Chapter 4 Section 1 The Development of a











- Slides: 34

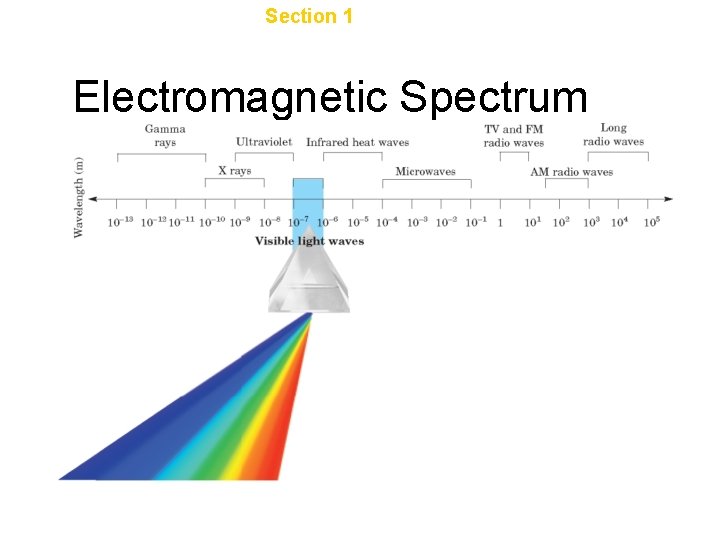
Chapter 4 Section 1 The Development of a New Atomic Model Electromagnetic Spectrum

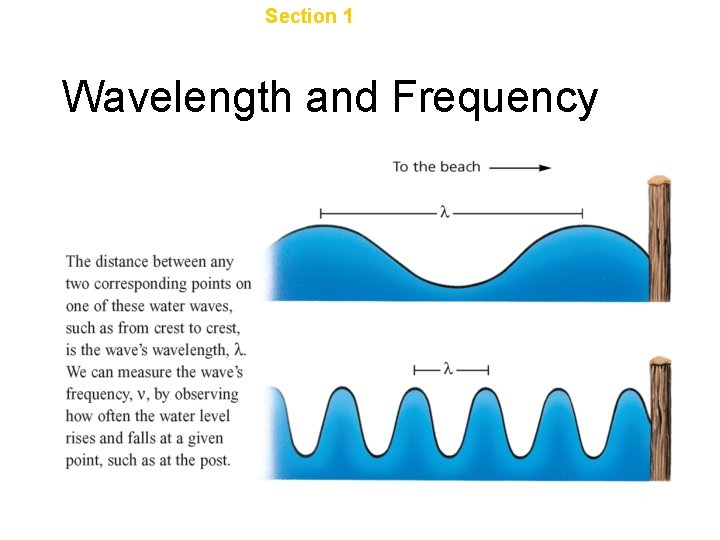
Chapter 4 Section 1 The Development of a New Atomic Model Wavelength and Frequency

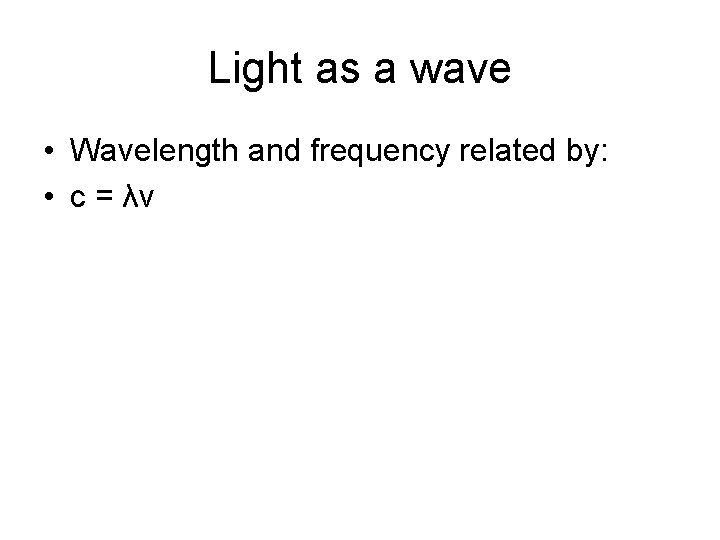
Light as a wave • Wavelength and frequency related by: • c = λv

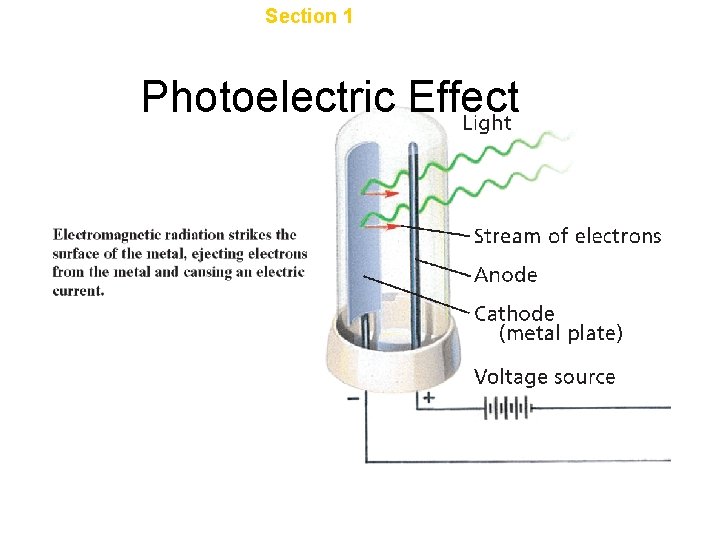
Chapter 4 Section 1 The Development of a New Atomic Model Photoelectric Effect

Light as a particle • • Photon Packet of energy E = hv Can be absorbed and emitted by atoms • Light has dual wave/particle nature

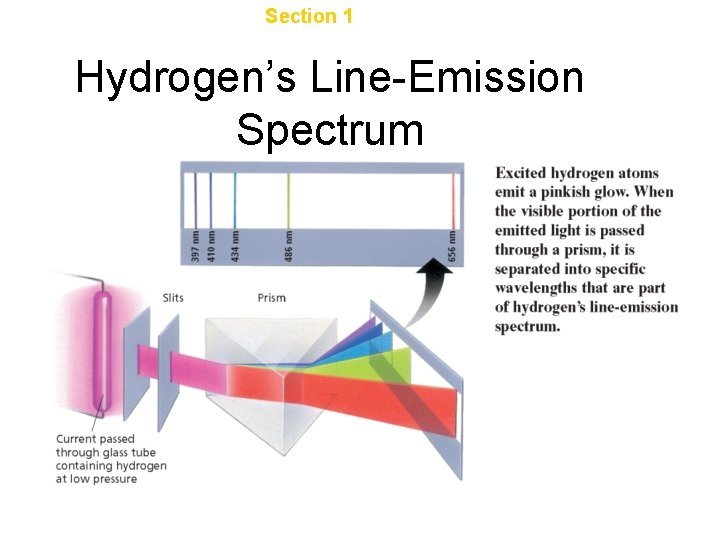
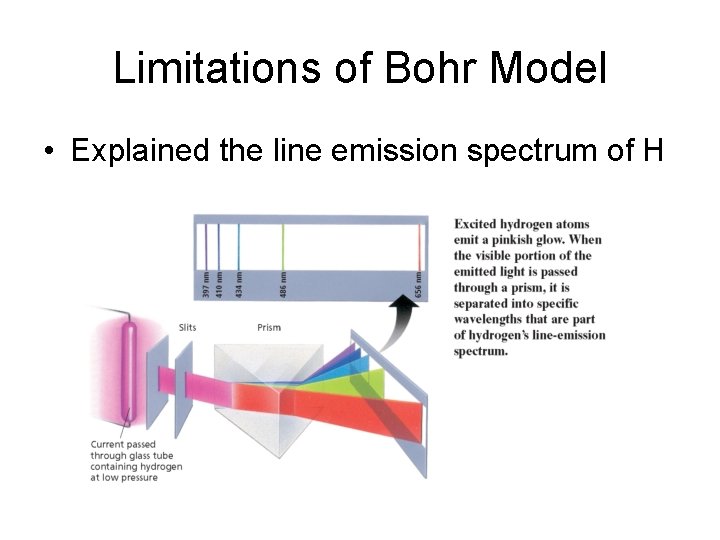
Chapter 4 Section 1 The Development of a New Atomic Model Hydrogen’s Line-Emission Spectrum

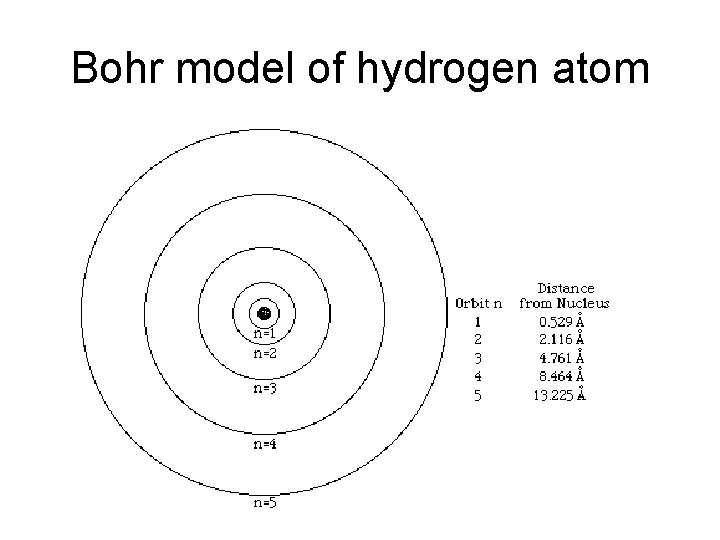
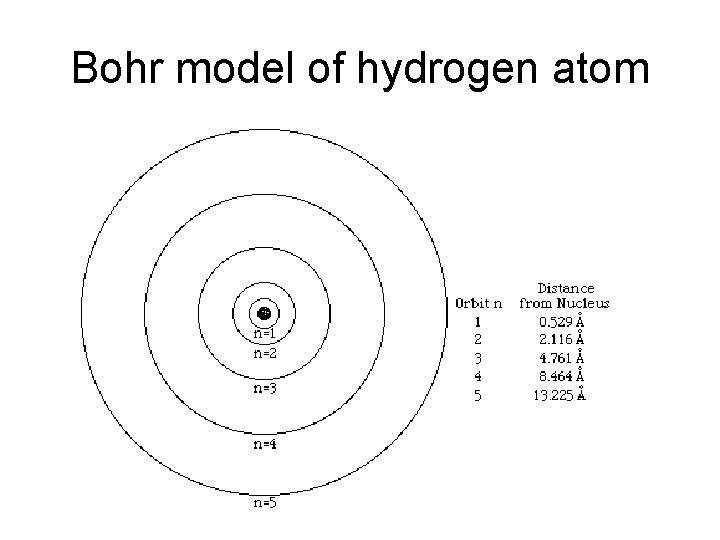
Bohr model of hydrogen atom

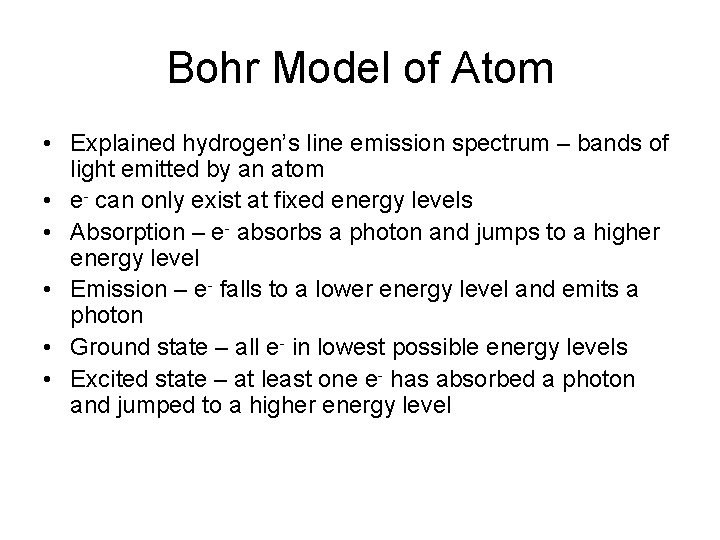
Bohr Model of Atom • Explained hydrogen’s line emission spectrum – bands of light emitted by an atom • e- can only exist at fixed energy levels • Absorption – e- absorbs a photon and jumps to a higher energy level • Emission – e- falls to a lower energy level and emits a photon • Ground state – all e- in lowest possible energy levels • Excited state – at least one e- has absorbed a photon and jumped to a higher energy level

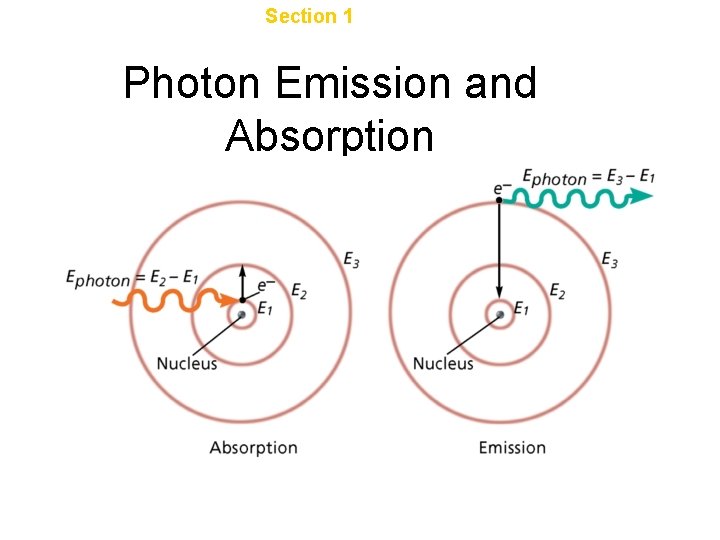
Chapter 4 Section 1 The Development of a New Atomic Model Photon Emission and Absorption

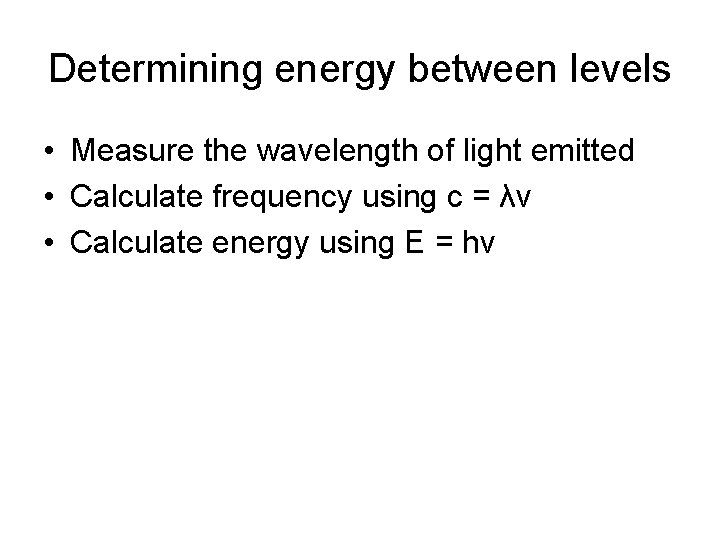
Determining energy between levels • Measure the wavelength of light emitted • Calculate frequency using c = λv • Calculate energy using E = hv

Bohr model of hydrogen atom

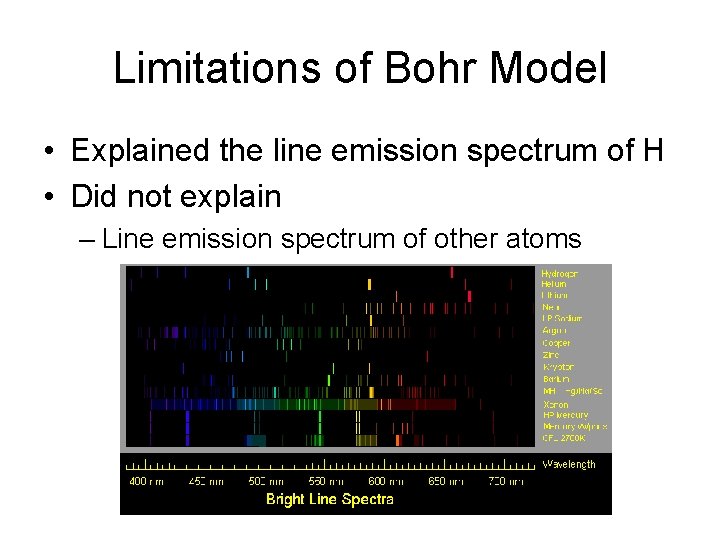
Limitations of Bohr Model

Limitations of Bohr Model • Explained the line emission spectrum of H

Limitations of Bohr Model • Explained the line emission spectrum of H • Did not explain

Limitations of Bohr Model • Explained the line emission spectrum of H • Did not explain – Line emission spectrum of other atoms

Limitations of Bohr Model • Explained the line emission spectrum of H • Did not explain – Line emission spectrum of other atoms – Chemical behavior of atoms

Limitations of Bohr Model • Explained the line emission spectrum of H • Did not explain – Line emission spectrum of other atoms – Chemical behavior of atoms – Why only certain energy levels existed

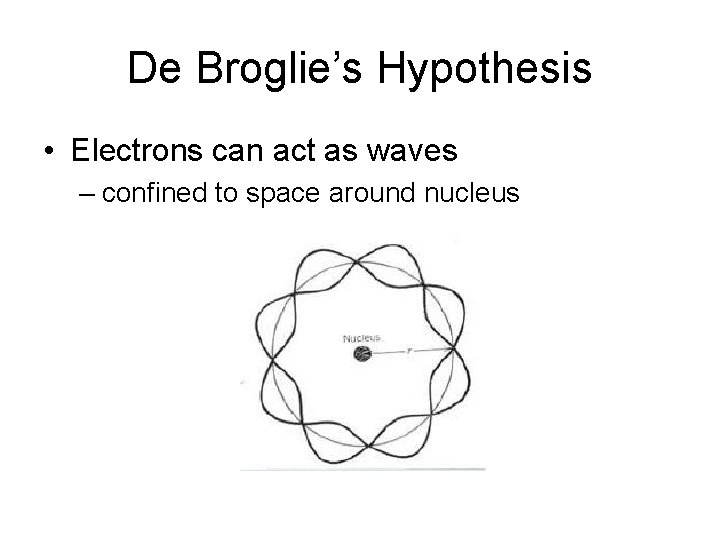
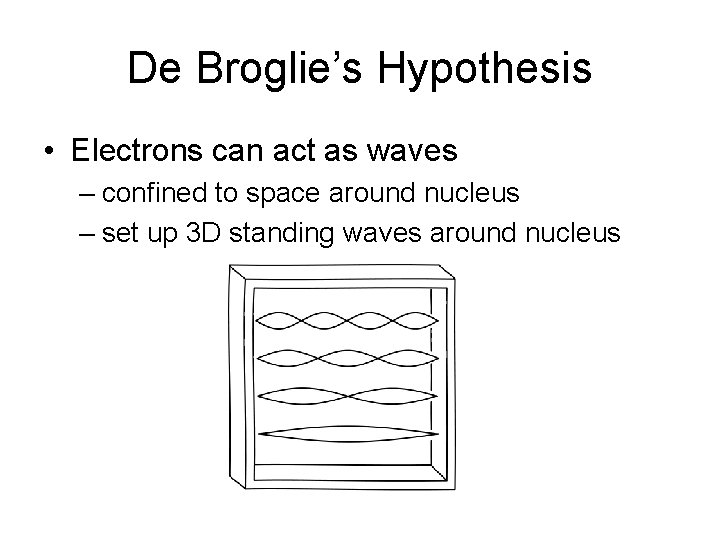
De Broglie’s Hypothesis

De Broglie’s Hypothesis • Electrons can act as waves

De Broglie’s Hypothesis • Electrons can act as waves – confined to space around nucleus

De Broglie’s Hypothesis • Electrons can act as waves – confined to space around nucleus – set up 3 D standing waves around nucleus

De Broglie’s Hypothesis • Electrons can act as waves – confined to space around nucleus – set up 3 D standing waves around nucleus – Only specific frequencies are allowed

De Broglie’s Hypothesis • Electrons can act as waves – confined to space around nucleus – set up 3 D standing waves around nucleus – Only specific frequencies are allowed – And, hence, only certain energy levels

Heisenberg Uncertainty Principle • Impossible to know both the position and velocity of an electron at the same time. • Electrons do not follow fixed paths. • Can only identify a region where an electron might exist.

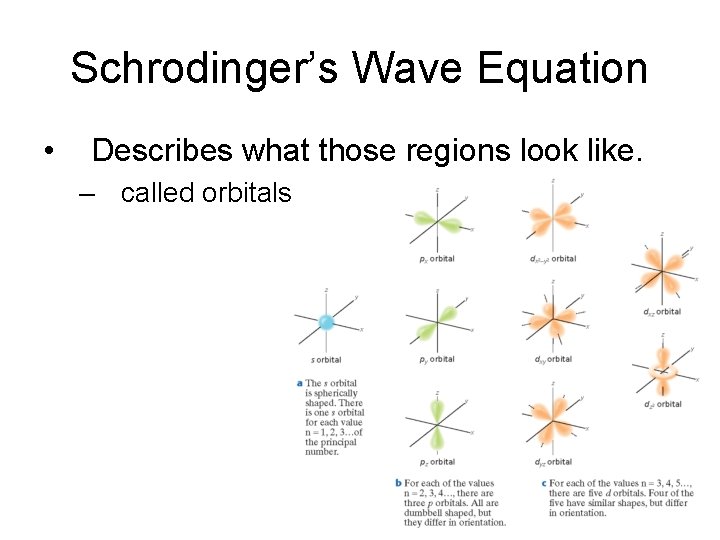
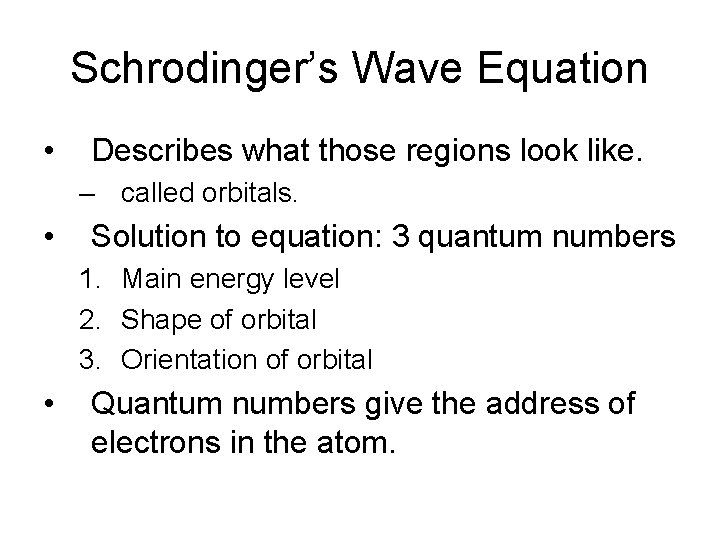
Schrodinger’s Wave Equation

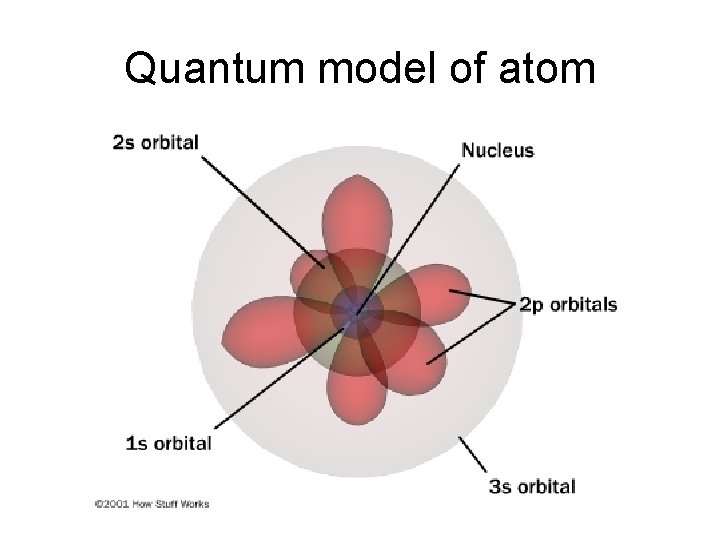
Schrodinger’s Wave Equation • Describes what those regions look like. – called orbitals

Schrodinger’s Wave Equation • Describes what those regions look like. – called orbitals. • Solution to equation: 3 quantum numbers

Schrodinger’s Wave Equation • Describes what those regions look like. – called orbitals. • Solution to equation: 3 quantum numbers 1. Main energy level

Schrodinger’s Wave Equation • Describes what those regions look like. – called orbitals. • Solution to equation: 3 quantum numbers 1. Main energy level 2. Shape of orbital

Schrodinger’s Wave Equation • Describes what those regions look like. – called orbitals. • Solution to equation: 3 quantum numbers 1. Main energy level 2. Shape of orbital 3. Orientation of orbital

Schrodinger’s Wave Equation • Describes what those regions look like. – called orbitals. • Solution to equation: 3 quantum numbers 1. Main energy level 2. Shape of orbital 3. Orientation of orbital • Quantum numbers give the address of electrons in the atom.

Quantum model of atom

Energy levels in the atom are like an upside down pyramid building.

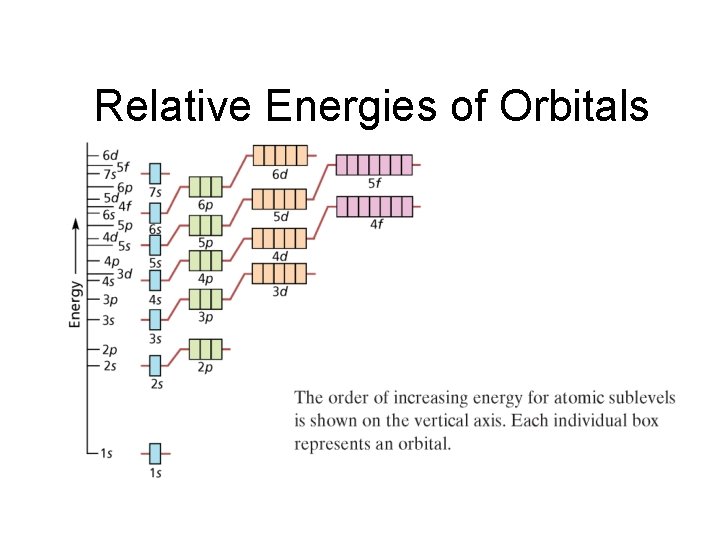
Chapter 4 Relative Energies of Orbitals