Chapter 3 Alkena dan Alkuna Nomenklatur dan Reaksinya










- Slides: 46

Chapter 3. Alkena dan Alkuna: Nomenklatur dan Reaksinya Tutik Dwi Wahyuningsih Jurusan Kimia FMIPA UGM 2011

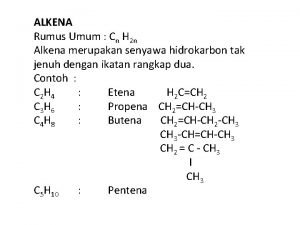
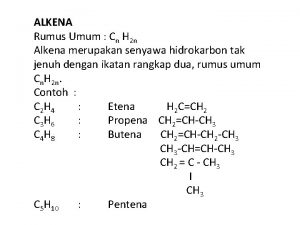
Alkena dan Alkuna Introduction: kegunaan alkena Struktur alkena Nomenklatur Alkena & Alkuna Nomenklatur E/Z Jenis/tipe ikatan rangkap dua Reaksi pada Alkena Adisi Substitusi Diels Alder Pemutusan 2

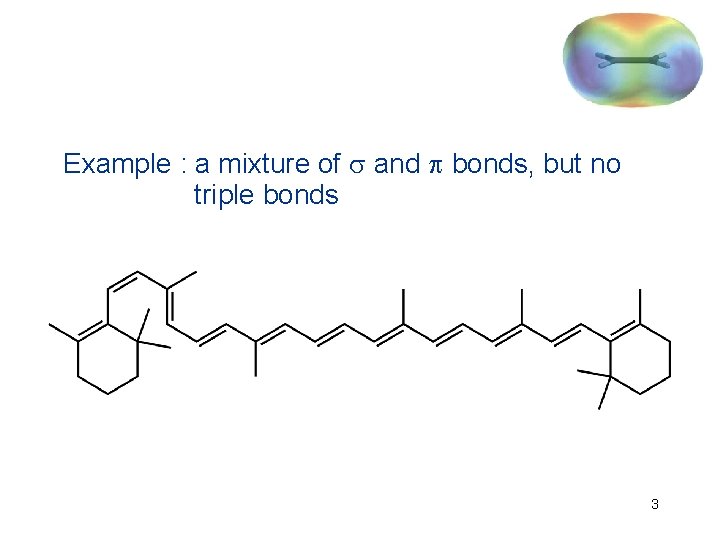
Example : a mixture of and bonds, but no triple bonds 3

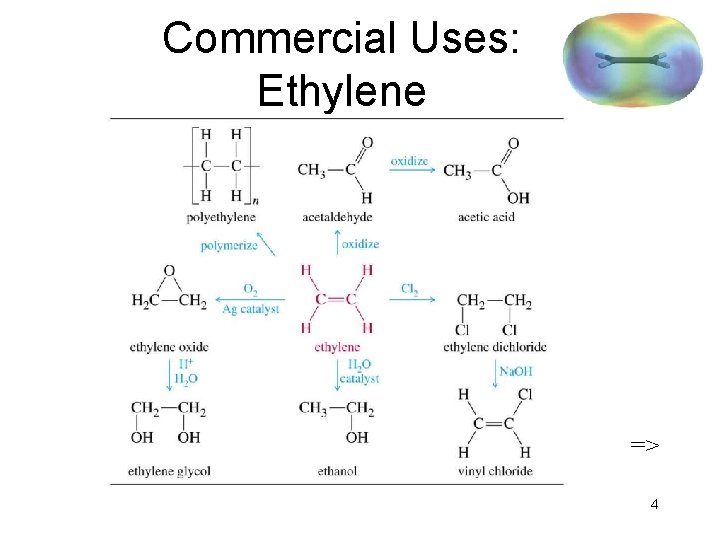
Commercial Uses: Ethylene => 4

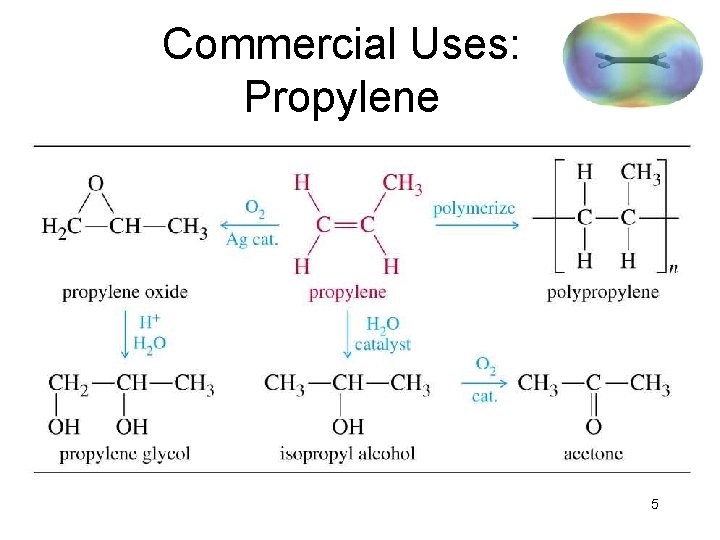
Commercial Uses: Propylene => 5

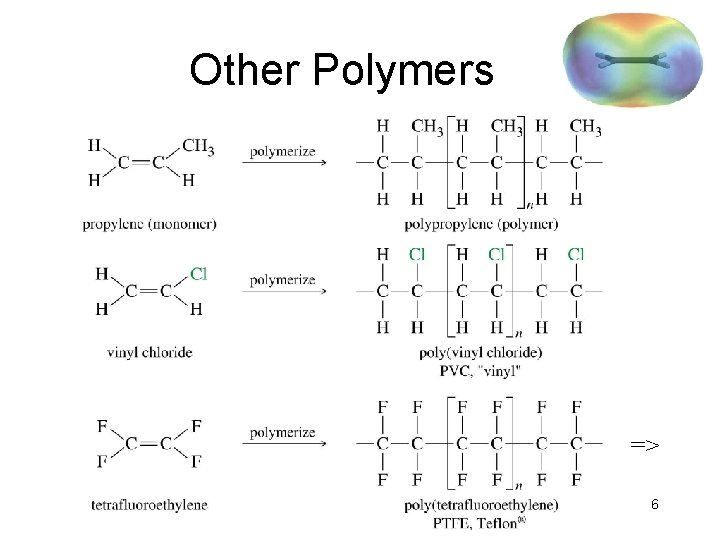
Other Polymers => 6

Industrial Methods • Catalytic cracking of petroleum ØLong-chain alkane is heated with a catalyst to produce an alkene and shorter alkane. ØComplex mixtures are produced. • Dehydrogenation of alkanes ØHydrogen (H 2) is removed with heat, catalyst. ØReaction is endothermic, but entropy-favored. • Neither method is suitable for lab synthesis => 7

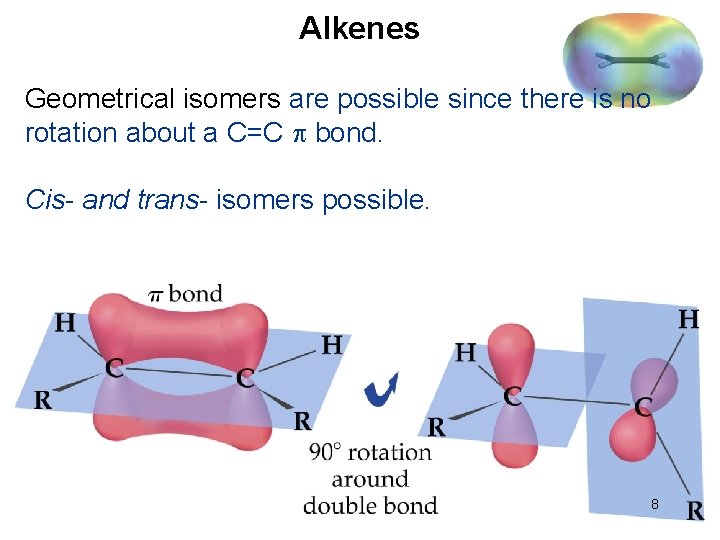
Alkenes Geometrical isomers are possible since there is no rotation about a C=C bond. Cis- and trans- isomers possible. 8

Functional Group • Pi bond is the functional group. • More reactive than sigma bond. 9

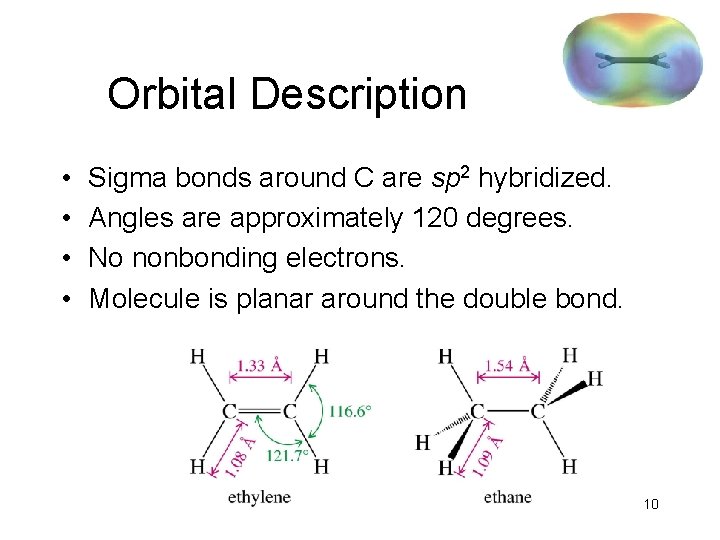
Orbital Description • • Sigma bonds around C are sp 2 hybridized. Angles are approximately 120 degrees. No nonbonding electrons. Molecule is planar around the double bond. 10

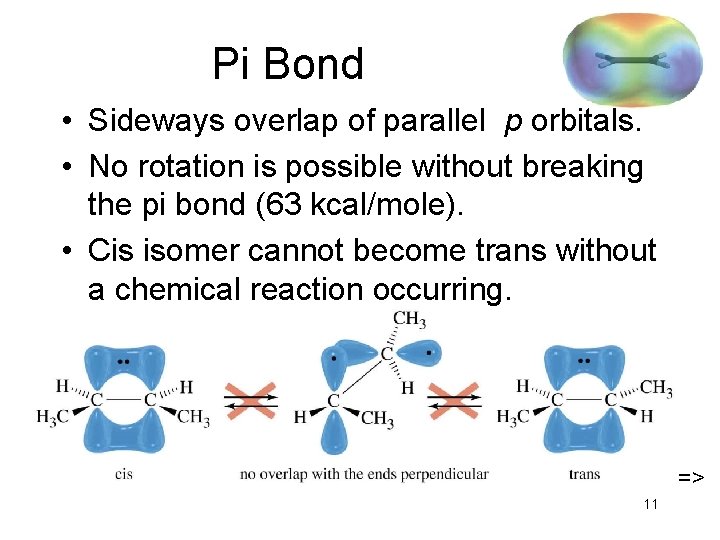
Pi Bond • Sideways overlap of parallel p orbitals. • No rotation is possible without breaking the pi bond (63 kcal/mole). • Cis isomer cannot become trans without a chemical reaction occurring. => 11

IUPAC Nomenclature • Parent is longest chain containing the double bond. • -ane changes to -ene. (or -diene, -triene) • Number the chain so that the double bond has the lowest possible number. • In a ring, the double bond is assumed to be between carbon 1 and carbon 2. => 12

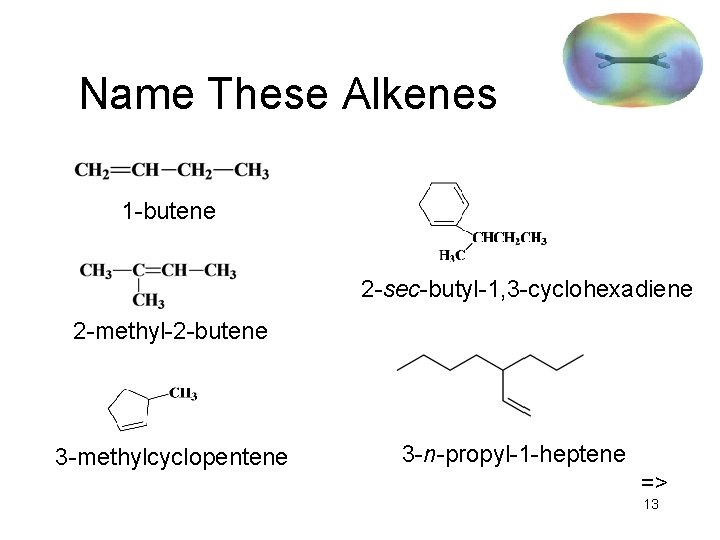
Name These Alkenes 1 -butene 2 -sec-butyl-1, 3 -cyclohexadiene 2 -methyl-2 -butene 3 -methylcyclopentene 3 -n-propyl-1 -heptene => 13

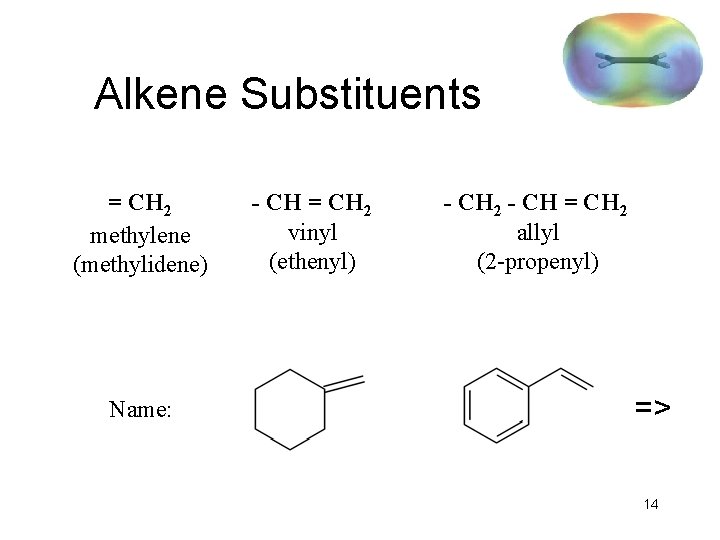
Alkene Substituents = CH 2 methylene (methylidene) Name: - CH = CH 2 vinyl (ethenyl) - CH 2 - CH = CH 2 allyl (2 -propenyl) => 14

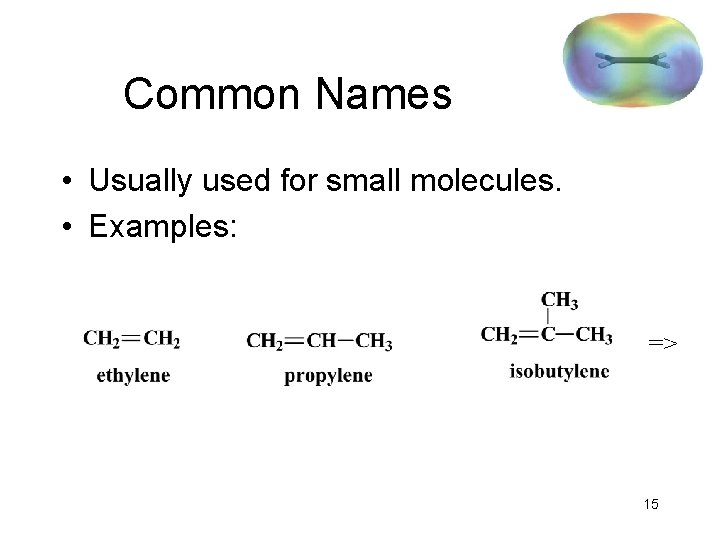
Common Names • Usually used for small molecules. • Examples: => 15

Cis-trans Isomerism • Similar groups on same side of double bond, alkene is cis. • Similar groups on opposite sides of double bond, alkene is trans. • Cycloalkenes are assumed to be cis. • Trans cycloalkenes are not stable unless the ring has at least 8 carbons. => 16

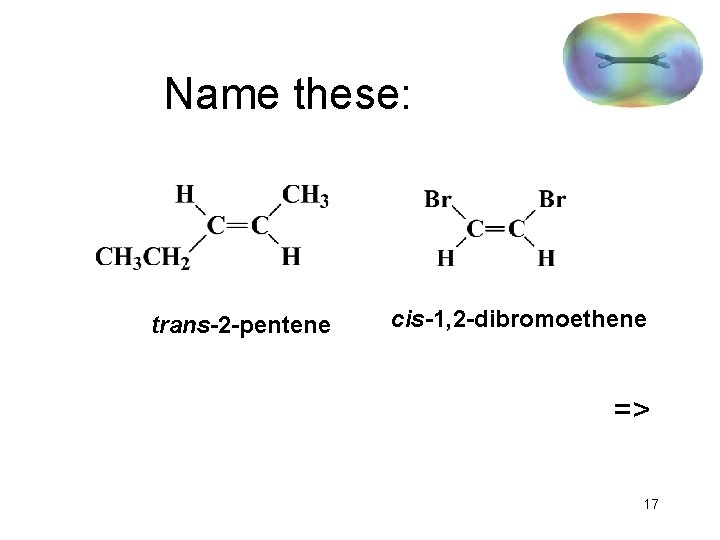
Name these: trans-2 -pentene cis-1, 2 -dibromoethene => 17

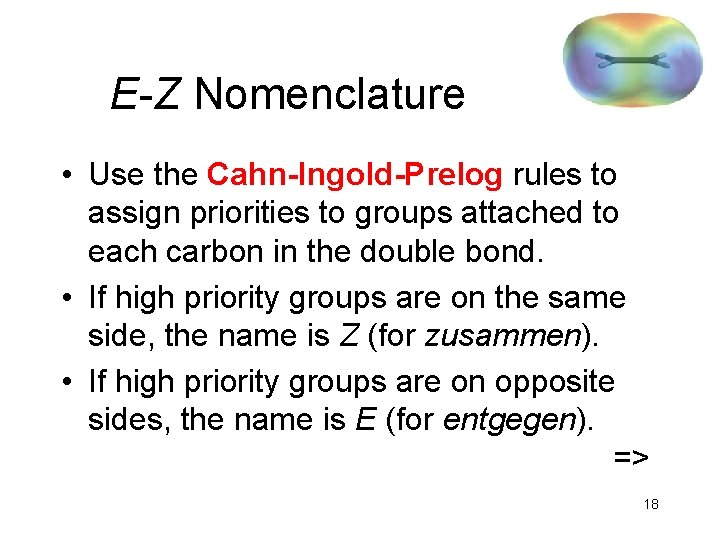
E-Z Nomenclature • Use the Cahn-Ingold-Prelog rules to assign priorities to groups attached to each carbon in the double bond. • If high priority groups are on the same side, the name is Z (for zusammen). • If high priority groups are on opposite sides, the name is E (for entgegen). => 18

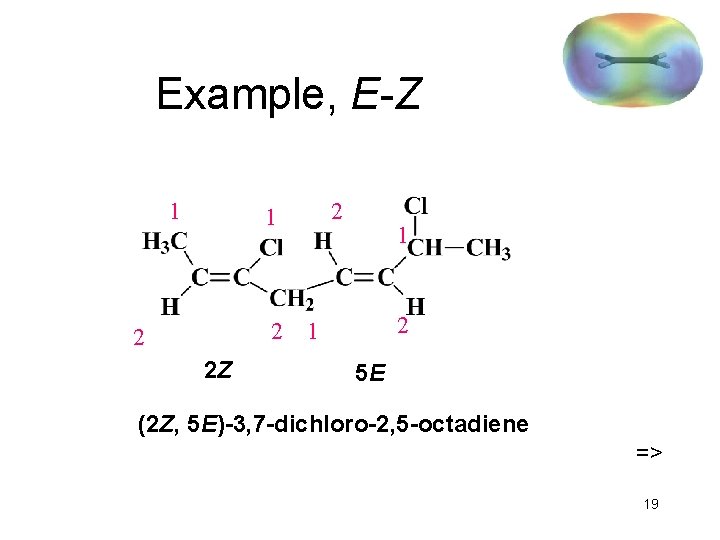
Example, E-Z 1 2 2 2 Z 1 2 1 5 E (2 Z, 5 E)-3, 7 -dichloro-2, 5 -octadiene => 19

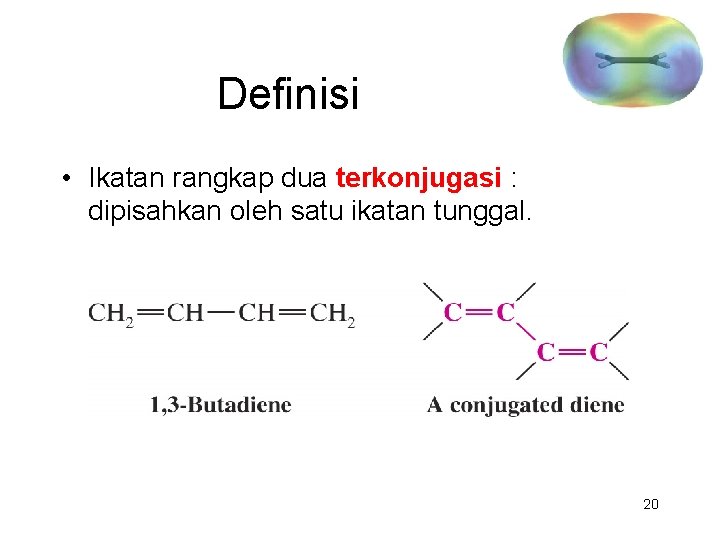
Definisi • Ikatan rangkap dua terkonjugasi : dipisahkan oleh satu ikatan tunggal. 20

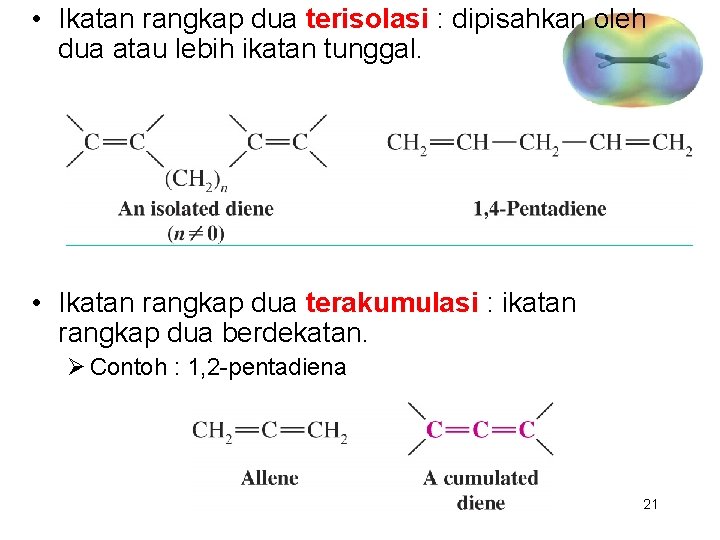
• Ikatan rangkap dua terisolasi : dipisahkan oleh dua atau lebih ikatan tunggal. • Ikatan rangkap dua terakumulasi : ikatan rangkap dua berdekatan. Ø Contoh : 1, 2 -pentadiena 21

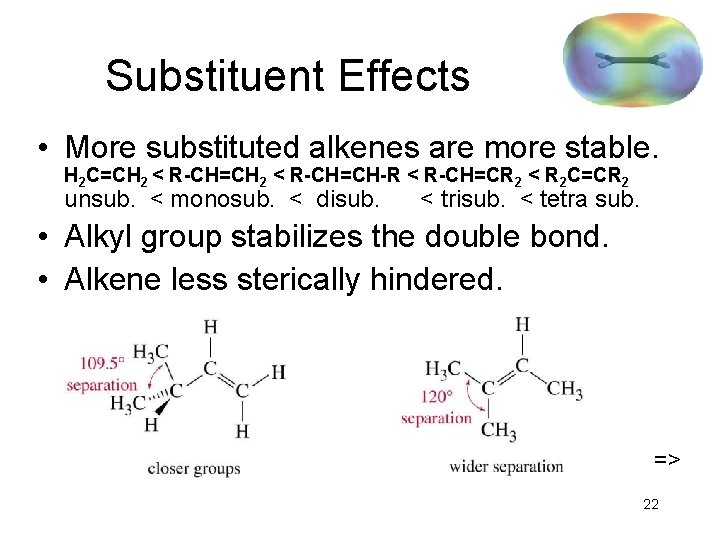
Substituent Effects • More substituted alkenes are more stable. H 2 C=CH 2 < R-CH=CH-R < R-CH=CR 2 < R 2 C=CR 2 unsub. < monosub. < disub. < trisub. < tetra sub. • Alkyl group stabilizes the double bond. • Alkene less sterically hindered. => 22

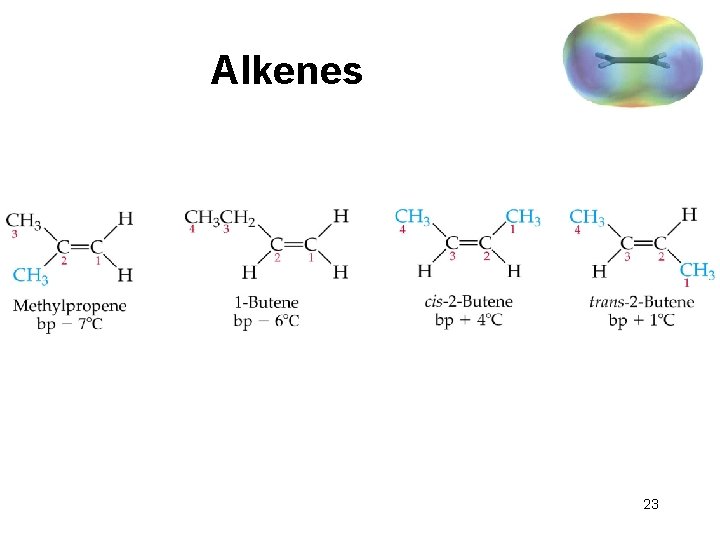
Alkenes 23

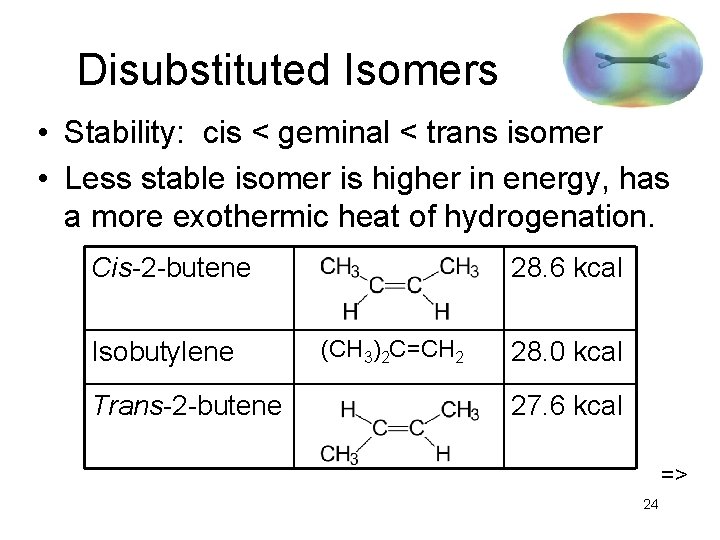
Disubstituted Isomers • Stability: cis < geminal < trans isomer • Less stable isomer is higher in energy, has a more exothermic heat of hydrogenation. Cis-2 -butene Isobutylene Trans-2 -butene 28. 6 kcal (CH 3)2 C=CH 2 28. 0 kcal 27. 6 kcal => 24

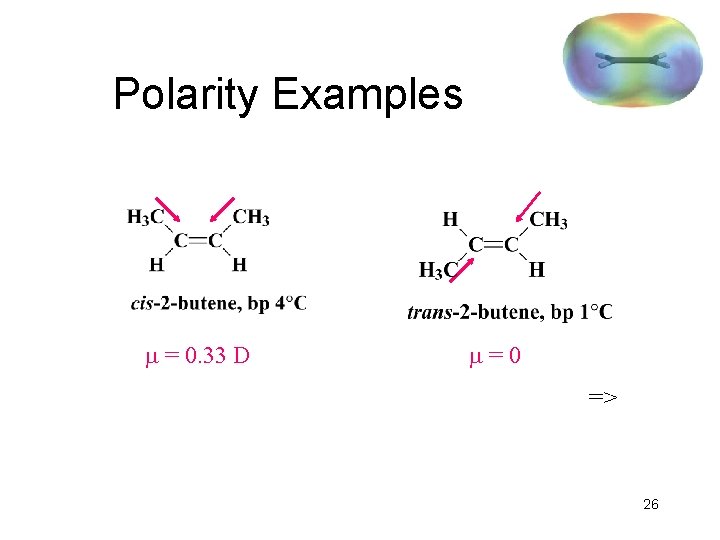
Physical Properties • • Low boiling points, increasing with mass. Branched alkenes have lower boiling points. Less dense than water. Slightly polar Ø Pi bond is polarizable, so instantaneous dipole interactions occur. Ø Alkyl groups are electron-donating toward the pi bond, so may have a small dipole moment. => 25

Polarity Examples = 0. 33 D =0 => 26

ADDITION REACTION An addition reaction is one in which the two reactants add together to make the product A + B AB with no other pieces lost or left over. 27

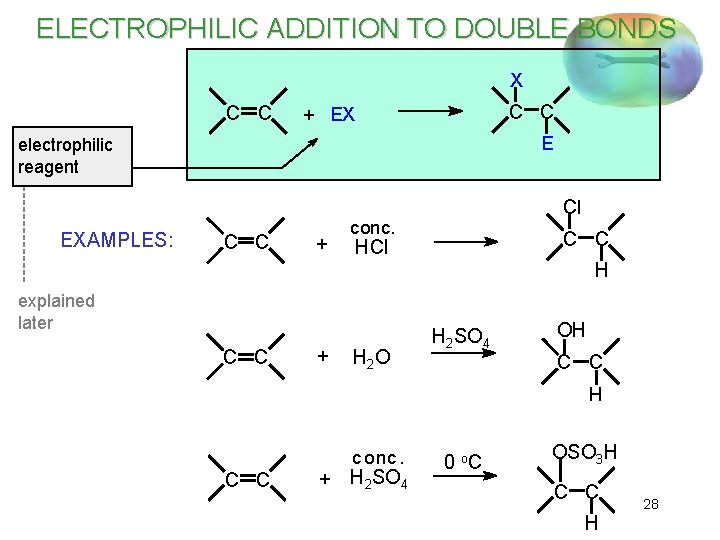
ELECTROPHILIC ADDITION TO DOUBLE BONDS X C C + EX E electrophilic reagent EXAMPLES: C C + Cl conc. C C HCl H explained later C C + H 2 O H 2 SO 4 OH C C H c onc. C C + H 2 SO 4 0 o. C OSO 3 H C C H 28

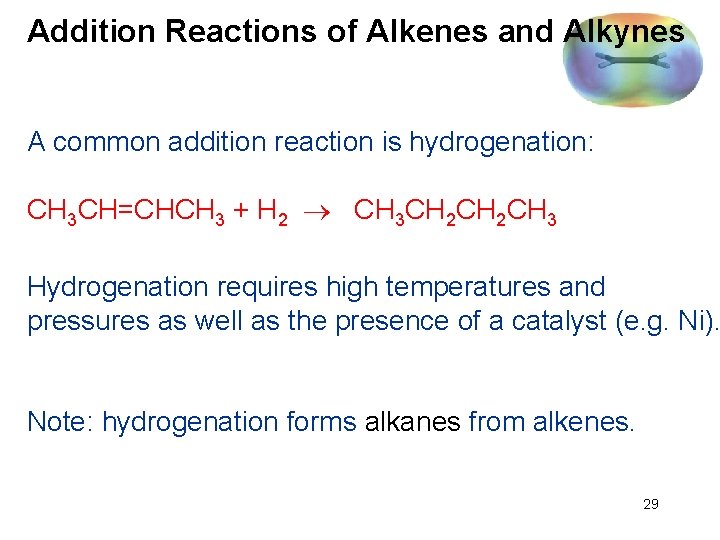
Addition Reactions of Alkenes and Alkynes A common addition reaction is hydrogenation: CH 3 CH=CHCH 3 + H 2 CH 3 CH 2 CH 3 Hydrogenation requires high temperatures and pressures as well as the presence of a catalyst (e. g. Ni). Note: hydrogenation forms alkanes from alkenes. 29

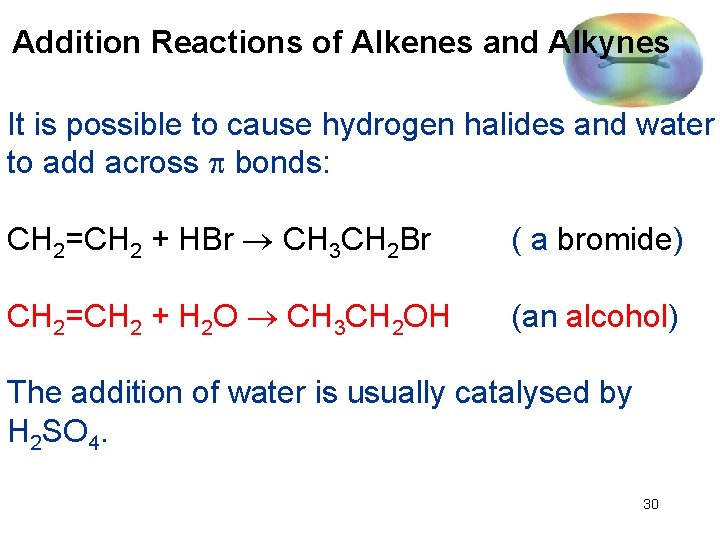
Addition Reactions of Alkenes and Alkynes It is possible to cause hydrogen halides and water to add across bonds: CH 2=CH 2 + HBr CH 3 CH 2 Br ( a bromide) CH 2=CH 2 + H 2 O CH 3 CH 2 OH (an alcohol) The addition of water is usually catalysed by H 2 SO 4. 30

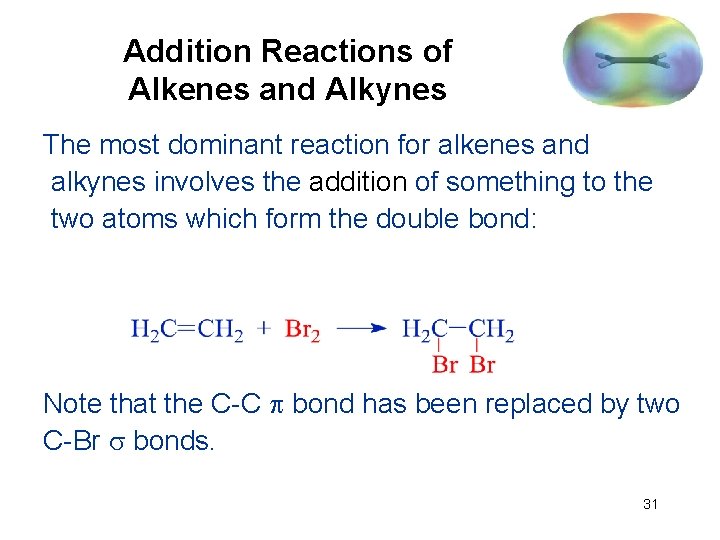
Addition Reactions of Alkenes and Alkynes The most dominant reaction for alkenes and alkynes involves the addition of something to the two atoms which form the double bond: Note that the C-C bond has been replaced by two C-Br bonds. 31

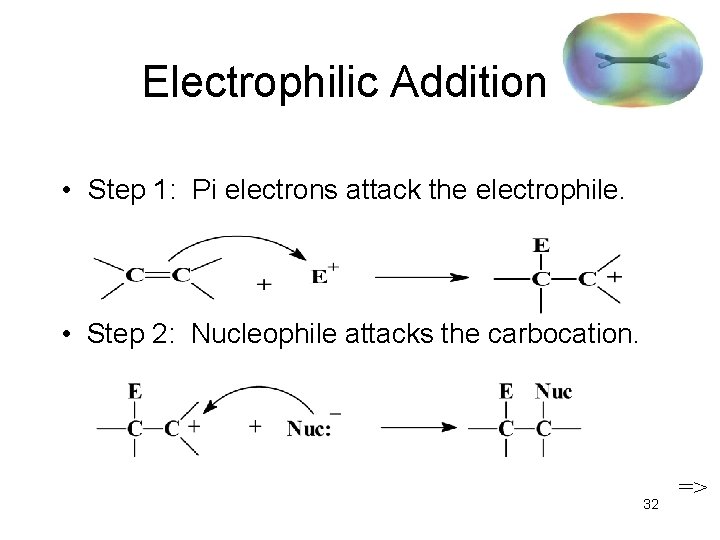
Electrophilic Addition • Step 1: Pi electrons attack the electrophile. • Step 2: Nucleophile attacks the carbocation. 32 =>

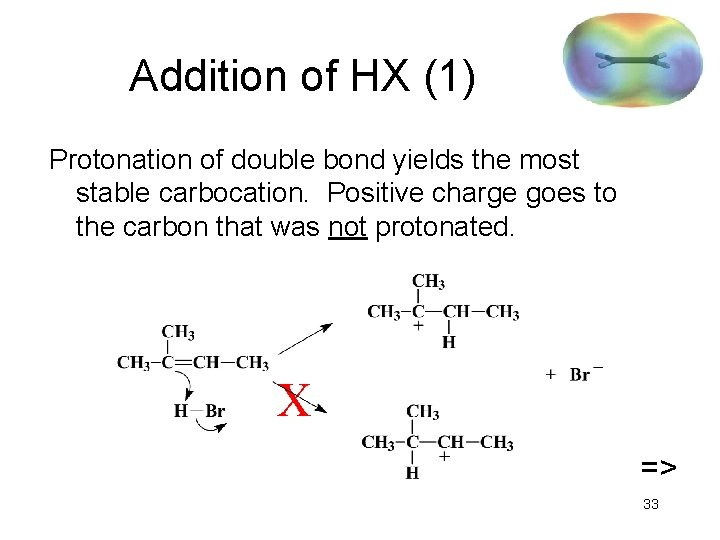
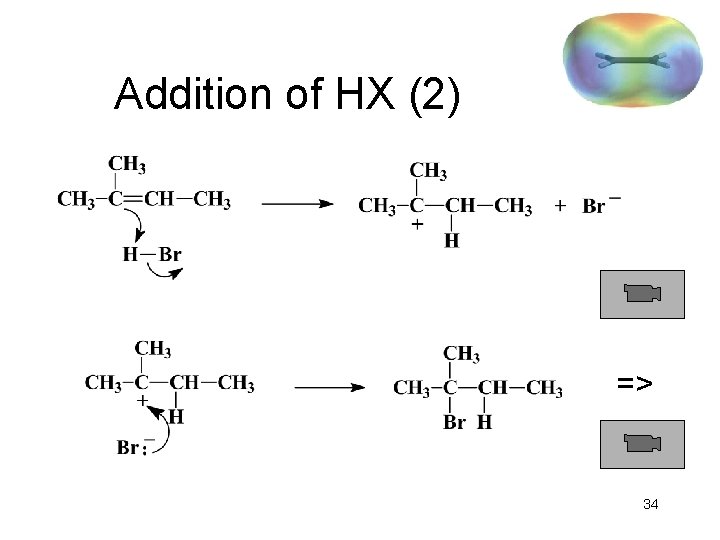
Addition of HX (1) Protonation of double bond yields the most stable carbocation. Positive charge goes to the carbon that was not protonated. X => 33

Addition of HX (2) => 34

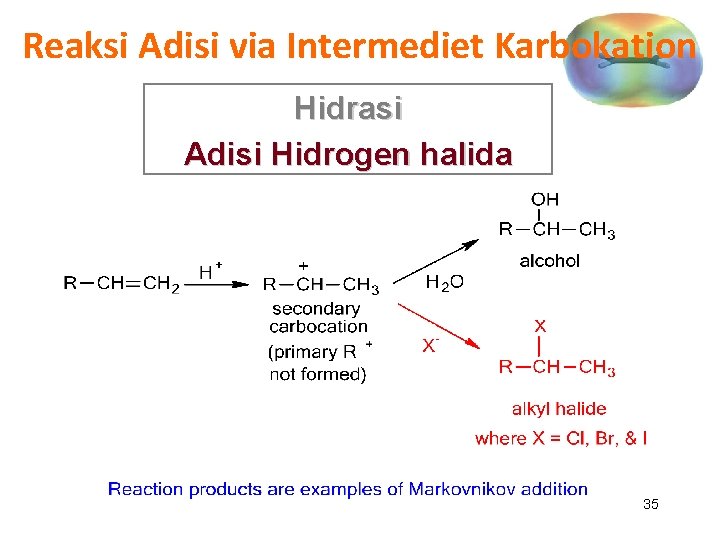
Reaksi Adisi via Intermediet Karbokation Hidrasi Adisi Hidrogen halida 35

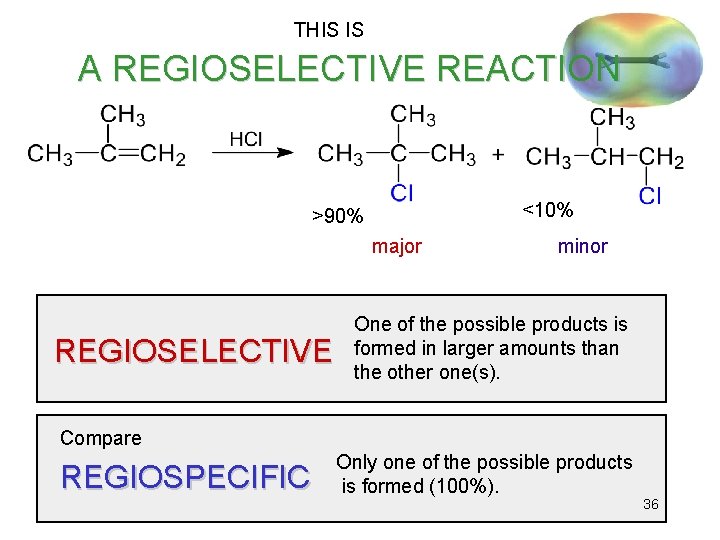
THIS IS A REGIOSELECTIVE REACTION <10% >90% major REGIOSELECTIVE minor One of the possible products is formed in larger amounts than the other one(s). Compare REGIOSPECIFIC Only one of the possible products is formed (100%). 36

Regiospecificity • Markovnikov’s Rule: The proton of an acid adds to the carbon in the double bond that already has the most H’s. “Rich get richer. ” • More general Markovnikov’s Rule: In an electrophilic addition to an alkene, the electrophile adds in such a way as to form the most stable intermediate. • HCl, HBr, and HI add to alkenes to form Markovnikov products. => 37

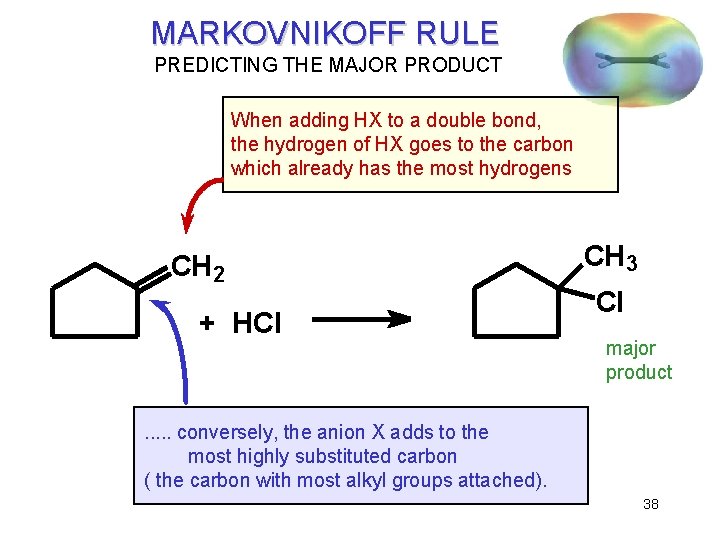
MARKOVNIKOFF RULE PREDICTING THE MAJOR PRODUCT When adding HX to a double bond, the hydrogen of HX goes to the carbon which already has the most hydrogens CH 2 + HCl CH 3 Cl major product . . . conversely, the anion X adds to the most highly substituted carbon ( the carbon with most alkyl groups attached). 38

AN “EMPIRICAL” RULE Markovnikoff formulated his rule by observing the results of hundreds of reactions that he performed. EMPIRICAL = DETERMINED BY OBSERVATION He had no idea why the reaction worked this way, only that as a general rule it did give the stated result. 39

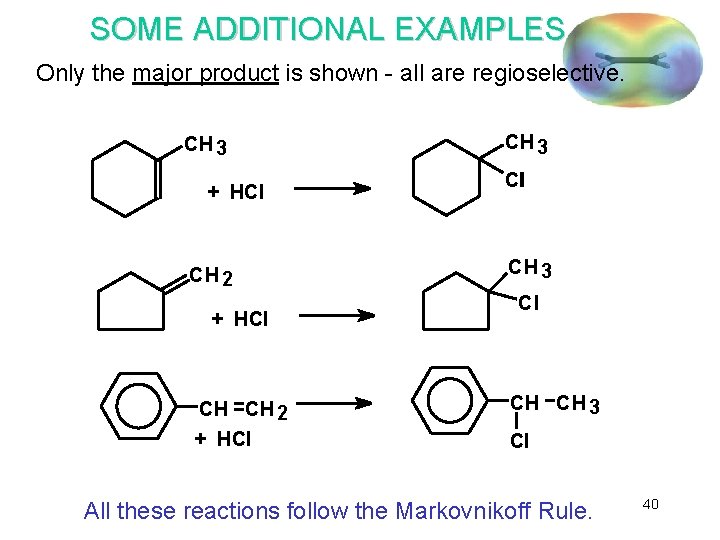
SOME ADDITIONAL EXAMPLES Only the major product is shown - all are regioselective. CH 3 + HCl CH 2 + HCl CH 3 Cll CH 3 Cl CH CH 3 Cl All these reactions follow the Markovnikoff Rule. 40

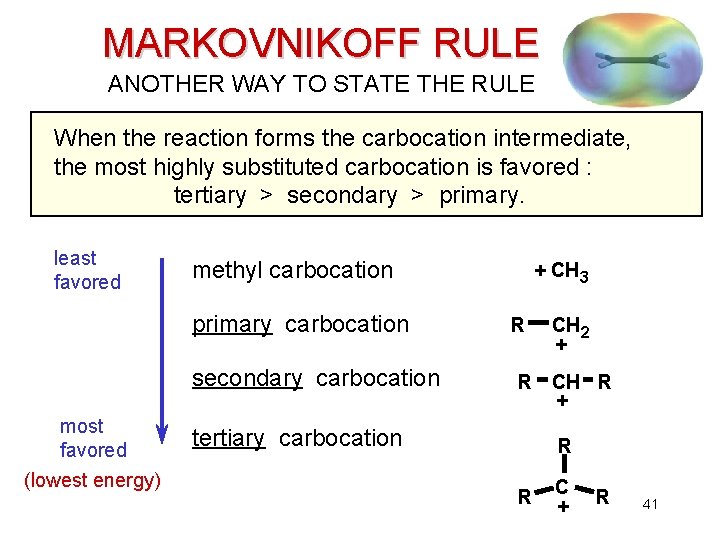
MARKOVNIKOFF RULE ANOTHER WAY TO STATE THE RULE When the reaction forms the carbocation intermediate, the most highly substituted carbocation is favored : tertiary > secondary > primary. least favored methyl carbocation primary carbocation secondary carbocation most favored (lowest energy) + CH 3 R R tertiary carbocation CH 2 + CH R + R R C + R 41

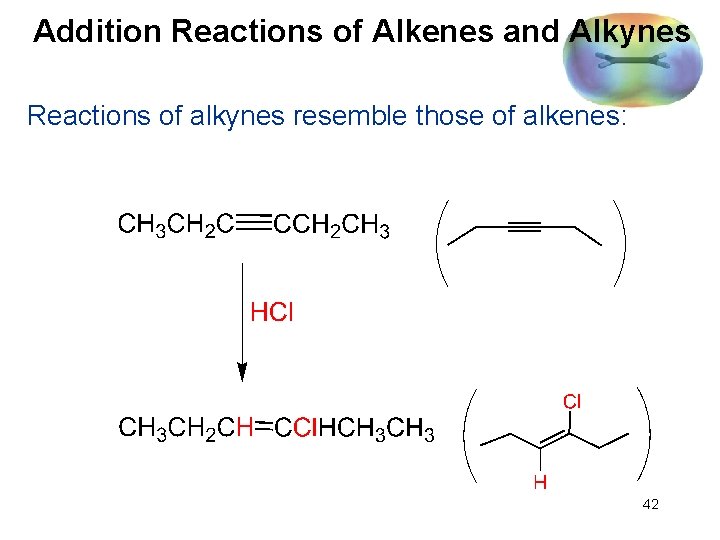
Addition Reactions of Alkenes and Alkynes Reactions of alkynes resemble those of alkenes: 42

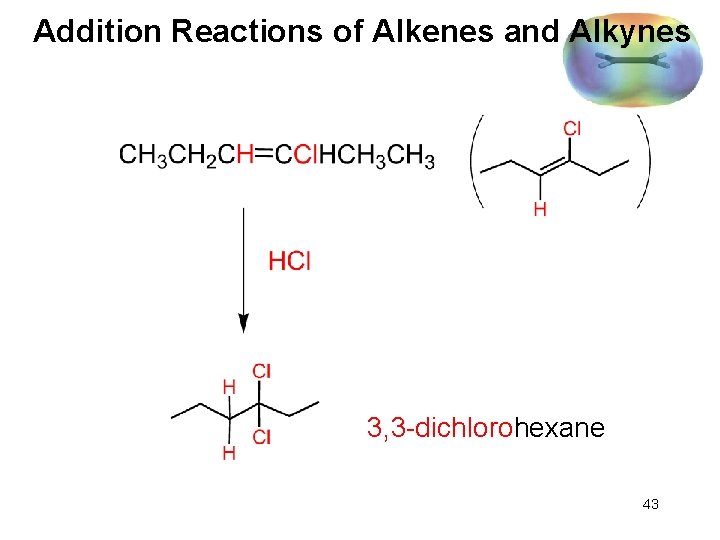
Addition Reactions of Alkenes and Alkynes 3, 3 -dichlorohexane 43

Alkene Synthesis Overview • • E 2 dehydrohalogenation (-HX) E 1 dehydrohalogenation (-HX) Dehalogenation of vicinal dibromides (-X 2) Dehydration of alcohols (-H 2 O) => 44

Dehydration of Alcohols • Reversible reaction • Use concentrated sulfuric or phosphoric acid, remove low-boiling alkene as it forms. • Protonation of OH converts it to a good leaving group, HOH • Carbocation intermediate, like E 1 • Protic solvent removes adjacent H+ =>45

End of Chapter 3 46
 Peta konsep alkana
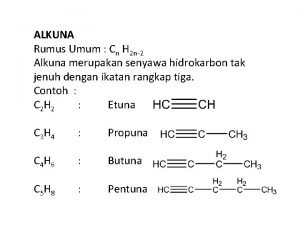
Peta konsep alkana Rumus umum alkuna
Rumus umum alkuna Permendagri tentang klasifikasi kodefikasi dan nomenklatur
Permendagri tentang klasifikasi kodefikasi dan nomenklatur Rumus bangun alkuna
Rumus bangun alkuna Keunikan alkuna
Keunikan alkuna Keunikan alkuna
Keunikan alkuna Esterbindung
Esterbindung Keton nomenklatur
Keton nomenklatur Ethansäureanhydrid
Ethansäureanhydrid Nomenklatur roda gigi
Nomenklatur roda gigi Elementare regelungstechnik
Elementare regelungstechnik Iupac nomenklatur
Iupac nomenklatur E z nomenklatur
E z nomenklatur Tata nama senyawa kimia
Tata nama senyawa kimia Oksidacija alkina
Oksidacija alkina Hidrogenizacija alkena
Hidrogenizacija alkena Polimerizacija alkena
Polimerizacija alkena Trans 1 2 dikloroetena
Trans 1 2 dikloroetena Imenovanje alkana
Imenovanje alkana Contoh soal stereokimia
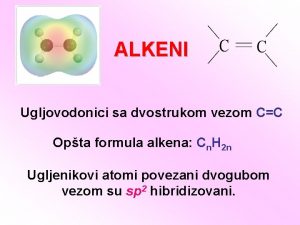
Contoh soal stereokimia Alkena
Alkena Aromatis homosiklis
Aromatis homosiklis 5–bromo–2–metilheks–3–ena
5–bromo–2–metilheks–3–ena Apa yang dimaksud dengan senyawa hidrokarbon? *
Apa yang dimaksud dengan senyawa hidrokarbon? * Hibridisasi alkena
Hibridisasi alkena Nomenklatura alkena
Nomenklatura alkena The red tent summary
The red tent summary Chapter 8 summary the great gatsby
Chapter 8 summary the great gatsby Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Stoichiometry chapter 11 study guide
Stoichiometry chapter 11 study guide Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Ratio and proportion for use after section 7-2
Ratio and proportion for use after section 7-2 Chapter 6 career readiness chapter review
Chapter 6 career readiness chapter review Chapter 7 ionic and metallic bonding chapter answer key
Chapter 7 ionic and metallic bonding chapter answer key Chapter 9 surface water answer key
Chapter 9 surface water answer key Chapter 2 assessment physics principles and problems
Chapter 2 assessment physics principles and problems The central science 14th edition
The central science 14th edition Chapter 7 ionic and metallic bonding answer key
Chapter 7 ionic and metallic bonding answer key Chapter 4 population ecology worksheet answer key
Chapter 4 population ecology worksheet answer key Chapter 2 chapter assessment
Chapter 2 chapter assessment Book of philippians background
Book of philippians background Properties of ionic compounds
Properties of ionic compounds 7 ionic and metallic bonding practice problems
7 ionic and metallic bonding practice problems 9 chemical names and formulas
9 chemical names and formulas Chapter 7 accounting
Chapter 7 accounting Pengertian gaudium et spes
Pengertian gaudium et spes Tipologi mazhab italia
Tipologi mazhab italia