Chemistry for Changing Times Thirteenth Edition Lecture Outlines


- Slides: 25

Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chapter 6 Gases, Liquids, Solids, and Intermolecular Forces John Singer, Jackson Community College © 2013 Pearson Education, Inc.

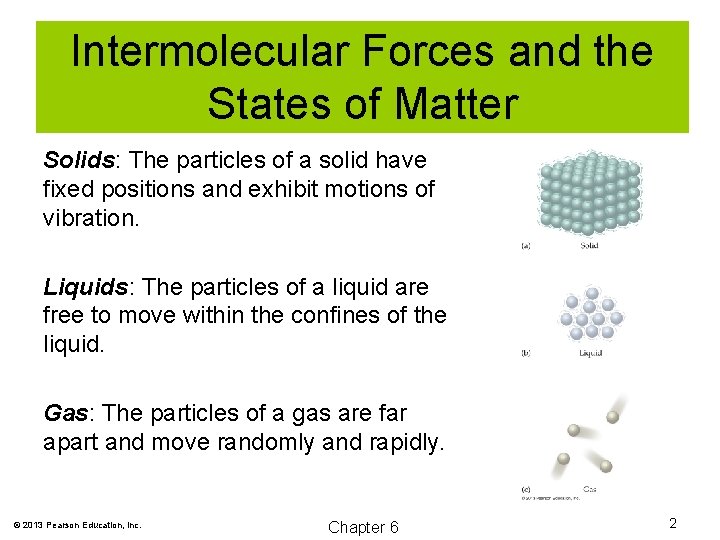
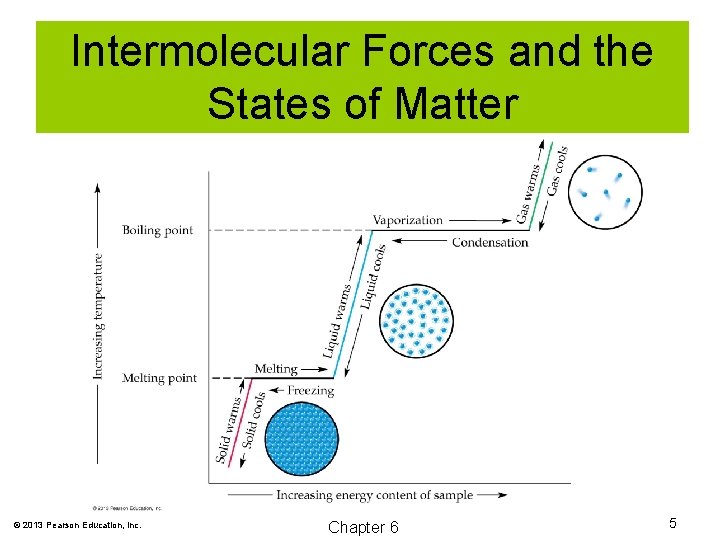
Intermolecular Forces and the States of Matter Solids: The particles of a solid have fixed positions and exhibit motions of vibration. Liquids: The particles of a liquid are free to move within the confines of the liquid. Gas: The particles of a gas are far apart and move randomly and rapidly. © 2013 Pearson Education, Inc. Chapter 6 2

Intermolecular Forces and the States of Matter Melting point: The temperature at which a solid becomes a liquid. Vaporization: The process of a liquid becoming a gas. Boiling point: The temperature at which the particles of a liquid escape and become a gas. © 2013 Pearson Education, Inc. Chapter 6 3

Intermolecular Forces and the States of Matter Condensation: The process by which a gas becomes a liquid. Freezing: The process by which a liquid becomes a solid. This occurs at the freezing point, which is the same as the melting point. Sublimation: When a solid changes directly from the solid to the gaseous state. © 2013 Pearson Education, Inc. Chapter 6 4

Intermolecular Forces and the States of Matter © 2013 Pearson Education, Inc. Chapter 6 5

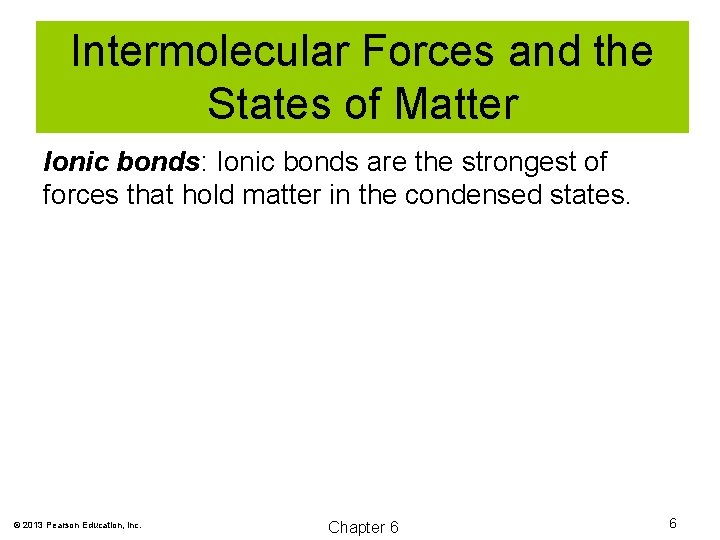
Intermolecular Forces and the States of Matter Ionic bonds: Ionic bonds are the strongest of forces that hold matter in the condensed states. © 2013 Pearson Education, Inc. Chapter 6 6

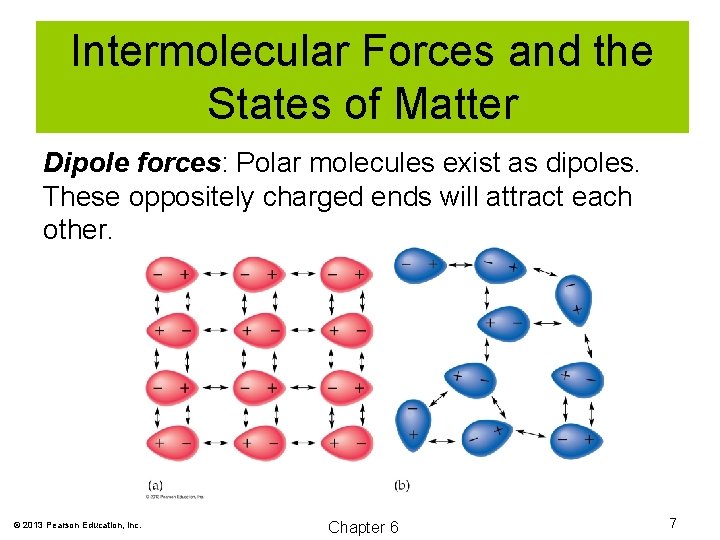
Intermolecular Forces and the States of Matter Dipole forces: Polar molecules exist as dipoles. These oppositely charged ends will attract each other. © 2013 Pearson Education, Inc. Chapter 6 7

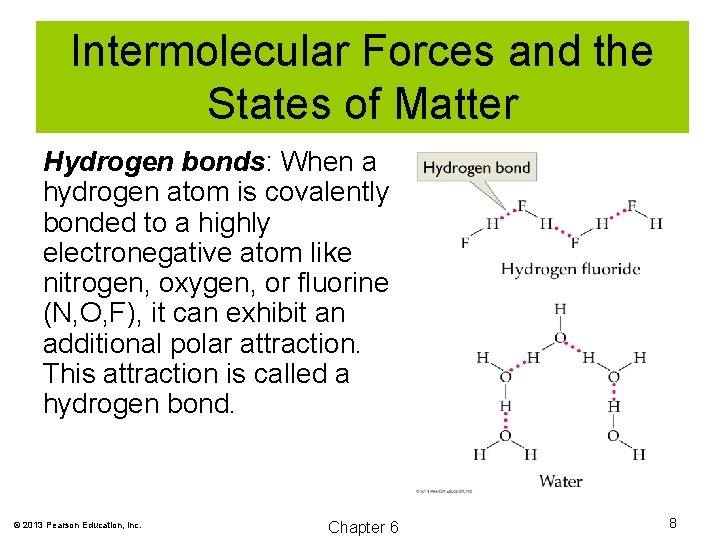
Intermolecular Forces and the States of Matter Hydrogen bonds: When a hydrogen atom is covalently bonded to a highly electronegative atom like nitrogen, oxygen, or fluorine (N, O, F), it can exhibit an additional polar attraction. This attraction is called a hydrogen bond. © 2013 Pearson Education, Inc. Chapter 6 8

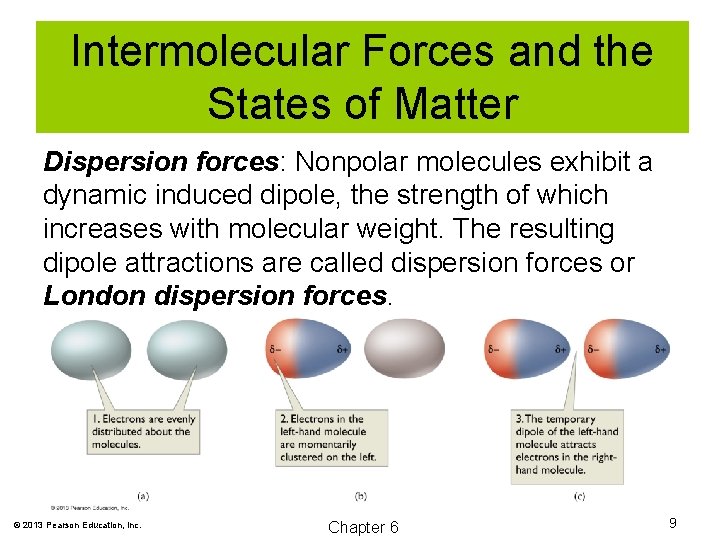
Intermolecular Forces and the States of Matter Dispersion forces: Nonpolar molecules exhibit a dynamic induced dipole, the strength of which increases with molecular weight. The resulting dipole attractions are called dispersion forces or London dispersion forces. © 2013 Pearson Education, Inc. Chapter 6 9

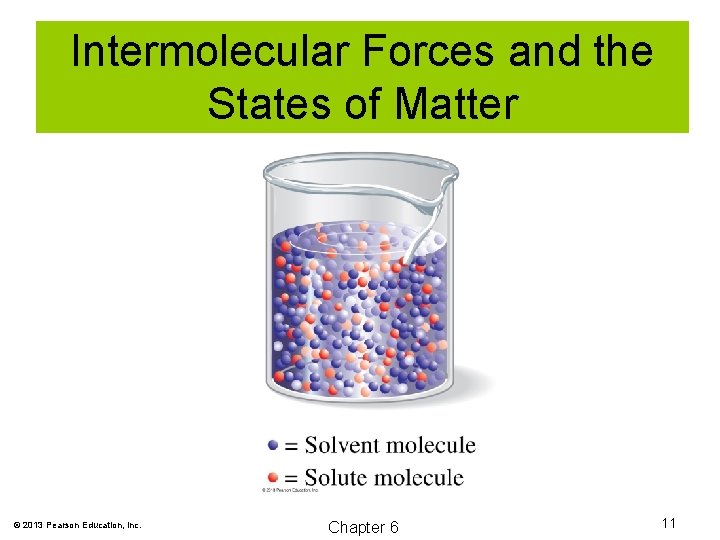
Intermolecular Forces and the States of Matter Solution: A homogeneous mixture of two or more substances. Solute: A substance that is dispersed in a solution. Solvent: The substance doing the dissolving, usually present in greatest quantity. © 2013 Pearson Education, Inc. Chapter 6 10

Intermolecular Forces and the States of Matter © 2013 Pearson Education, Inc. Chapter 6 11

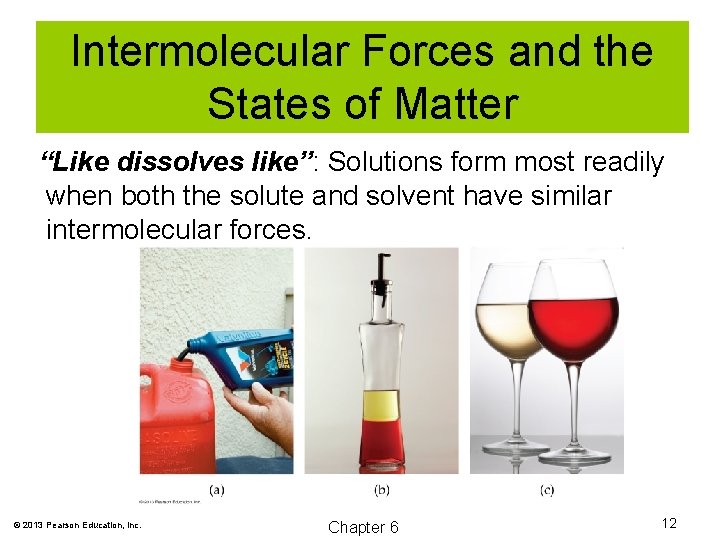
Intermolecular Forces and the States of Matter “Like dissolves like”: Solutions form most readily when both the solute and solvent have similar intermolecular forces. © 2013 Pearson Education, Inc. Chapter 6 12

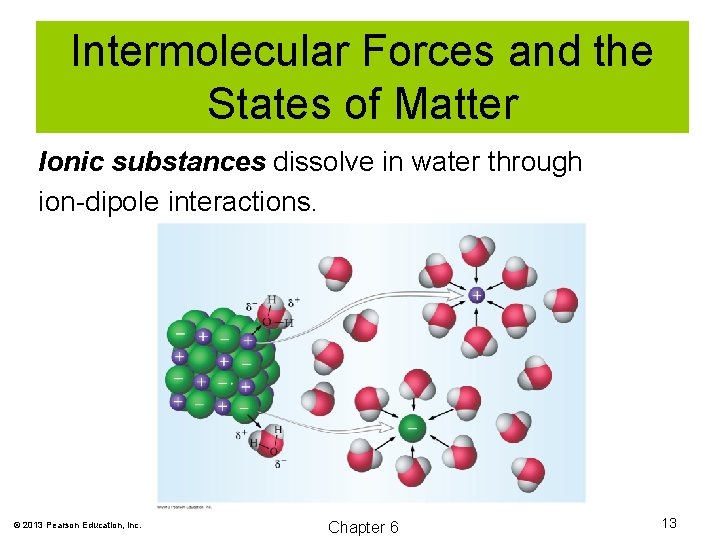
Intermolecular Forces and the States of Matter Ionic substances dissolve in water through ion-dipole interactions. © 2013 Pearson Education, Inc. Chapter 6 13

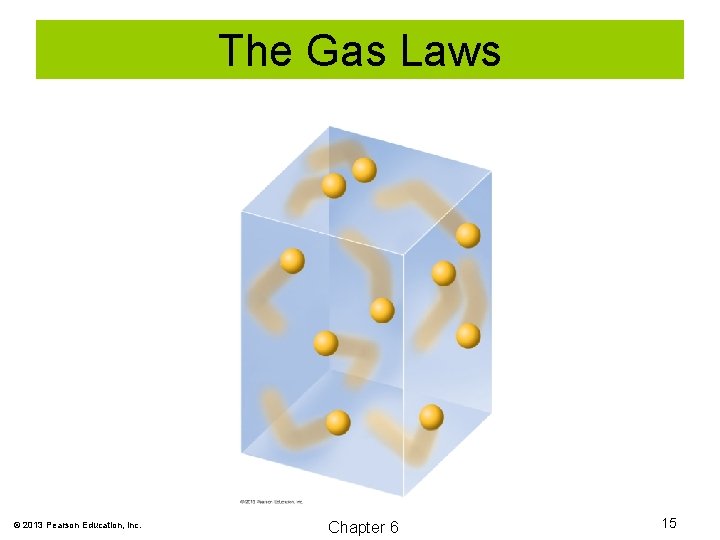
The Gas Laws Kinetic Molecular Theory of a Gas Postulates: 1. The particles of a gas are in rapid constant motion. 2. The particles of a gas are tiny compared to the distance between them. 3. There is little attraction between the particles of a gas. 4. Collisions between gas molecules are perfectly elastic. 5. Temperature is a measure of the average kinetic energy of gas molecules. © 2013 Pearson Education, Inc. Chapter 6 14

The Gas Laws © 2013 Pearson Education, Inc. Chapter 6 15

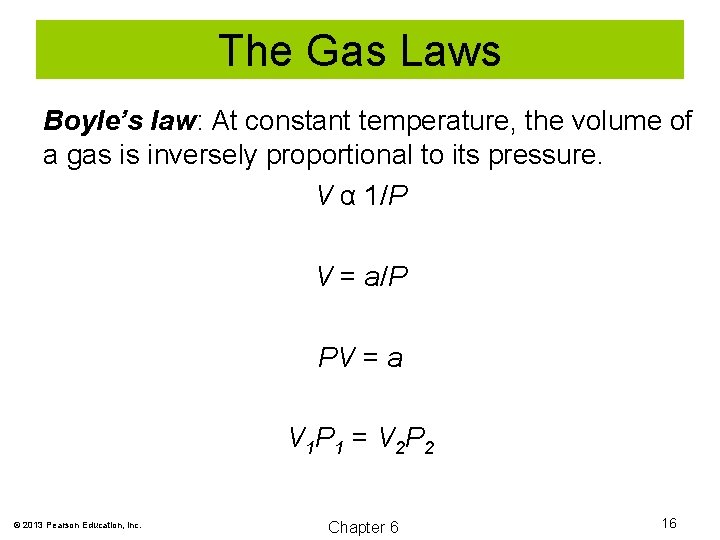
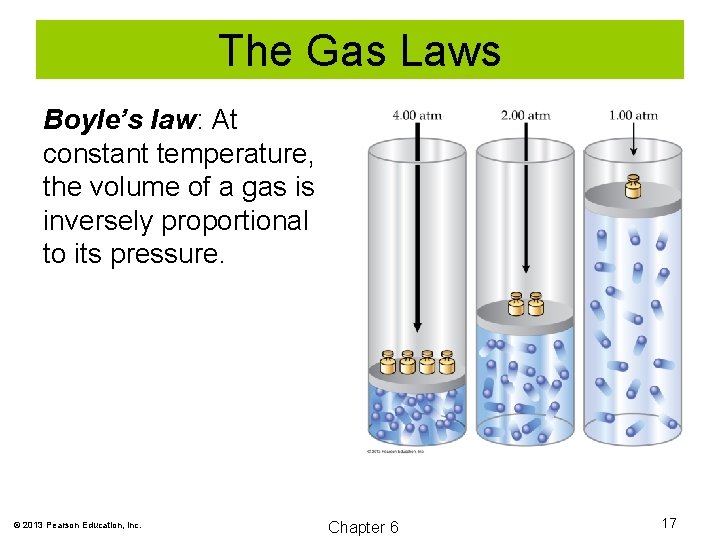
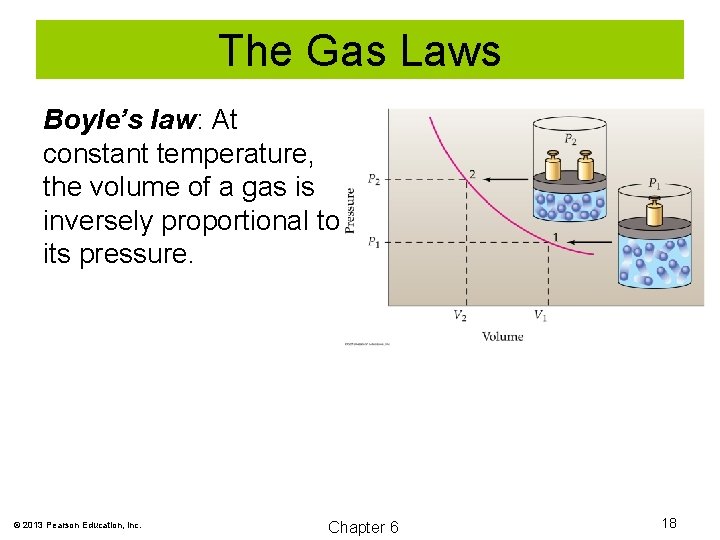
The Gas Laws Boyle’s law: At constant temperature, the volume of a gas is inversely proportional to its pressure. V α 1/P V = a/P PV = a V 1 P 1 = V 2 P 2 © 2013 Pearson Education, Inc. Chapter 6 16

The Gas Laws Boyle’s law: At constant temperature, the volume of a gas is inversely proportional to its pressure. © 2013 Pearson Education, Inc. Chapter 6 17

The Gas Laws Boyle’s law: At constant temperature, the volume of a gas is inversely proportional to its pressure. © 2013 Pearson Education, Inc. Chapter 6 18

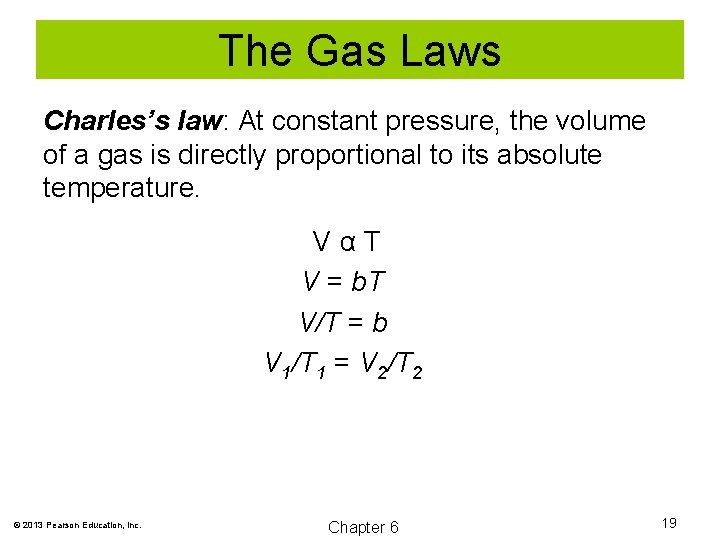
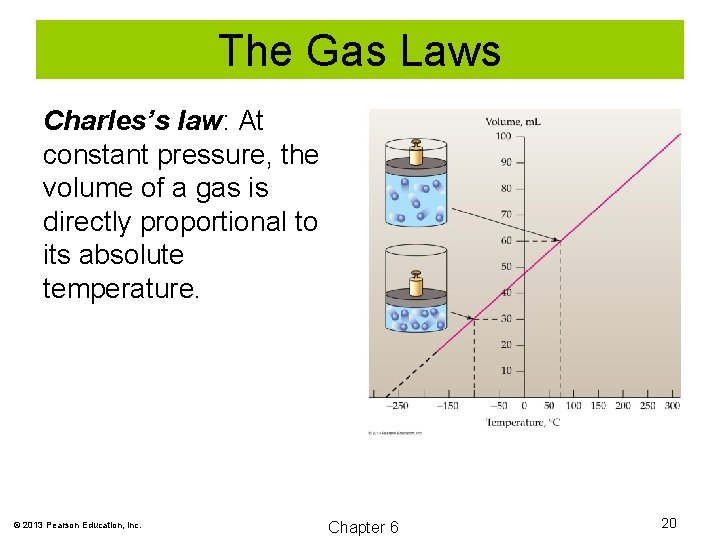
The Gas Laws Charles’s law: At constant pressure, the volume of a gas is directly proportional to its absolute temperature. VαT V = b. T V/T = b V 1/T 1 = V 2/T 2 © 2013 Pearson Education, Inc. Chapter 6 19

The Gas Laws Charles’s law: At constant pressure, the volume of a gas is directly proportional to its absolute temperature. © 2013 Pearson Education, Inc. Chapter 6 20

The Gas Laws Charles’s Law © 2013 Pearson Education, Inc. Chapter 6 21

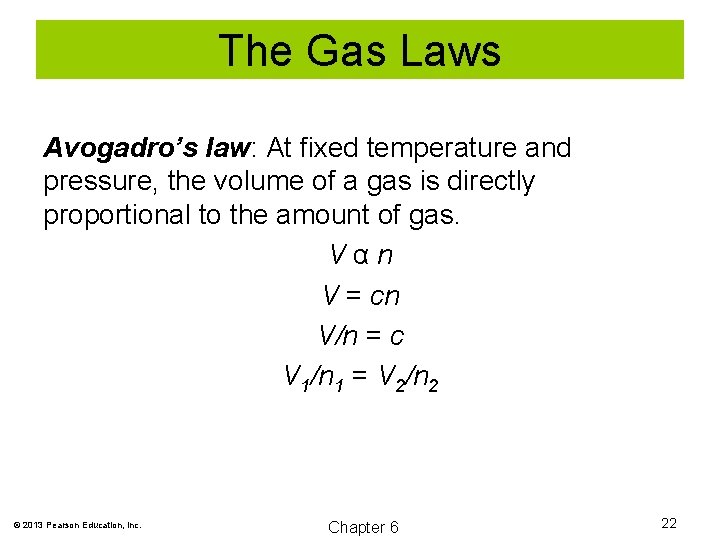
The Gas Laws Avogadro’s law: At fixed temperature and pressure, the volume of a gas is directly proportional to the amount of gas. Vαn V = cn V/n = c V 1/n 1 = V 2/n 2 © 2013 Pearson Education, Inc. Chapter 6 22

The Gas Laws Standard temperature and pressure: Standard temperature = 0 o. C Standard pressure = 1 atm A mole of any gas at STP occupies 22. 4 L © 2013 Pearson Education, Inc. Chapter 6 23

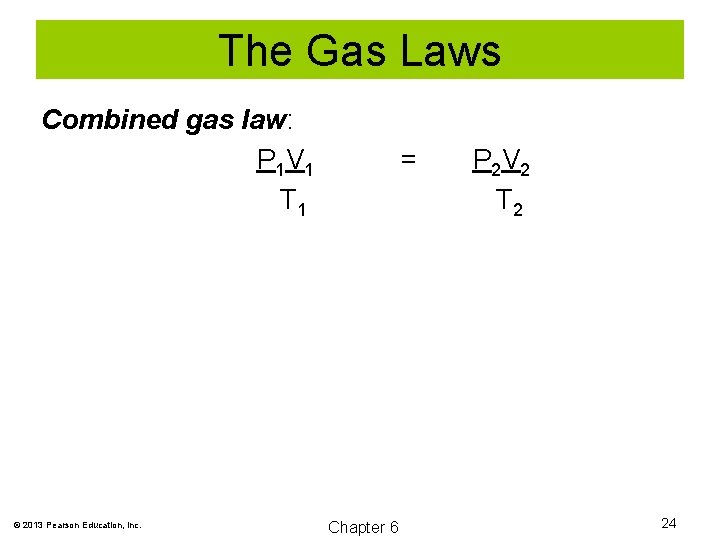
The Gas Laws Combined gas law: P 1 V 1 T 1 © 2013 Pearson Education, Inc. = Chapter 6 P 2 V 2 T 2 24

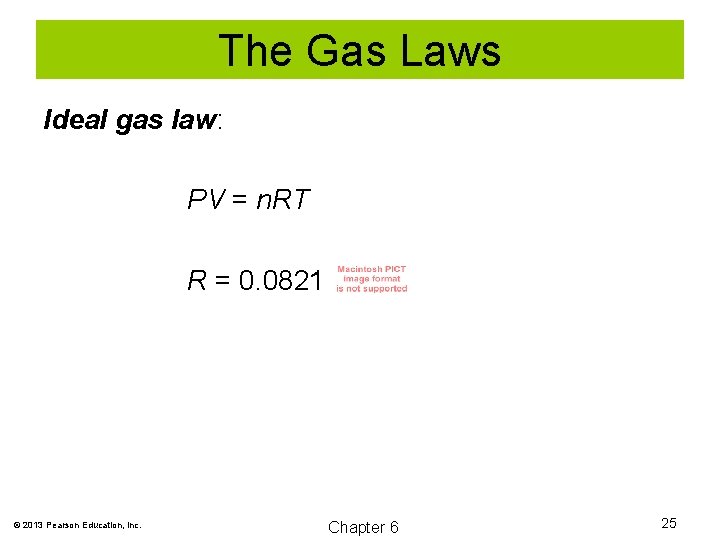
The Gas Laws Ideal gas law: PV = n. RT R = 0. 0821 © 2013 Pearson Education, Inc. Chapter 6 25
 Carsonian nightmare
Carsonian nightmare Changing times essay
Changing times essay Activity 1 changing times
Activity 1 changing times Bob dylan the times they are a-changin' lyrics
Bob dylan the times they are a-changin' lyrics Factors of 168
Factors of 168 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Parallelism outline
Parallelism outline Outline learning objectives
Outline learning objectives Kairos talk outlines
Kairos talk outlines Commercial law outline
Commercial law outline Four main components for effective outlines
Four main components for effective outlines A business plan is a document that outlines
A business plan is a document that outlines Pyelonephritis
Pyelonephritis 2 kings 4 8 17
2 kings 4 8 17 Anime outline character
Anime outline character Two types of outlines
Two types of outlines A haunted house virginia woolf analysis
A haunted house virginia woolf analysis Ksf outlines
Ksf outlines Mucoepidermoid carcinoma histology
Mucoepidermoid carcinoma histology High level outline
High level outline Acts 4 outline
Acts 4 outline Exegetical outline example
Exegetical outline example Cjis security training
Cjis security training Mun position paper outline
Mun position paper outline Define visible
Define visible Crohn's disease pathology outlines
Crohn's disease pathology outlines