inc.

Atoms: The Greek Idea ~384 B. C. E. , Aristotle: All matter is composed of four elements and all matter is continuous, not atomistic. © 2013 Pearson Education, Inc. Chapter 2 2

Atoms: The Greek Idea ~ 450 B. C. E. , Leucippus and Democritus Atomos: The point at which matter can no longer be subdivided. © 2013 Pearson Education, Inc. Chapter 2 3

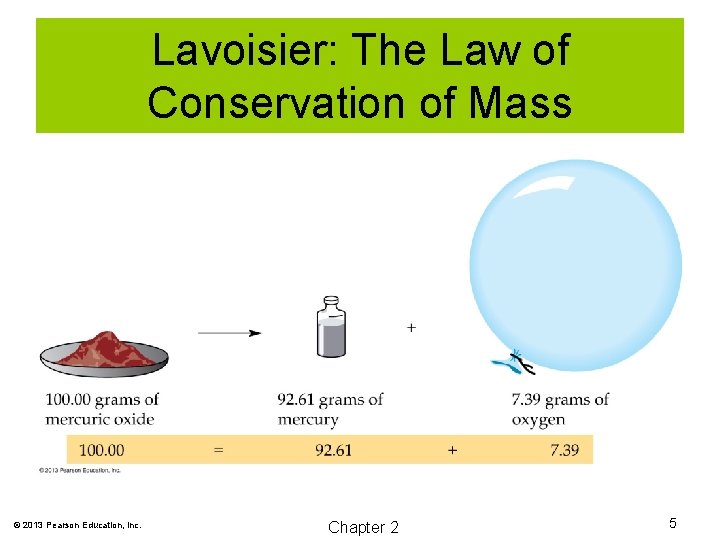
Lavoisier: The Law of Conservation of Mass Early 1700 s Lavoisier Law of Conservation of Mass: During a chemical change, matter is neither created nor destroyed. © 2013 Pearson Education, Inc. Chapter 2 4

Lavoisier: The Law of Conservation of Mass © 2013 Pearson Education, Inc. Chapter 2 5

Proust: The Law of Definite Proportions 1799, Proust Law of Definite Proportions: A compound always contains the same elements in certain definite proportions. © 2013 Pearson Education, Inc. Chapter 2 6

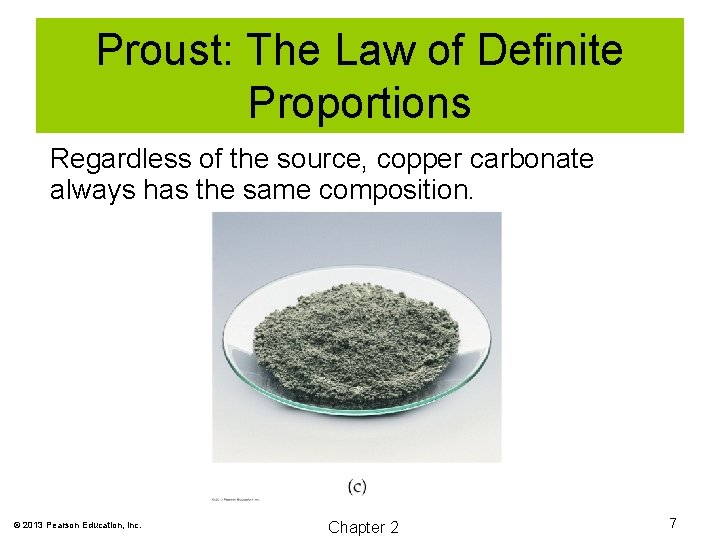
Proust: The Law of Definite Proportions Regardless of the source, copper carbonate always has the same composition. © 2013 Pearson Education, Inc. Chapter 2 7

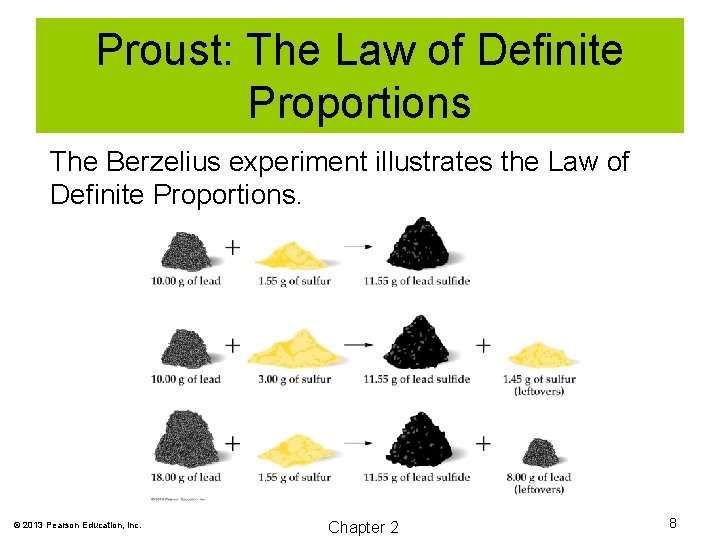
Proust: The Law of Definite Proportions The Berzelius experiment illustrates the Law of Definite Proportions. © 2013 Pearson Education, Inc. Chapter 2 8

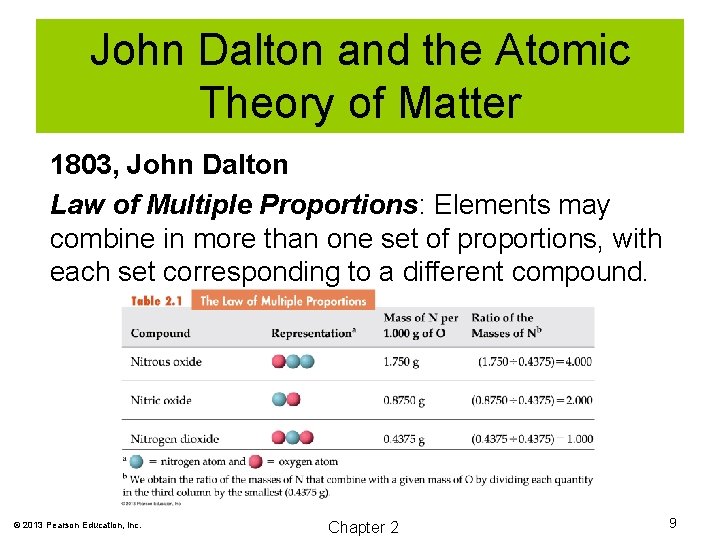
John Dalton and the Atomic Theory of Matter 1803, John Dalton Law of Multiple Proportions: Elements may combine in more than one set of proportions, with each set corresponding to a different compound. © 2013 Pearson Education, Inc. Chapter 2 9

John Dalton and the Atomic Theory of Matter © 2013 Pearson Education, Inc. Chapter 2 10

John Dalton and the Atomic Theory of Matter • All matter is composed of extremely small particles called atoms. • All atoms of a given element are alike and differ from the atoms of any other element. • Compounds are formed when atoms of different elements combine in fixed proportions. • A chemical reaction involves the rearrangement of atoms. © 2013 Pearson Education, Inc. Chapter 2 11

Isotopes Much of John Dalton’s atomic theory has been modified. For example, John Dalton assumed that all atoms of an element are alike. He did not understand the existence of isotopes. Isotopes are atoms of the same element with different relative masses. © 2013 Pearson Education, Inc. Chapter 2 12

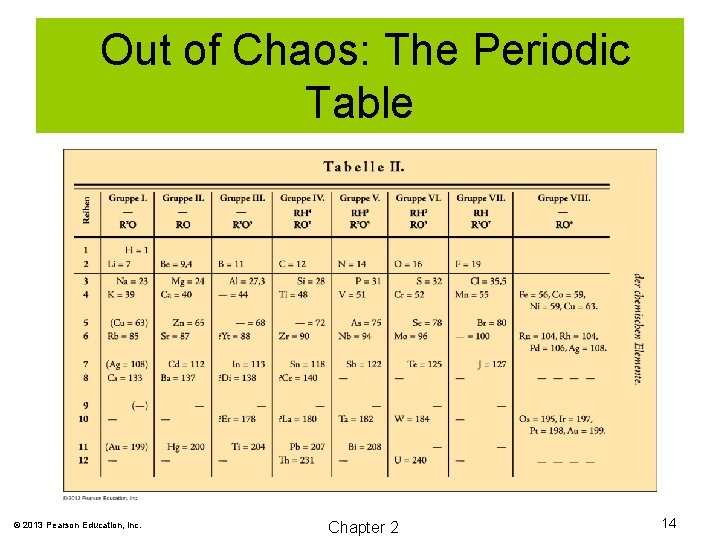
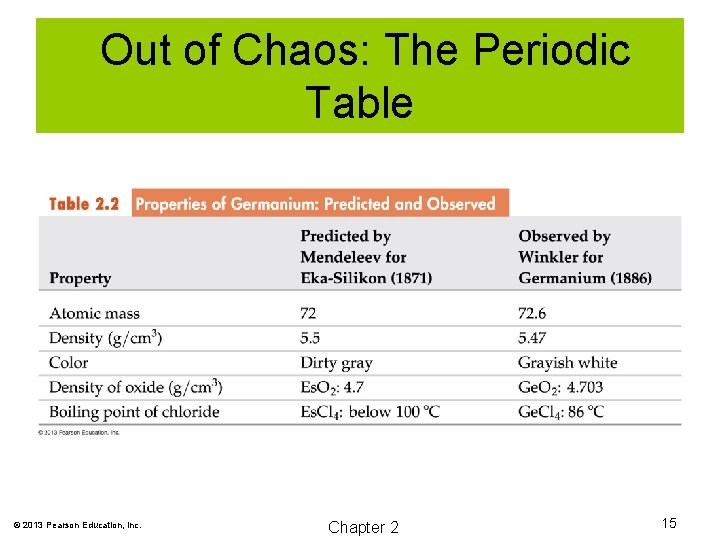
Out of Chaos: The Periodic Table 1869, Dmitri Mendeleev arranged the elements in order of increasing atomic mass. He left gaps for yet undiscovered elements. He also predicted the properties of those elements. When those elements were eventually discovered, many of his predictions were found to be accurate. © 2013 Pearson Education, Inc. Chapter 2 13

Out of Chaos: The Periodic Table © 2013 Pearson Education, Inc. Chapter 2 14

Out of Chaos: The Periodic Table © 2013 Pearson Education, Inc. Chapter 2 15

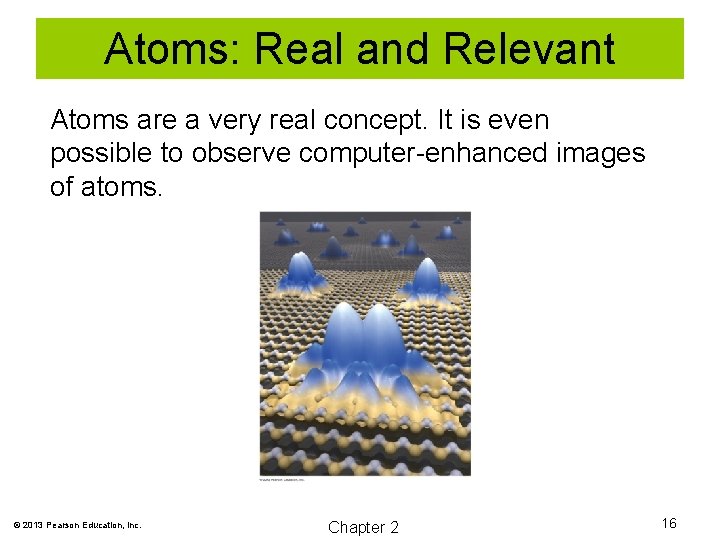
Atoms: Real and Relevant Atoms are a very real concept. It is even possible to observe computer-enhanced images of atoms. © 2013 Pearson Education, Inc. Chapter 2 16

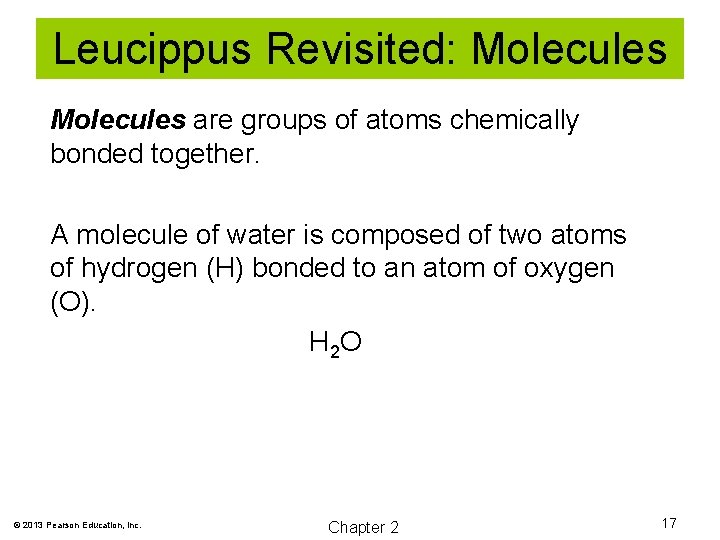
Leucippus Revisited: Molecules are groups of atoms chemically bonded together. A molecule of water is composed of two atoms of hydrogen (H) bonded to an atom of oxygen (O). H 2 O © 2013 Pearson Education, Inc. Chapter 2 17
 Carsonian nightmare
Carsonian nightmare Changing times essay
Changing times essay Activity 1 changing times
Activity 1 changing times Bob dylan the times they are a-changin' lyrics
Bob dylan the times they are a-changin' lyrics 15 times 15 times 20
15 times 15 times 20 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad What is outlining?
What is outlining? Tujuan garis besar adalah
Tujuan garis besar adalah Kairos program manual
Kairos program manual Commercial law outline
Commercial law outline Four main components for effective outlines
Four main components for effective outlines A business plan is a document that outlines
A business plan is a document that outlines Benign nephrosclerosis pathology outlines
Benign nephrosclerosis pathology outlines 2 kings 4 8 17
2 kings 4 8 17 Anime outline
Anime outline Two types of outlines
Two types of outlines Summary of a haunted house by virginia woolf
Summary of a haunted house by virginia woolf Ksf outlines
Ksf outlines