Chemical kinetics The area of chemistry that is

![Example: Consider a reaction A + B Cfor which rate =k[A][B]2. Each of the Example: Consider a reaction A + B Cfor which rate =k[A][B]2. Each of the](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-14.jpg)

![(b) Rate = k[A]2[B]0 rate K= [A]2 -5 4. 0 10 = [0. 100 (b) Rate = k[A]2[B]0 rate K= [A]2 -5 4. 0 10 = [0. 100](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-21.jpg)
![(b) Rate = k[NO]2[H 2] rate K= [NO]2[H 2] -3 1. 23 10 = (b) Rate = k[NO]2[H 2] rate K= [NO]2[H 2] -3 1. 23 10 =](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-23.jpg)
![ln[A]t = kt + ln[A]0 This equation has the form of the general equation ln[A]t = kt + ln[A]0 This equation has the form of the general equation](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-25.jpg)
![1 [A]t = k. t + y = m. x + b 1 [A]0 1 [A]t = k. t + y = m. x + b 1 [A]0](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-29.jpg)

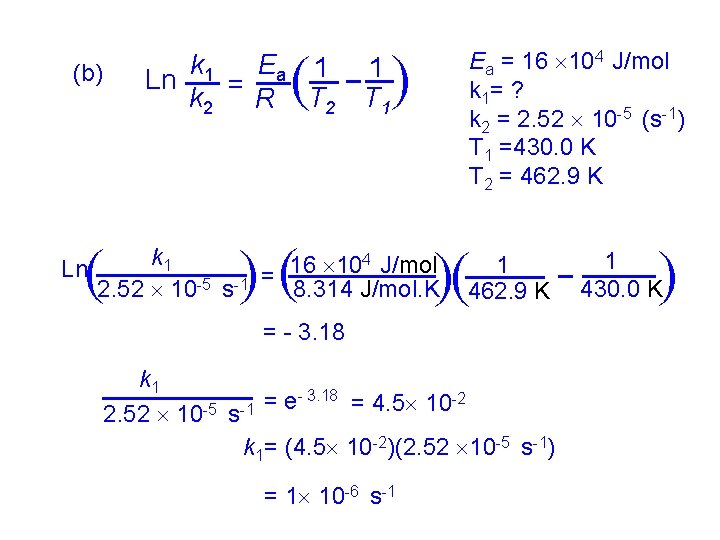
- Slides: 46

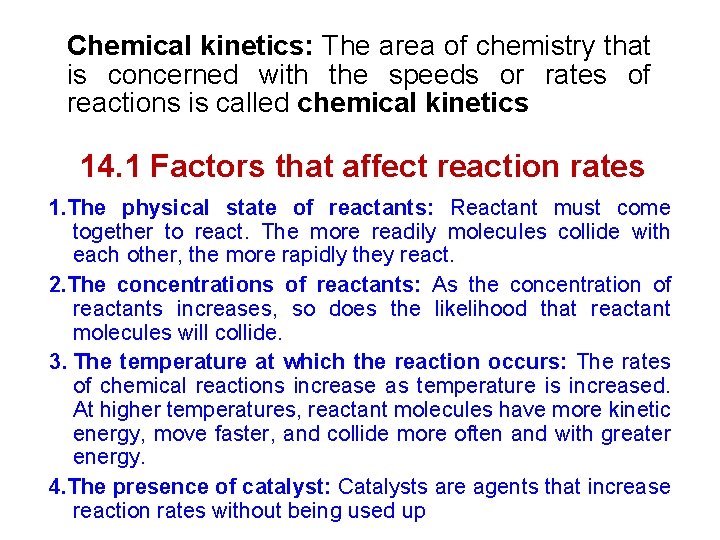
Chemical kinetics: The area of chemistry that is concerned with the speeds or rates of reactions is called chemical kinetics 14. 1 Factors that affect reaction rates 1. The physical state of reactants: Reactant must come together to react. The more readily molecules collide with each other, the more rapidly they react. 2. The concentrations of reactants: As the concentration of reactants increases, so does the likelihood that reactant molecules will collide. 3. The temperature at which the reaction occurs: The rates of chemical reactions increase as temperature is increased. At higher temperatures, reactant molecules have more kinetic energy, move faster, and collide more often and with greater energy. 4. The presence of catalyst: Catalysts are agents that increase reaction rates without being used up

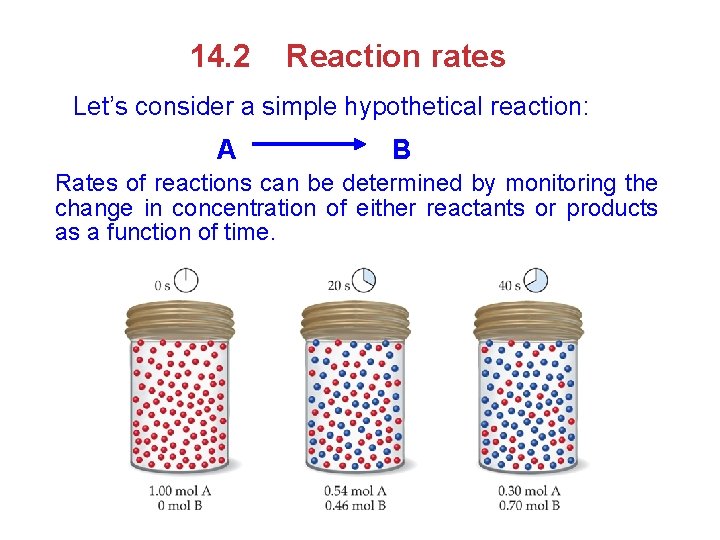
14. 2 Reaction rates Let’s consider a simple hypothetical reaction: A B Rates of reactions can be determined by monitoring the change in concentration of either reactants or products as a function of time.

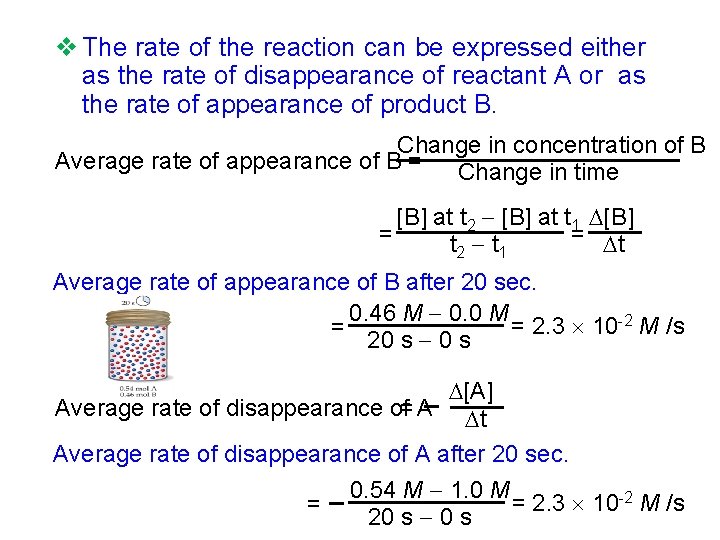
v The rate of the reaction can be expressed either as the rate of disappearance of reactant A or as the rate of appearance of product B. Change in concentration of B Average rate of appearance of B = Change in time [B] at t 2 [B] at t 1 [B] = = t t 2 t 1 Average rate of appearance of B after 20 sec. 0. 46 M 0. 0 M = 2. 3 10 -2 M /s = 20 s [A] Average rate of disappearance of= A t Average rate of disappearance of A after 20 sec. = 0. 54 M 1. 0 M = 2. 3 10 -2 M /s 20 s

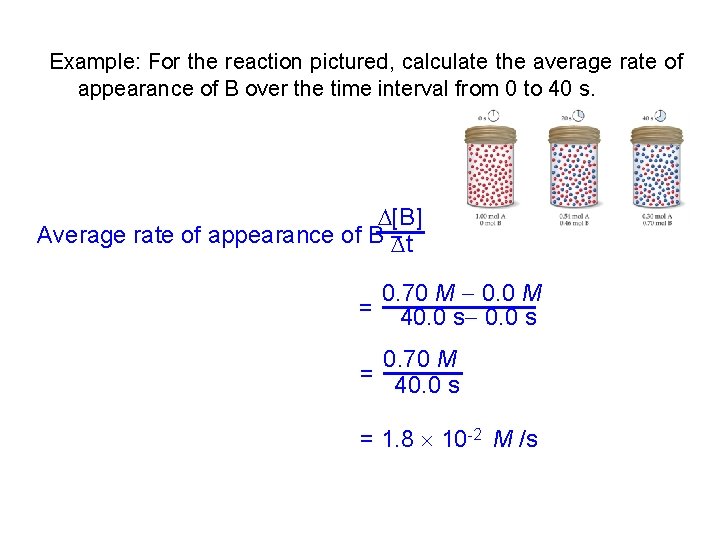
Example: For the reaction pictured, calculate the average rate of appearance of B over the time interval from 0 to 40 s. [B] Average rate of appearance of B = t 0. 70 M 0. 0 M = 40. 0 s 0. 70 M = 40. 0 s = 1. 8 10 -2 M /s

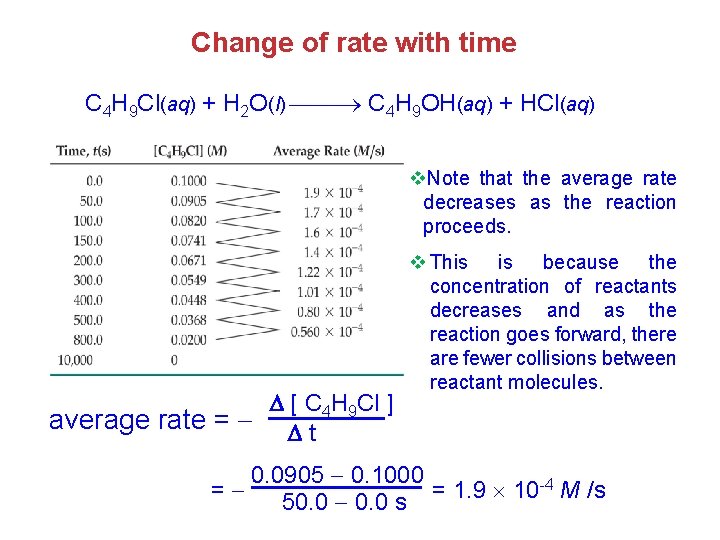
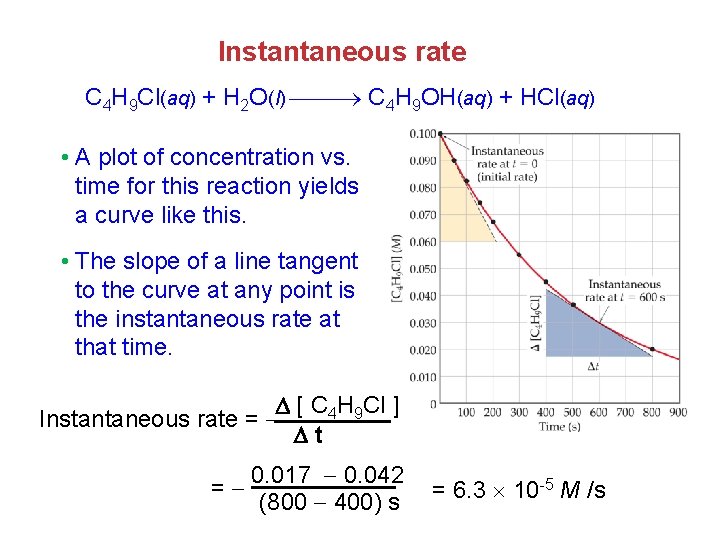
Change of rate with time C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) v. Note that the average rate decreases as the reaction proceeds. [ C 4 H 9 Cl ] average rate = t v This is because the concentration of reactants decreases and as the reaction goes forward, there are fewer collisions between reactant molecules. 0. 0905 0. 1000 -4 M /s = = 1. 9 10 50. 0 s

Instantaneous rate C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) • A plot of concentration vs. time for this reaction yields a curve like this. • The slope of a line tangent to the curve at any point is the instantaneous rate at that time. [ C 4 H 9 Cl ] Instantaneous rate = t 0. 017 0. 042 = (800 400) s = 6. 3 10 -5 M /s

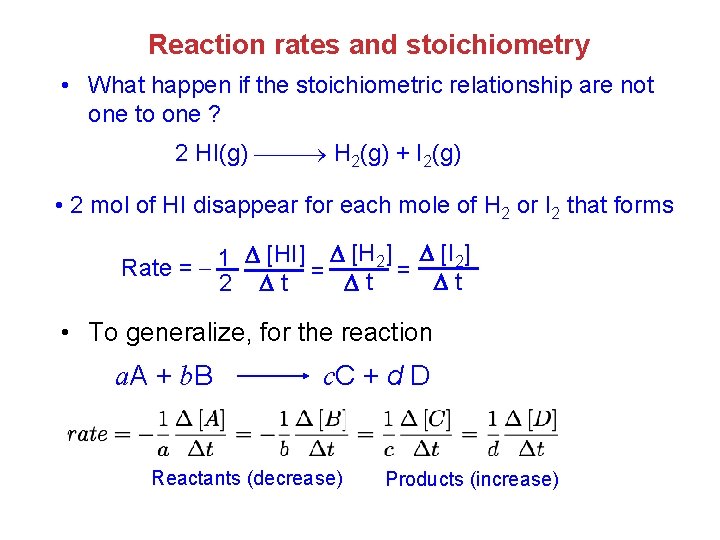
Reaction rates and stoichiometry • What happen if the stoichiometric relationship are not one to one ? 2 HI(g) H 2(g) + I 2(g) • 2 mol of HI disappear for each mole of H 2 or I 2 that forms Rate = 1 2 [HI] [H 2] [I 2] = t t • To generalize, for the reaction a. A + b. B c. C + d D Reactants (decrease) Products (increase)

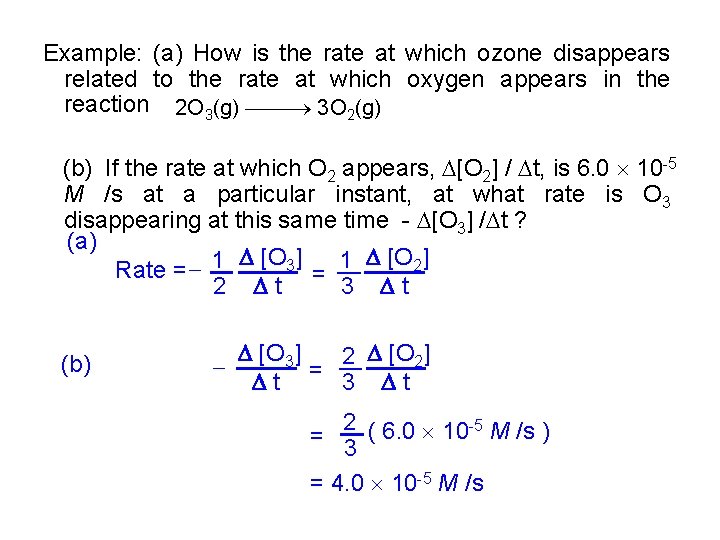
Example: (a) How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction 2 O 3(g) 3 O 2(g) (b) If the rate at which O 2 appears, [O 2] / t, is 6. 0 10 -5 M /s at a particular instant, at what rate is O 3 disappearing at this same time - [O 3] / t ? (a) [O 3] 1 [O 2] Rate = 1 2 t = 3 t (b) [O 3] 2 [O 2] t = 3 t 2 -5 = 3 ( 6. 0 10 M /s ) = 4. 0 10 -5 M /s

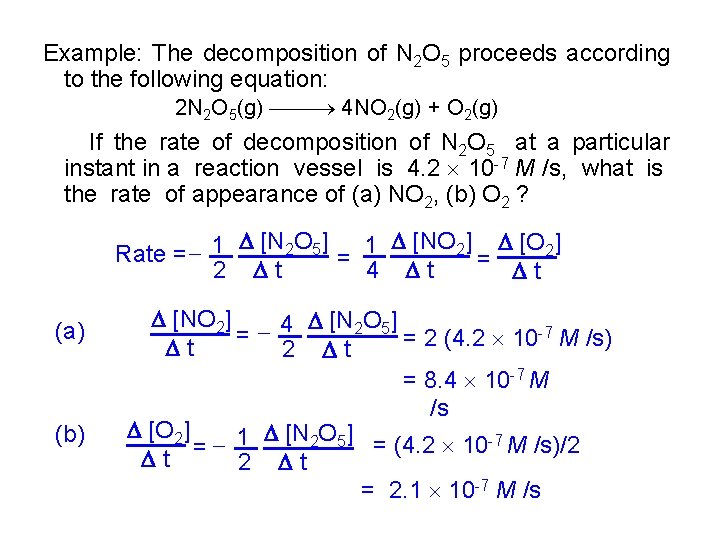
Example: The decomposition of N 2 O 5 proceeds according to the following equation: 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) If the rate of decomposition of N 2 O 5 at a particular instant in a reaction vessel is 4. 2 10 -7 M /s, what is the rate of appearance of (a) NO 2, (b) O 2 ? [N 2 O 5] 1 [NO 2] [O 2] Rate = 1 = 4 t = t 2 t (a) (b) [NO 2] [N 2 O 5] -7 M /s) = 4 = 2 (4. 2 10 t 2 t = 8. 4 10 -7 M /s [O 2] 1 [N 2 O 5] = (4. 2 10 -7 M /s)/2 = t 2 t = 2. 1 10 -7 M /s

14. 3 The rate law: The effect of concentration on rate Rate Law - A mathematical equation that shows how the rate of a reaction depends on the concentration of reactants NH 4+(aq) + NO 2 (aq) N 2(g) + 2 H 2 O(l) Rate = k[NH 4+][NO 2 ] • k is a constant, called “rate constant” that has a specific value for each reaction. • The value of k is determined experimentally. • k is independent of reactant concentrations but is dependent on temperature.

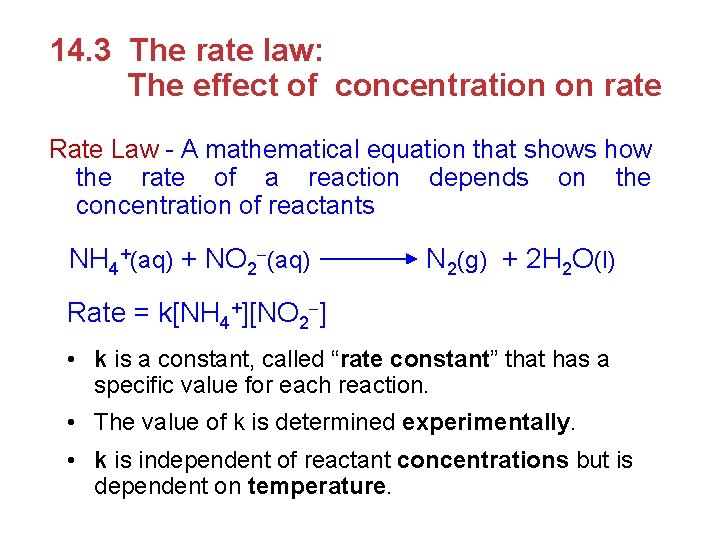
For a general reaction, a. A+b. B c. C + d. D the rate law has the general form reaction rate = k [A]m [B ]n • exponents m and n are determined experimentally, and are typically small whole numbers (usually 0, 1, or 2) • [The rate law exponents are usually not the same as the coefficients in the balanced chemical equation. ] • The rate law for a reaction cannot be predicted from the chemical equation. It must be experimentally determined.

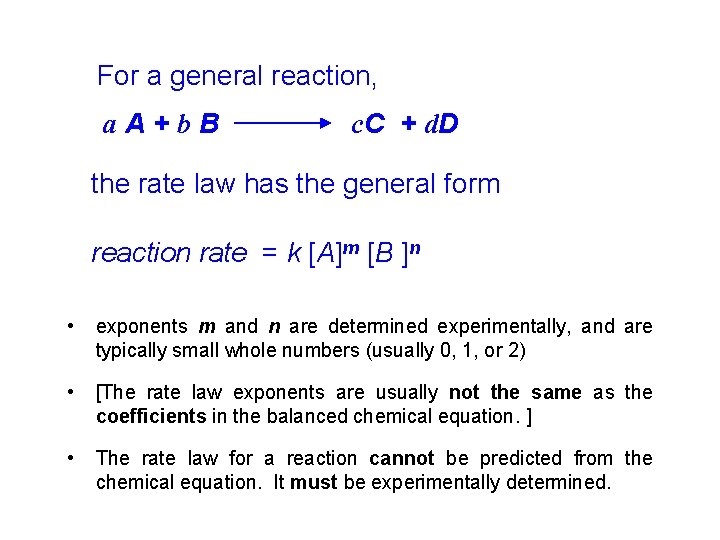
Concentration and Rate This equation is called the rate law, and k is the rate constant.

Reaction orders: The exponents in the rate law The rate laws for most reactions have the general form Rate = k[reactant 1]m[reactant 2]n ……… The exponents m and n in the rate law are called reaction orders with respect to each reactant • This reaction is First-order in [NH 4+] First-order in [NO 2−] • The overall reaction order can be found by adding (1+1=2) the exponents on the reactants in the rate law. • This reaction is second-order overall.
![Example Consider a reaction A B Cfor which rate kAB2 Each of the Example: Consider a reaction A + B Cfor which rate =k[A][B]2. Each of the](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-14.jpg)
Example: Consider a reaction A + B Cfor which rate =k[A][B]2. Each of the following boxes represents a reaction mixtures in which A is shown as red spheres and B as purple ones. Rank these mixtures in order of increasing rate of reaction. (1) (2) (3) rate =k[A][B]2 Box 1: rate = k(5)(5)2 =125 k Box 2: rate = k(7)(3)2 =63 k Box 3: rate = k(3)(7)2 =147 k Rate in order: box 2 < box 1 < box 3

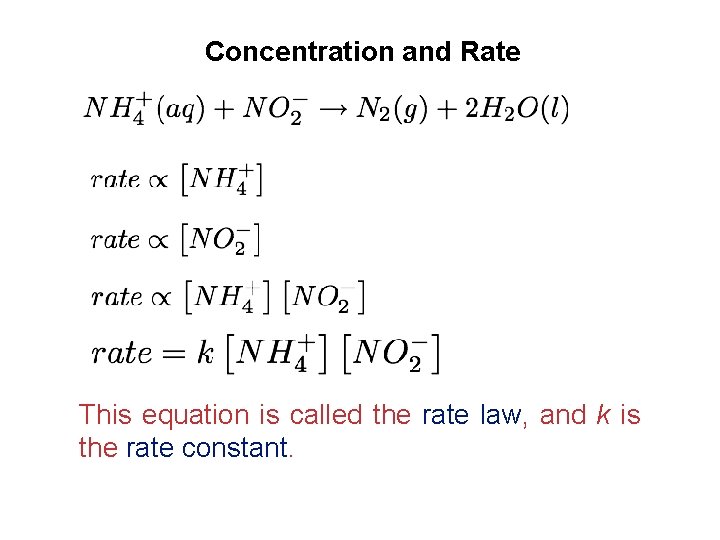
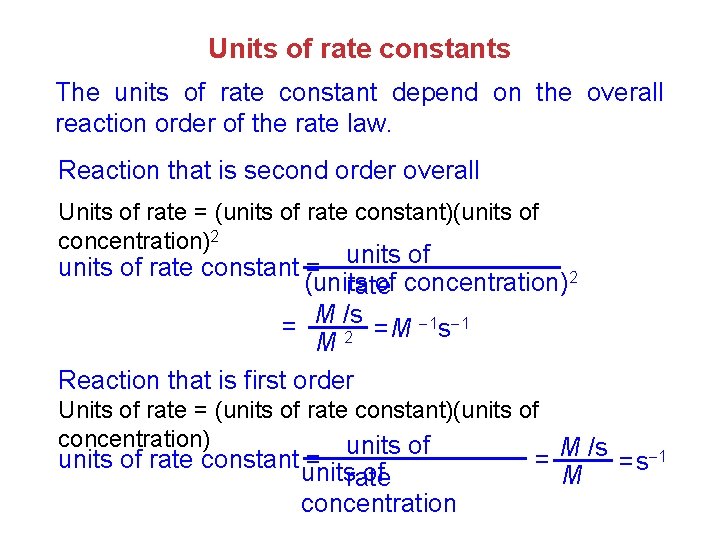
Units of rate constants The units of rate constant depend on the overall reaction order of the rate law. Reaction that is second order overall Units of rate = (units of rate constant)(units of concentration)2 units of rate constant = (units of concentration)2 rate = M /s =M 1 s 1 2 M Reaction that is first order Units of rate = (units of rate constant)(units of concentration) units of = M /s units of rate constant = unitsrate of concentration M =s 1

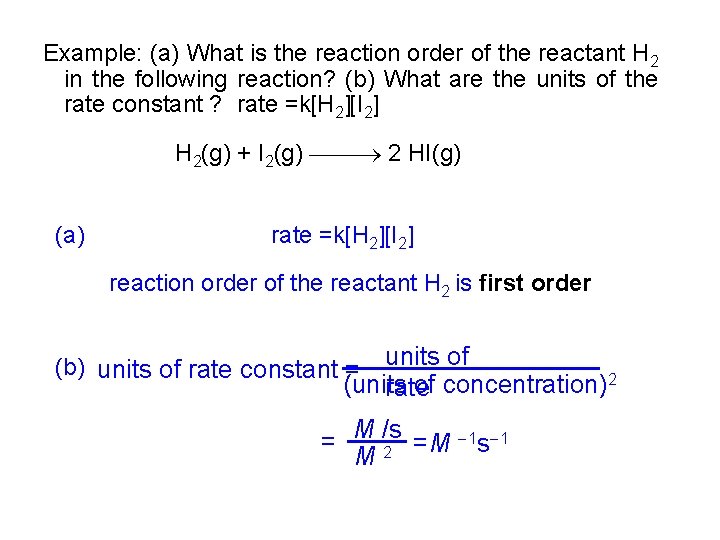
Example: (a) What is the reaction order of the reactant H 2 in the following reaction? (b) What are the units of the rate constant ? rate =k[H 2][I 2] H 2(g) + I 2(g) 2 HI(g) (a) rate =k[H 2][I 2] reaction order of the reactant H 2 is first order units of (units of concentration)2 rate (b) units of rate constant = 1 s 1 = M /s =M M 2

Using initial rates to determine rate laws The rate laws for most reactions have the general form = k[reactant 1]m[reactant 2]n Rate ……… The exponents m and n in the rate law are called reaction orders with respect to each reactant In most reactions the reaction orders are 0, 1 or 2 v If a reaction is zero order in a particular reactant, changing its concentration will have no effect on rate v When a reaction is first order in a reactant, changes in the concentration of that reactant will produce proportional changes in the rate v When a reaction is second order in a reactant, doubling or tripling its concentration increases the rate by a factor 22=4 or 32=9

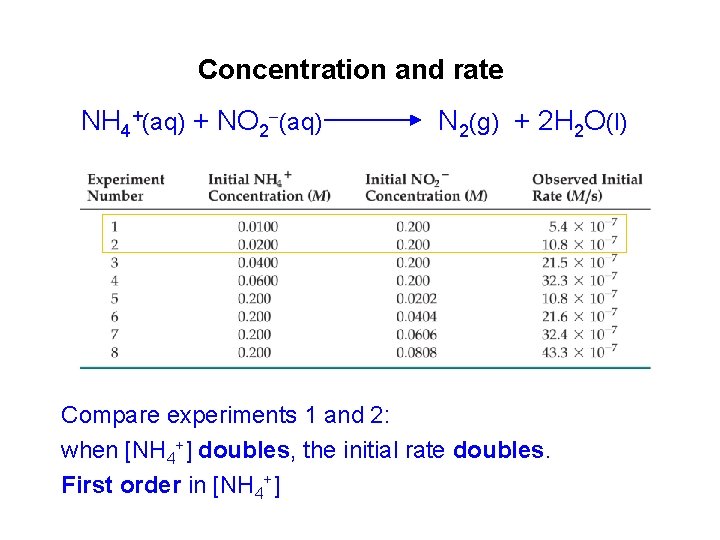
Concentration and rate NH 4+(aq) + NO 2 (aq) N 2(g) + 2 H 2 O(l) Compare experiments 1 and 2: when [NH 4+] doubles, the initial rate doubles. First order in [NH 4+]

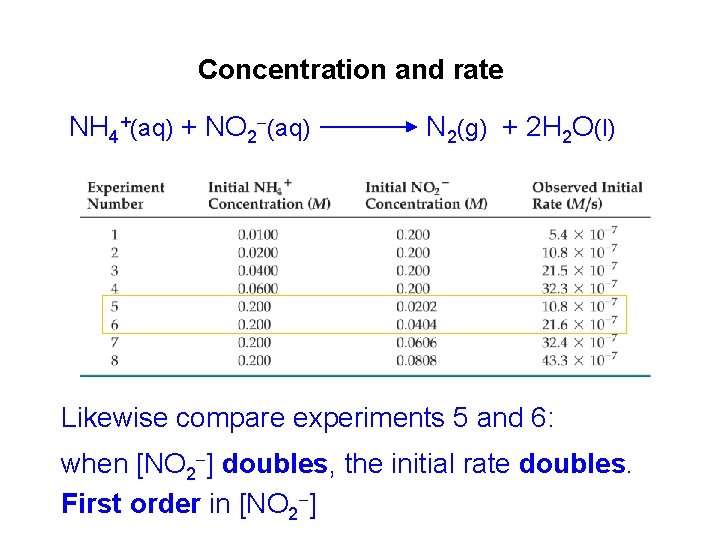
Concentration and rate NH 4+(aq) + NO 2 (aq) N 2(g) + 2 H 2 O(l) Likewise compare experiments 5 and 6: when [NO 2 ] doubles, the initial rate doubles. First order in [NO 2 ]

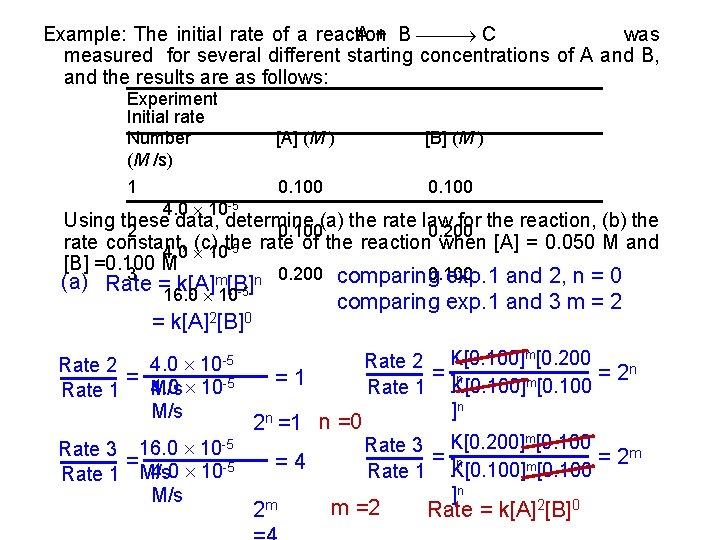
Example: The initial rate of a reaction was A + B C measured for several different starting concentrations of A and B, and the results are as follows: Experiment Initial rate Number (M /s) [A] (M ) [B] (M ) 1 0. 100 4. 0 10 -5 Using these data, determine (a) the rate law for the reaction, (b) the 2 0. 100 0. 200 rate constant, (c) the rate of the reaction when [A] = 0. 050 M and 4. 0 10 -5 [B] =0. 100 M 3 0. 200 0. 100 comparing exp. 1 and 2, n = 0 comparing exp. 1 and 3 m = 2 n (a) Rate = k[A]m[B] -5 16. 0 10 = k[A]2[B]0 4. 0 10 -5 Rate 2 = M/s 4. 0 10 -5 Rate 1 M/s Rate 3 16. 0 10 -5 = 4. 0 10 -5 Rate 1 M/s =1 2 n =1 n =0 =4 2 m Rate 2 K[0. 100]m[0. 200 = ]n = 2 n m Rate 1 K[0. 100] [0. 100 ]n Rate 3 K[0. 200]m[0. 100 = ]n = 2 m m Rate 1 K[0. 100] [0. 100 ]n 2 0 m =2 Rate = k[A] [B]
![b Rate kA2B0 rate K A2 5 4 0 10 0 100 (b) Rate = k[A]2[B]0 rate K= [A]2 -5 4. 0 10 = [0. 100](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-21.jpg)
(b) Rate = k[A]2[B]0 rate K= [A]2 -5 4. 0 10 = [0. 100 M]2 M/s = 4. 0 10 -3 M-1 s 1 (c) Rate = k[A]2[B]0 = (4. 0 10 -3 M-1 s-1)(0. 050 M)2 = 1. 0 10 -5 M/s

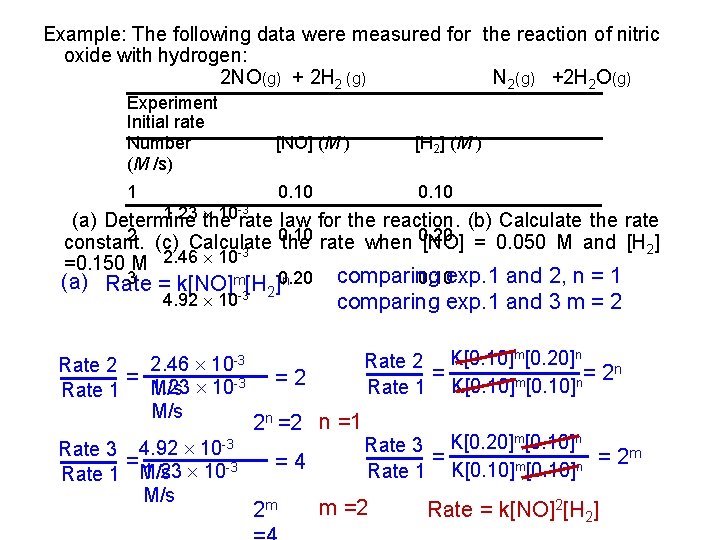
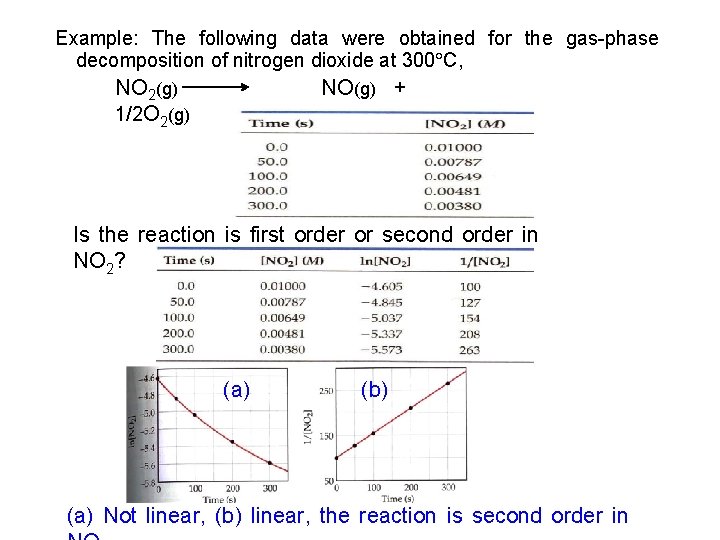
Example: The following data were measured for the reaction of nitric oxide with hydrogen: 2 NO(g) + 2 H 2 (g) N 2(g) +2 H 2 O(g) Experiment Initial rate Number (M /s) [NO] (M ) [H 2] (M ) 1 0. 10 1. 23 10 rate law for the reaction. (b) Calculate the rate (a) Determine the 2 (c) Calculate 0. 10 0. 20 constant. the rate when [NO] = 0. 050 M and [H 2] -3 2. 46 10 =0. 150 M -3 3 = k[NO]m[H ]0. 20 n (a) Rate 2 -3 4. 92 10 2. 46 10 -3 Rate 2 = M/s 1. 23 10 -3 Rate 1 M/s Rate 3 4. 92 10 -3 = 1. 23 10 -3 Rate 1 M/s comparing 0. 10 exp. 1 and 2, n = 1 comparing exp. 1 and 3 m = 2 Rate 2 K[0. 10]m[0. 20]n = = 2 n m n Rate 1 K[0. 10] =2 2 n =2 n =1 =4 2 m Rate 3 K[0. 20]m[0. 10]n = = 2 m m n Rate 1 K[0. 10] m =2 Rate = k[NO]2[H 2]
![b Rate kNO2H 2 rate K NO2H 2 3 1 23 10 (b) Rate = k[NO]2[H 2] rate K= [NO]2[H 2] -3 1. 23 10 =](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-23.jpg)
(b) Rate = k[NO]2[H 2] rate K= [NO]2[H 2] -3 1. 23 10 = [0. 10 M]2[0. 10 M/s M] = 1. 2 M-2 s -1 (c) Rate = k[NO]2[H 2] = (1. 2 M-2 s-1)(0. 050 M)2(0. 150 M) = 4. 5 10 -4 M/s

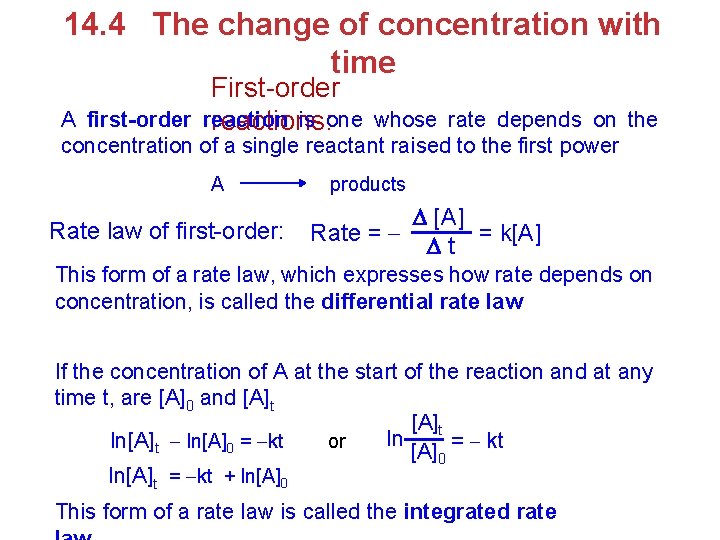
14. 4 The change of concentration with time First-order reaction is one reactions: A first-order whose rate depends on the concentration of a single reactant raised to the first power A Rate law of first-order: products [A] Rate = = k[A] t This form of a rate law, which expresses how rate depends on concentration, is called the differential rate law If the concentration of A at the start of the reaction and at any time t, are [A]0 and [A]t ln = kt ln[A]t ln[A]0 = kt or [A]0 ln[A]t = kt + ln[A]0 This form of a rate law is called the integrated rate
![lnAt kt lnA0 This equation has the form of the general equation ln[A]t = kt + ln[A]0 This equation has the form of the general equation](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-25.jpg)
ln[A]t = kt + ln[A]0 This equation has the form of the general equation for a straight line, y +b We can use pressure as a unit of ln[A] = k. t =+mx ln[A] t 0 concentration for a gas because from the ideal-gas law the pressure is directly proportion to the number of moles per unit volume therefore, a graph of ln[A] versus y = m. x + b For a first-order reaction, t time gives a straight line with a slope of –k and y-intercept of ln[A]0. A reaction that is not first order will not yield a straight line (b) (a) Variation in the partial pressure of methyl isonitrile (CH 3 NC) with time during the reaction CH 3 NC CH 3 CN (b) A plat of natural logarithm of the CH 3 NC pressure as a function of time

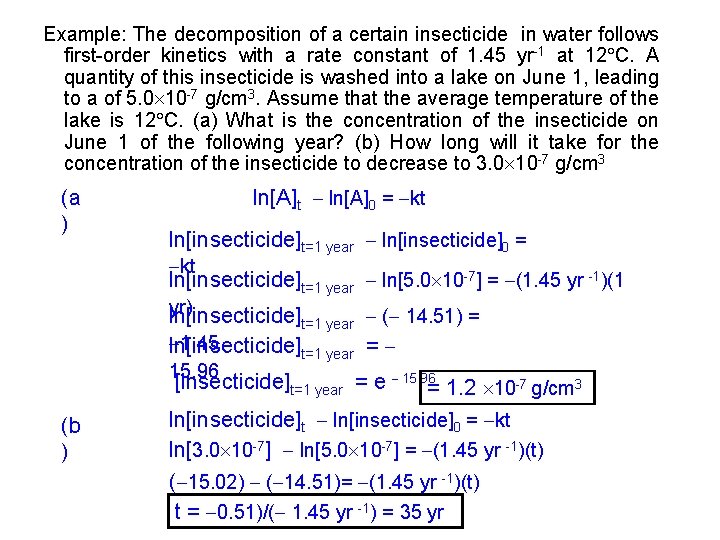
Example: The decomposition of a certain insecticide in water follows first-order kinetics with a rate constant of 1. 45 yr-1 at 12 C. A quantity of this insecticide is washed into a lake on June 1, leading to a of 5. 0 10 -7 g/cm 3. Assume that the average temperature of the lake is 12 C. (a) What is the concentration of the insecticide on June 1 of the following year? (b) How long will it take for the concentration of the insecticide to decrease to 3. 0 10 -7 g/cm 3 (a ) ln[A]t ln[A]0 = kt ln[insecticide]t=1 year ln[insecticide]0 = kt ln[insecticide]t=1 year ln[5. 0 10 -7] = (1. 45 yr -1)(1 yr) ln[insecticide] t=1 year 1. 45 ln[insecticide]t=1 year 15. 96 ( 14. 51) = = [insecticide]t=1 year = e 15. 96= 1. 2 10 -7 g/cm 3 (b ) ln[insecticide]t ln[insecticide]0 = kt ln[3. 0 10 -7] ln[5. 0 10 -7] = (1. 45 yr -1)(t) ( 15. 02) ( 14. 51)= (1. 45 yr -1)(t) t = 0. 51)/( 1. 45 yr -1) = 35 yr

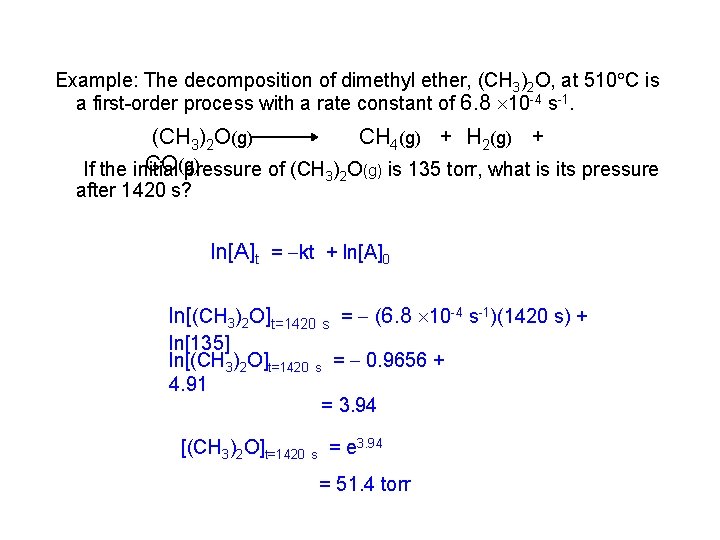
Example: The decomposition of dimethyl ether, (CH 3)2 O, at 510 C is a first-order process with a rate constant of 6. 8 10 -4 s-1. (CH 3)2 O(g) CH 4(g) + H 2(g) + CO(g) If the initial pressure of (CH 3)2 O(g) is 135 torr, what is its pressure after 1420 s? ln[A]t = kt + ln[A]0 ln[(CH 3)2 O]t=1420 s = (6. 8 10 -4 s-1)(1420 s) + ln[135] ln[(CH 3)2 O]t=1420 s = 0. 9656 + 4. 91 = 3. 94 [(CH 3)2 O]t=1420 s = e 3. 94 = 51. 4 torr

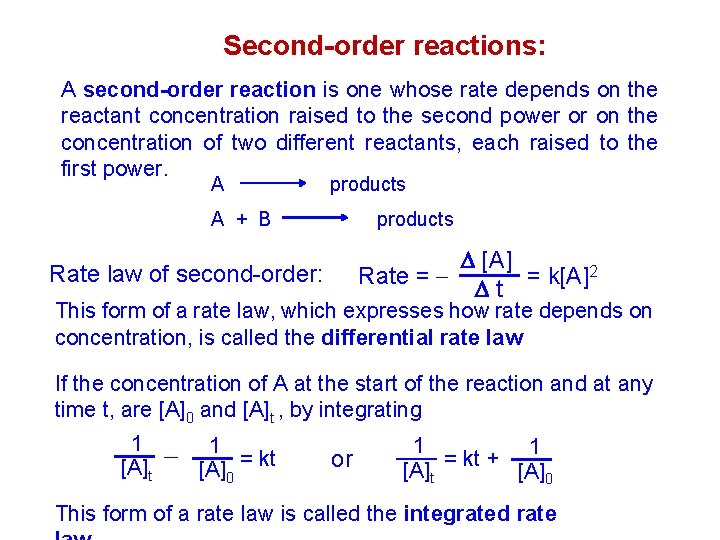
Second-order reactions: A second-order reaction is one whose rate depends on the reactant concentration raised to the second power or on the concentration of two different reactants, each raised to the first power. A products A + B products [A] Rate = = k[A]2 t Rate law of second-order: This form of a rate law, which expresses how rate depends on concentration, is called the differential rate law If the concentration of A at the start of the reaction and at any time t, are [A]0 and [A]t , by integrating 1 [A]t 1 = kt [A]0 or 1 1 = kt + [A]t [A]0 This form of a rate law is called the integrated rate
![1 At k t y m x b 1 A0 1 [A]t = k. t + y = m. x + b 1 [A]0](https://slidetodoc.com/presentation_image/482688021dbc1e37d38c843ab9c227b1/image-29.jpg)
1 [A]t = k. t + y = m. x + b 1 [A]0 For a second-order reaction, a graph of 1/[A]t versus time gives a straight line with a slope equal to k and y -intercept of 1/[A]0. v One way to distinguish between first- and secondorder rate laws is to graph both ln[A]t and 1/[A]t against t. If the ln[A]t ploet is linear, the reactions is first order, if the 1/[A]t plot is linear, the reaction is second order.

Example: The following data were obtained for the gas-phase decomposition of nitrogen dioxide at 300 C, NO 2(g) 1/2 O 2(g) NO(g) + Is the reaction is first order or second order in NO 2? (a) (b) (a) Not linear, (b) linear, the reaction is second order in

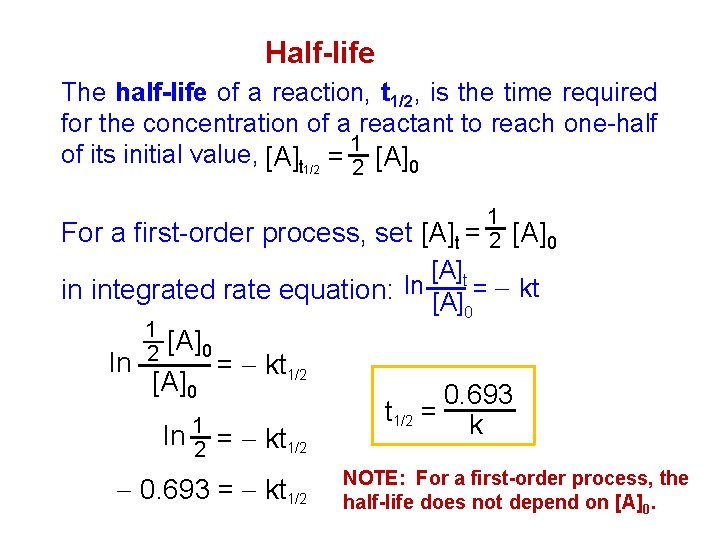
Half-life The half-life of a reaction, t 1/2, is the time required for the concentration of a reactant to reach one-half of its initial value, [A]t 1/2 = 12 [A]0 For a first-order process, set 1 [A]t = 2 [A]0 [A]t in integrated rate equation: ln [A] = kt 0 1 2 [A]0 ln = kt 1/2 [A]0 ln 12 = kt 1/2 0. 693 = kt 1/2 0. 693 t 1/2 = k NOTE: For a first-order process, the half-life does not depend on [A]0.

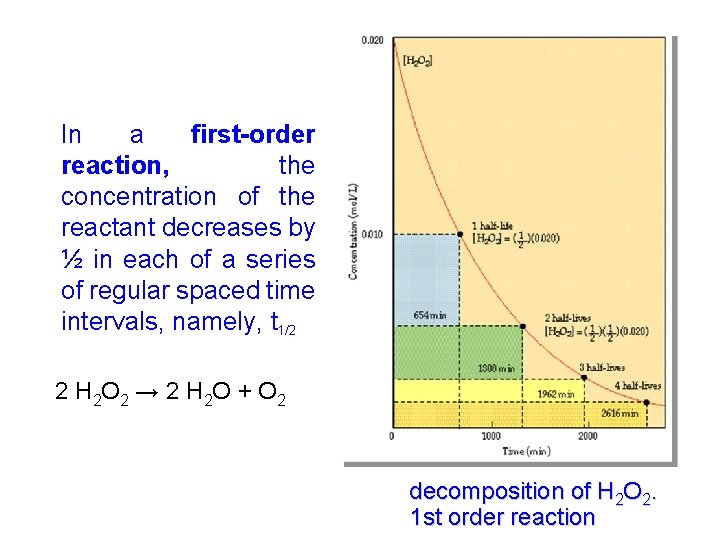
In a first-order reaction, the concentration of the reactant decreases by ½ in each of a series of regular spaced time intervals, namely, t 1/2 2 H 2 O 2 → 2 H 2 O + O 2 decomposition of H 2 O 2. 1 st order reaction

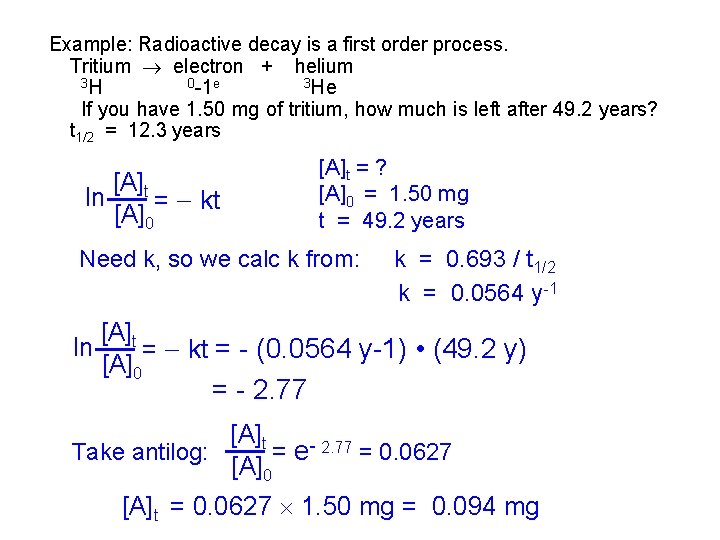
Example: Radioactive decay is a first order process. Tritium electron + helium 3 H 0 -1 e 3 He If you have 1. 50 mg of tritium, how much is left after 49. 2 years? t 1/2 = 12. 3 years [A]t ln = kt [A]0 [A]t = ? [A]0 = 1. 50 mg t = 49. 2 years Need k, so we calc k from: ln k = 0. 693 / t 1/2 k = 0. 0564 y -1 [A]t = kt = - (0. 0564 y-1) • (49. 2 y) [A]0 = - 2. 77 [A]t Take antilog: = e- 2. 77 = 0. 0627 [A]0 [A]t = 0. 0627 1. 50 mg = 0. 094 mg

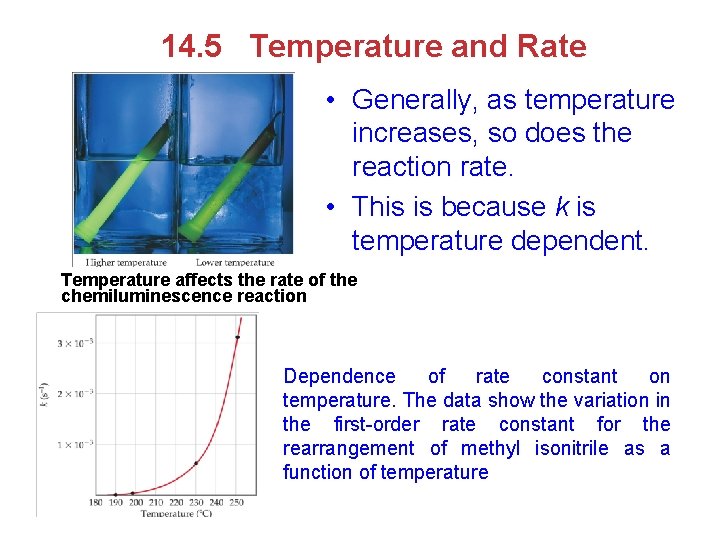
14. 5 Temperature and Rate • Generally, as temperature increases, so does the reaction rate. • This is because k is temperature dependent. Temperature affects the rate of the chemiluminescence reaction Dependence of rate constant on temperature. The data show the variation in the first-order rate constant for the rearrangement of methyl isonitrile as a function of temperature

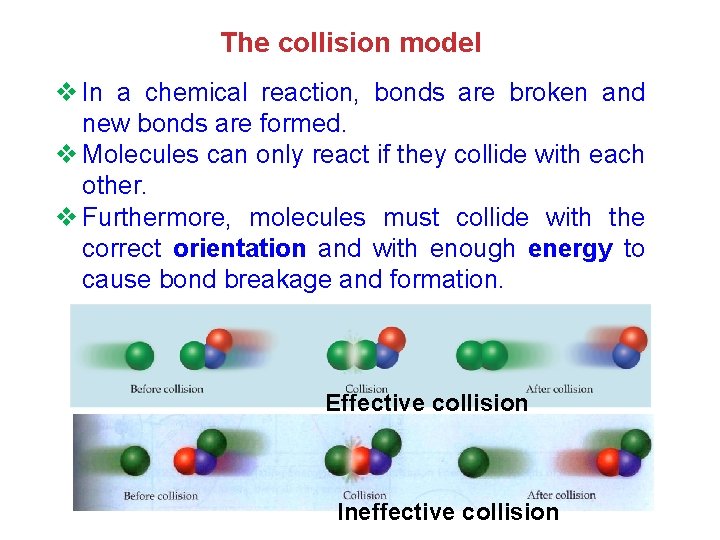
The collision model v In a chemical reaction, bonds are broken and new bonds are formed. v Molecules can only react if they collide with each other. v Furthermore, molecules must collide with the correct orientation and with enough energy to cause bond breakage and formation. Effective collision Ineffective collision

Activation energy v The minimum energy required to initiate a chemical reaction is called the activation energy, Ea. v The value of Ea varies from reaction to reaction v Just as a ball cannot get over a hill if it does not roll up the hill with enough energy, a reaction cannot occur unless the molecules possess sufficient energy to get over the activation energy barrier.

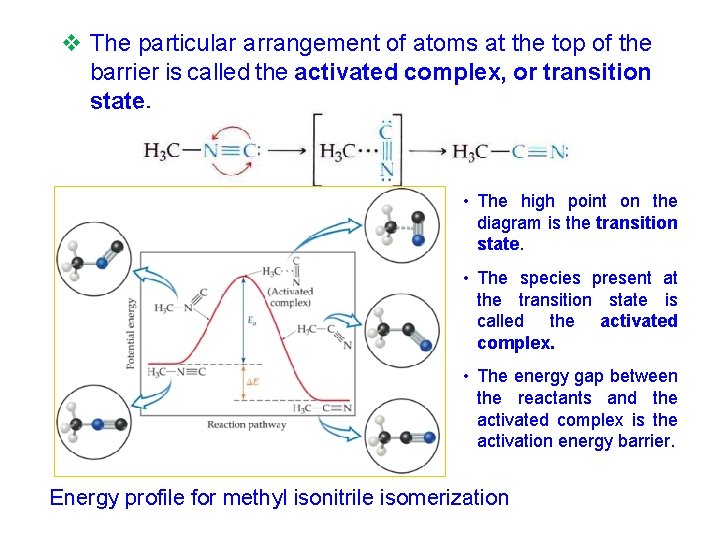
v The particular arrangement of atoms at the top of the barrier is called the activated complex, or transition state. • The high point on the diagram is the transition state. • The species present at the transition state is called the activated complex. • The energy gap between the reactants and the activated complex is the activation energy barrier. Energy profile for methyl isonitrile isomerization

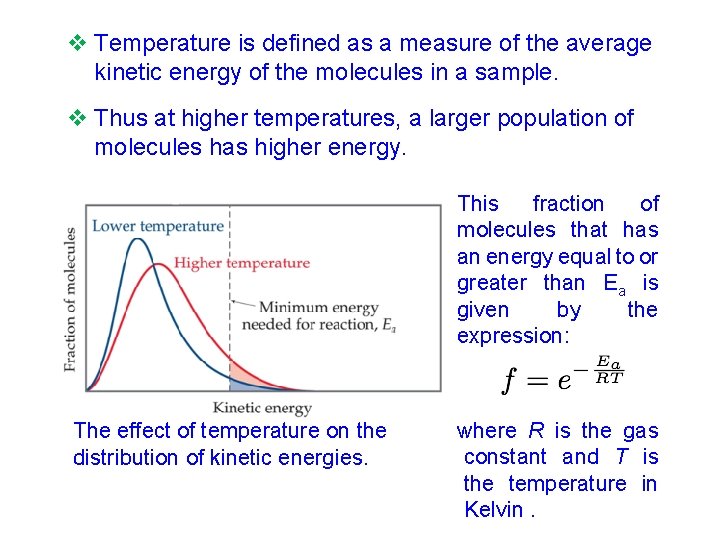
v Temperature is defined as a measure of the average kinetic energy of the molecules in a sample. v Thus at higher temperatures, a larger population of molecules has higher energy. This fraction of molecules that has an energy equal to or greater than Ea is given by the expression: The effect of temperature on the distribution of kinetic energies. where R is the gas constant and T is the temperature in Kelvin.

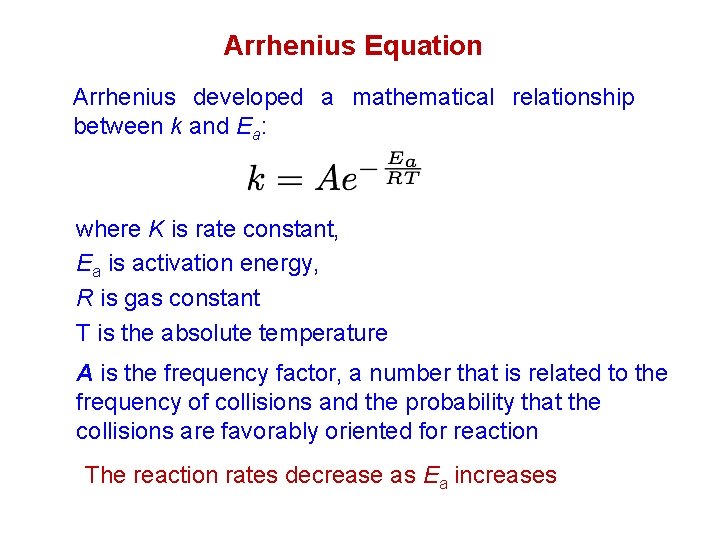
Arrhenius Equation Arrhenius developed a mathematical relationship between k and Ea: where K is rate constant, Ea is activation energy, R is gas constant T is the absolute temperature A is the frequency factor, a number that is related to the frequency of collisions and the probability that the collisions are favorably oriented for reaction The reaction rates decrease as Ea increases

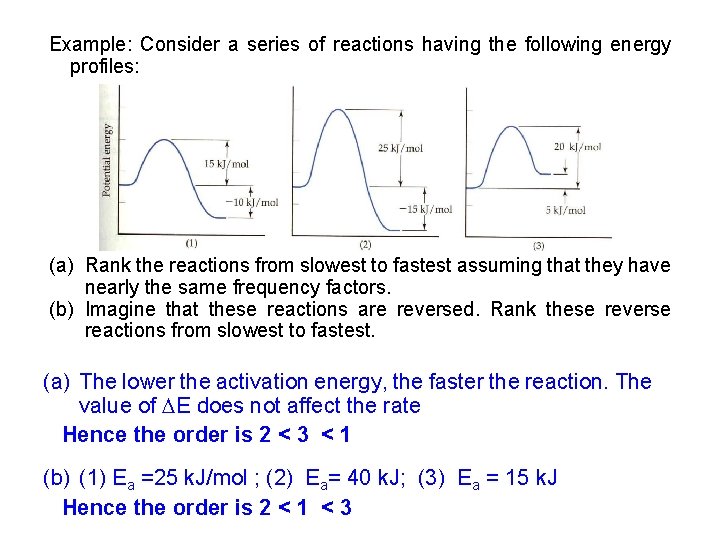
Example: Consider a series of reactions having the following energy profiles: (a) Rank the reactions from slowest to fastest assuming that they have nearly the same frequency factors. (b) Imagine that these reactions are reversed. Rank these reverse reactions from slowest to fastest. (a) The lower the activation energy, the faster the reaction. The value of E does not affect the rate Hence the order is 2 < 3 < 1 (b) (1) Ea =25 k. J/mol ; (2) Ea= 40 k. J; (3) Ea = 15 k. J Hence the order is 2 < 1 < 3

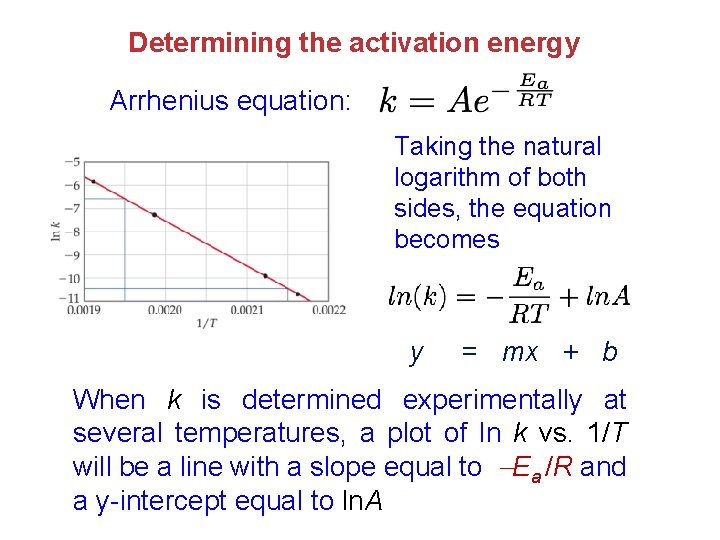
Determining the activation energy Arrhenius equation: Taking the natural logarithm of both sides, the equation becomes y = mx + b When k is determined experimentally at several temperatures, a plot of ln k vs. 1/T will be a line with a slope equal to Ea /R and a y-intercept equal to ln. A

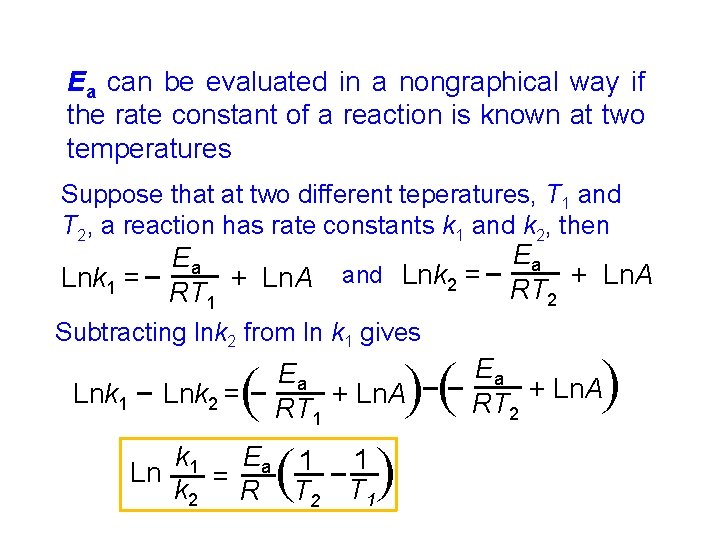
Ea can be evaluated in a nongraphical way if the rate constant of a reaction is known at two temperatures Suppose that at two different teperatures, T 1 and T 2, a reaction has rate constants k 1 and k 2, then and Lnk 2 = Subtracting lnk 2 from ln k 1 gives ( ( k 1 Ea 1 Ln k 2 = R T 2 1 T 1 ( Lnk 2 = ( Lnk 1 Ea + Ln. A RT 1 ( Ea + Ln. A RT 2 ( Lnk 1 = Ea + Ln. A RT 1

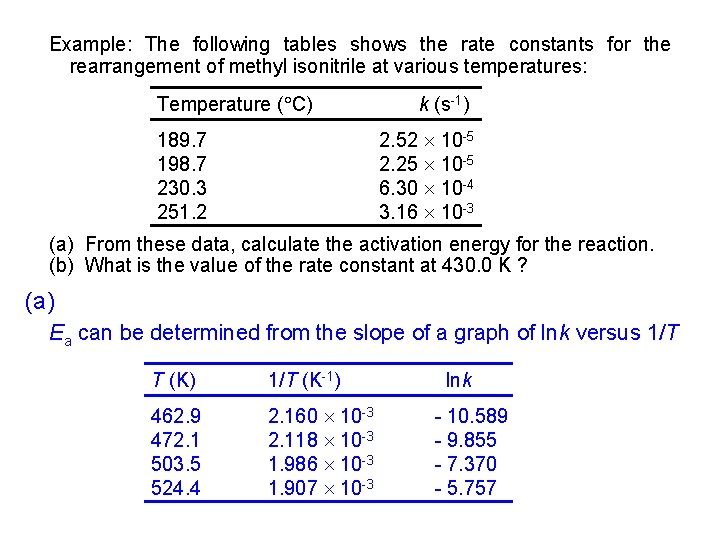
Example: The following tables shows the rate constants for the rearrangement of methyl isonitrile at various temperatures: Temperature ( C) 189. 7 198. 7 230. 3 251. 2 k (s-1) 2. 52 2. 25 6. 30 3. 16 10 -5 10 -4 10 -3 (a) From these data, calculate the activation energy for the reaction. (b) What is the value of the rate constant at 430. 0 K ? (a) Ea can be determined from the slope of a graph of lnk versus 1/T T (K) 1/T (K-1) 462. 9 472. 1 503. 5 524. 4 2. 160 10 -3 2. 118 10 -3 1. 986 10 -3 1. 907 10 -3 lnk - 10. 589 - 9. 855 - 7. 370 - 5. 757

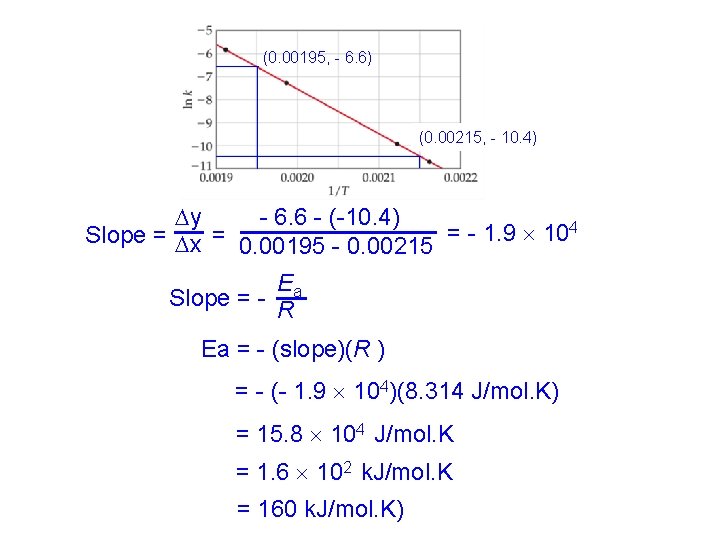
(0. 00195, - 6. 6) (0. 00215, - 10. 4) - 6. 6 - (-10. 4) y Slope = x = 0. 00195 - 0. 00215 = - 1. 9 104 Ea Slope = - R Ea = - (slope)(R ) = - (- 1. 9 104)(8. 314 J/mol. K) = 15. 8 104 J/mol. K = 1. 6 102 k. J/mol. K = 160 k. J/mol. K)
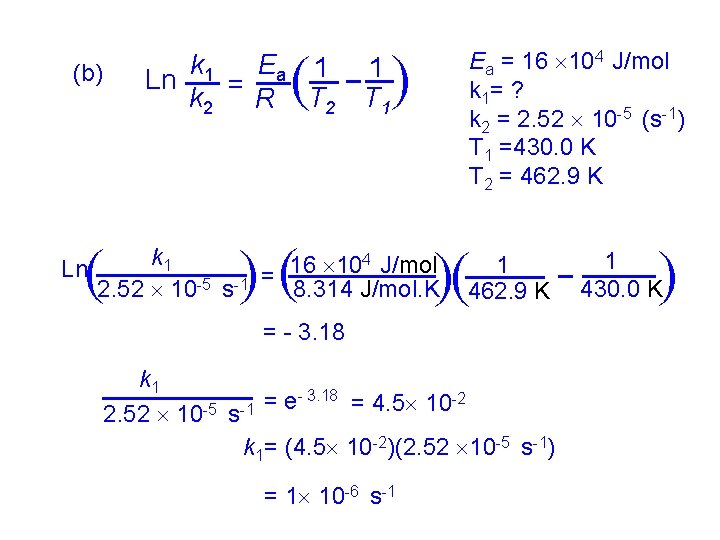
( ( 4 J/mol k 1 16 10 = 8. 314 J/mol. K 2. 52 10 -5 s-1 ( = - 3. 18 ( ( Ln 1 T 1 1 462. 9 K k 1 = e- 3. 18 = 4. 5 10 -2 -5 -1 2. 52 10 s k 1= (4. 5 10 -2)(2. 52 10 -5 s-1) = 1 10 -6 s-1 1 430. 0 K ( (b) Ea = 16 104 J/mol k 1= ? k 2 = 2. 52 10 -5 (s-1) T 1 =430. 0 K T 2 = 462. 9 K ( ( k 1 Ea 1 Ln k 2 = R T 2

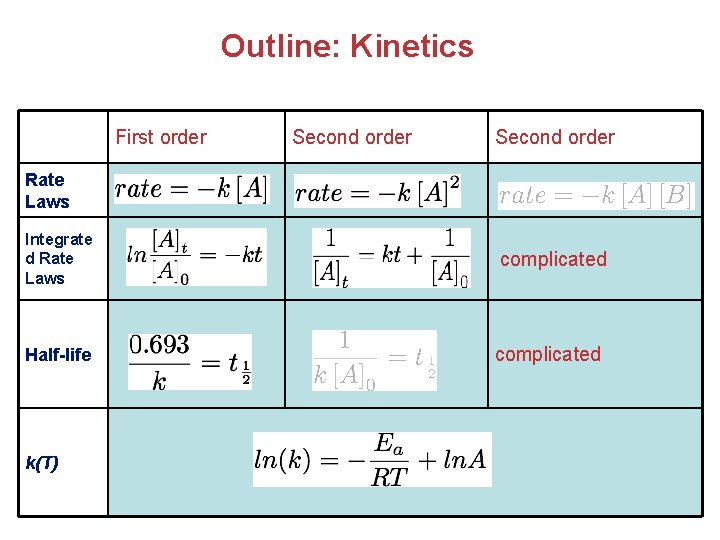
Outline: Kinetics First order Second order Rate Laws Integrate d Rate Laws Half-life k(T) complicated
 Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Types of reactions grade 11
Types of reactions grade 11 Half life chemical kinetics
Half life chemical kinetics Ap chemistry kinetics
Ap chemistry kinetics Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state
Steady state Phân độ lown
Phân độ lown Premature atrial contraction
Premature atrial contraction Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Kinetics and equilibrium
Kinetics and equilibrium Ion trapping
Ion trapping Planar kinetics of a rigid body
Planar kinetics of a rigid body Difference between 1st order and zero order kinetics
Difference between 1st order and zero order kinetics Metaboloism
Metaboloism Collision theory of kinetics
Collision theory of kinetics Kinetics flotation reagents
Kinetics flotation reagents Kinetics of rigid bodies
Kinetics of rigid bodies Kinetics of rigid body
Kinetics of rigid body Kinetics of a particle force and acceleration
Kinetics of a particle force and acceleration Kinetics of crystal violet fading
Kinetics of crystal violet fading Octet kinetics
Octet kinetics Km in enzyme kinetics
Km in enzyme kinetics Pseudo 1st order reaction
Pseudo 1st order reaction Kinetics of particles newton's second law
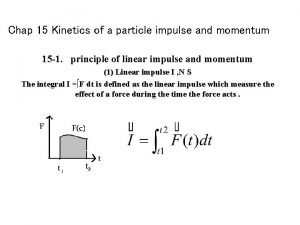
Kinetics of particles newton's second law Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Dynafit kinetics
Dynafit kinetics Enzyme kinetics
Enzyme kinetics Ebevlet
Ebevlet Kinematics and kinetics of rigid bodies
Kinematics and kinetics of rigid bodies Data kinetics ltd
Data kinetics ltd Kcat
Kcat Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Kinetics reaction
Kinetics reaction Growth kinetics
Growth kinetics Kinetics is the branch of:
Kinetics is the branch of: Fermenter
Fermenter