CHAPTER 12 Concentration Terms for Solutions Concentration Amount






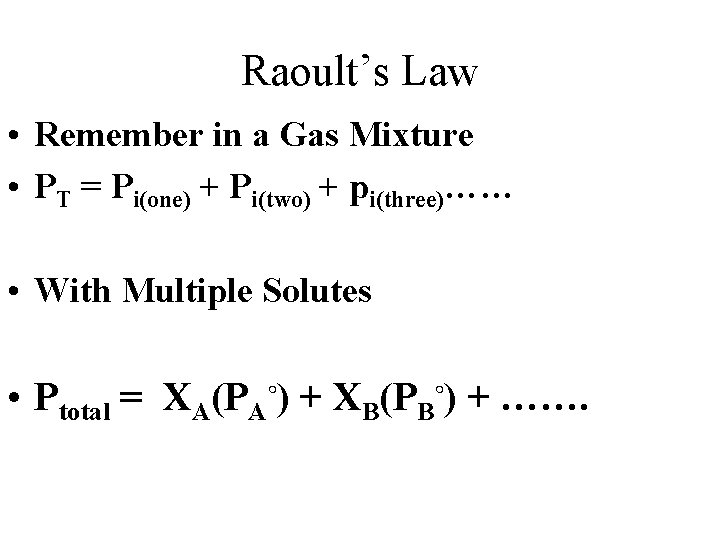















- Slides: 42

CHAPTER 12: Concentration Terms for Solutions • Concentration = Amount solute/amount solvent • Some Concentration Terms are Temperature Sensitive

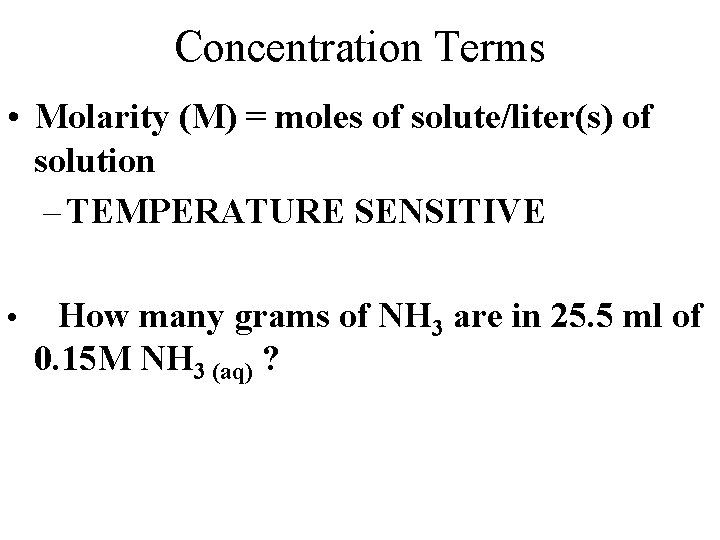
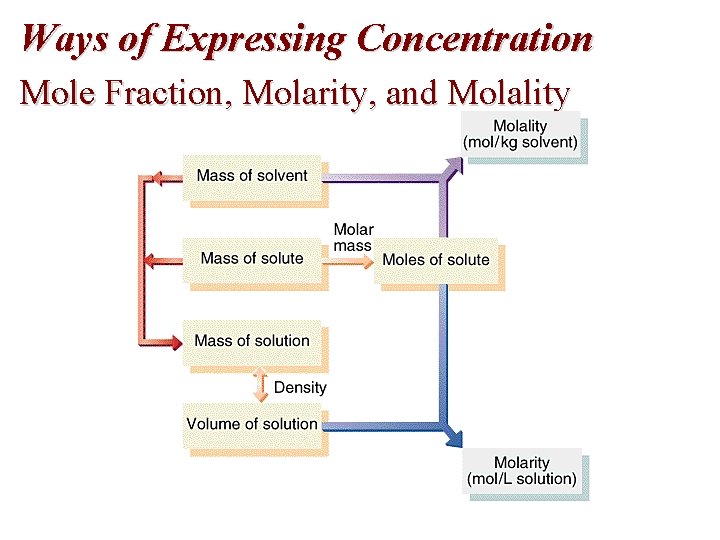
Concentration Terms • Molarity (M) = moles of solute/liter(s) of solution – TEMPERATURE SENSITIVE • How many grams of NH 3 are in 25. 5 ml of 0. 15 M NH 3 (aq) ?

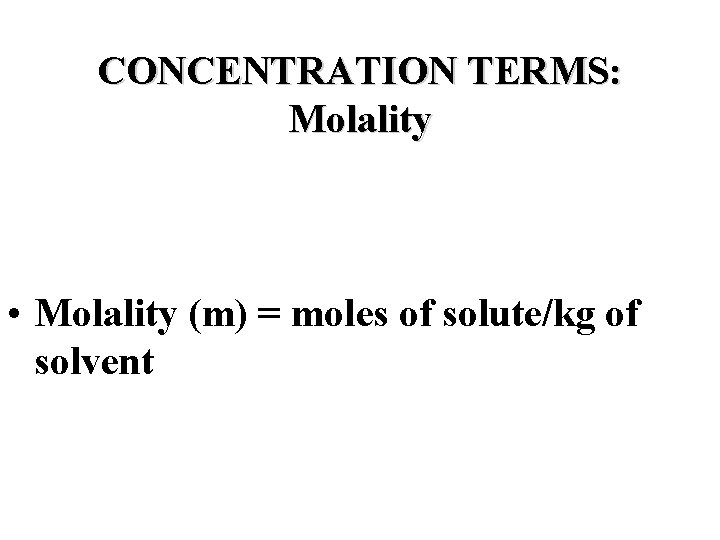
CONCENTRATION TERMS: Molality • Molality (m) = moles of solute/kg of solvent

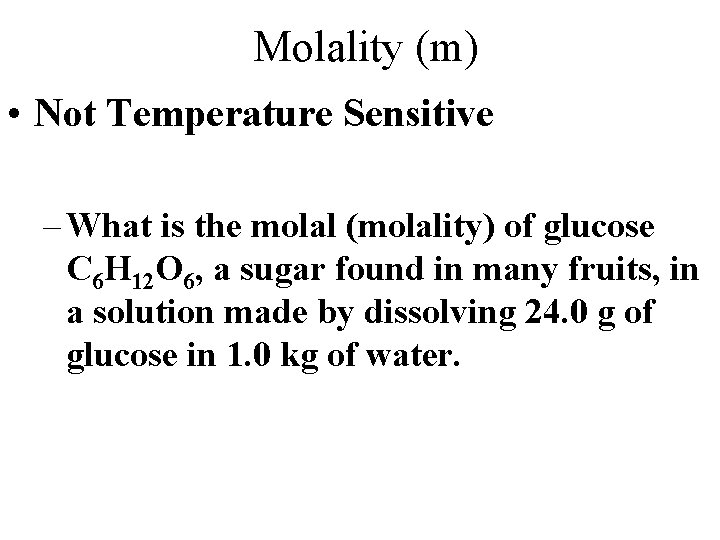
Molality (m) • Not Temperature Sensitive – What is the molal (molality) of glucose C 6 H 12 O 6, a sugar found in many fruits, in a solution made by dissolving 24. 0 g of glucose in 1. 0 kg of water.

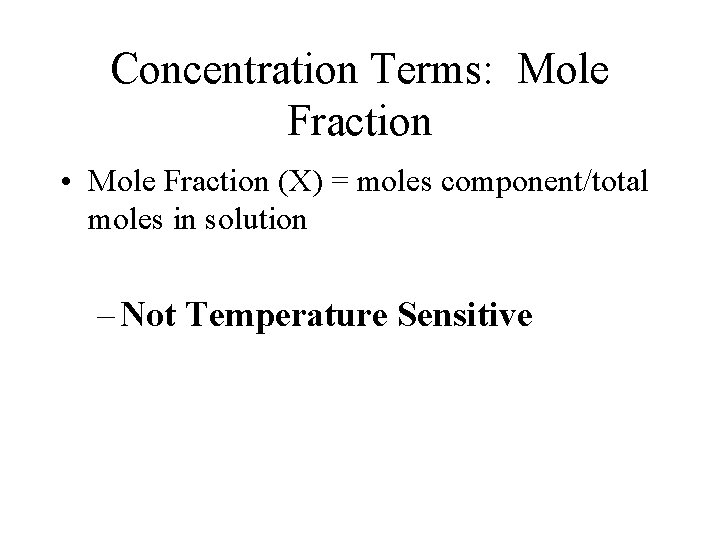
Concentration Terms: Mole Fraction • Mole Fraction (X) = moles component/total moles in solution – Not Temperature Sensitive

Ways of Expressing Concentration Mole Fraction, Molarity, and Molality

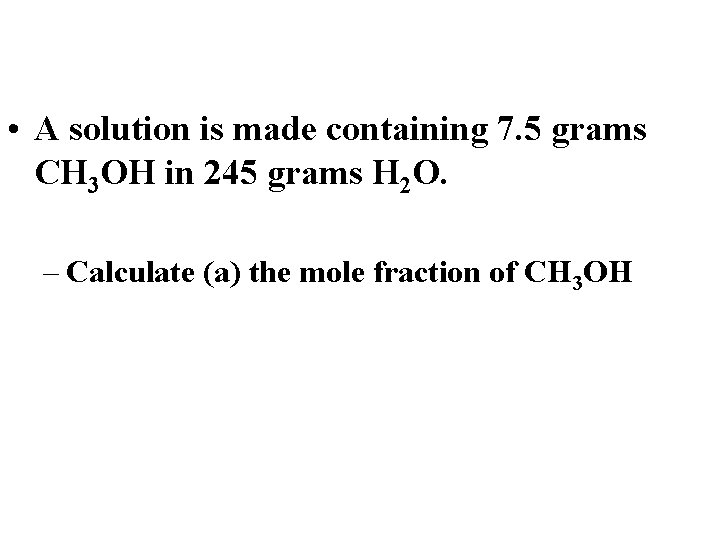
• A solution is made containing 7. 5 grams CH 3 OH in 245 grams H 2 O. – Calculate (a) the mole fraction of CH 3 OH

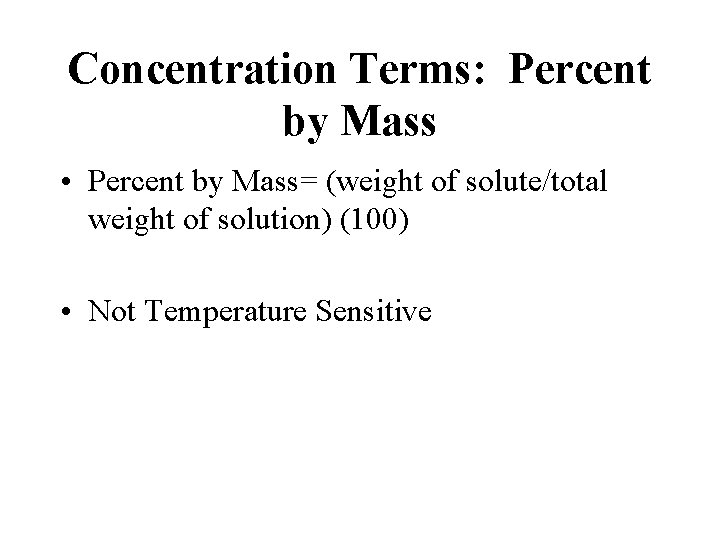
Concentration Terms: Percent by Mass • Percent by Mass= (weight of solute/total weight of solution) (100) • Not Temperature Sensitive

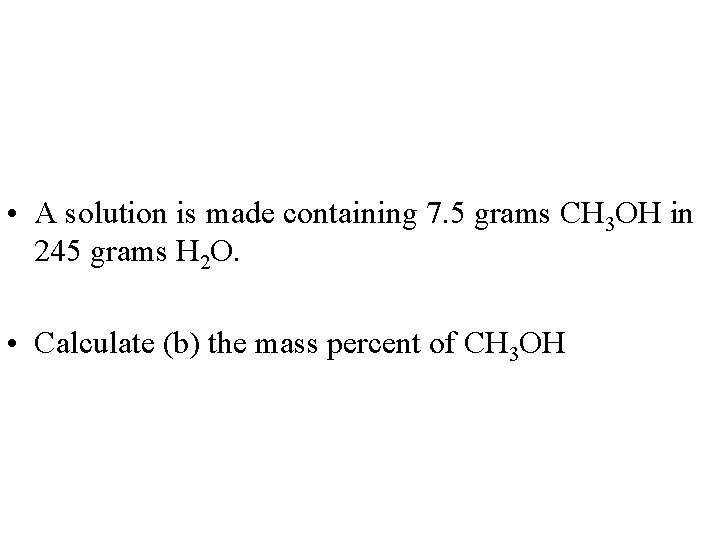
• A solution is made containing 7. 5 grams CH 3 OH in 245 grams H 2 O. • Calculate (b) the mass percent of CH 3 OH

Chang Text Problem 12. 22: Page 546 The concentrated sulfuric acid we use in the laboratory is 98. 0 percent H 2 SO 4 by mass. Calculate the molality and molarity of the acid solution. The density of the solution is 1. 83 g/ml.

Chang 12. 24: Page 547 • The density of an aqueous solution containing 10 percent of ethanol (C 2 H 5 OH) by mass is 0. 984 g/ml. (a) Calculate the molality; (b) Calculate the molarity; (C ) What volume of the solution would contain 0. 125 mole of ethanol?

Problem 12. 22: Page 535 1. Assume you have 100 grams of solution. 2. Mass % = [mass solute/mass solute + mass H 2 O] 100 3. Have 98 grams of solute (98%). Have 2. 0 grams of H 2 O. 4. Molality = moles of solute/kg of solvent

Problem 12. 22: Page 535 • Molarity = moles solute/liters of solution Have 100 g of solution. Use density of the sulfuric acid solution to calculate volume. Calculate moles of sulfuric in 98. 0 grams

Colligative Properties • Physical properties of a solvent which depend upon the amount of solute present in a solution. (1) Lowering the Vapor Pressure • Non-volatile solvents reduce the ability of the surface solvent molecules to escape the liquid. • TEMPERATURE SENSITIVE • The amount of vapor pressure lowering depends on the amount of solute.

Colligative Properties Of Nonelectrolyte Solutions: Vapor Pressure Raoult’s Law • Raoult’s Law: PA is the vapor pressure with solute, PA is the vapor pressure without solvent, and A is the mole fraction of A, then • Recall Dalton’s Law:
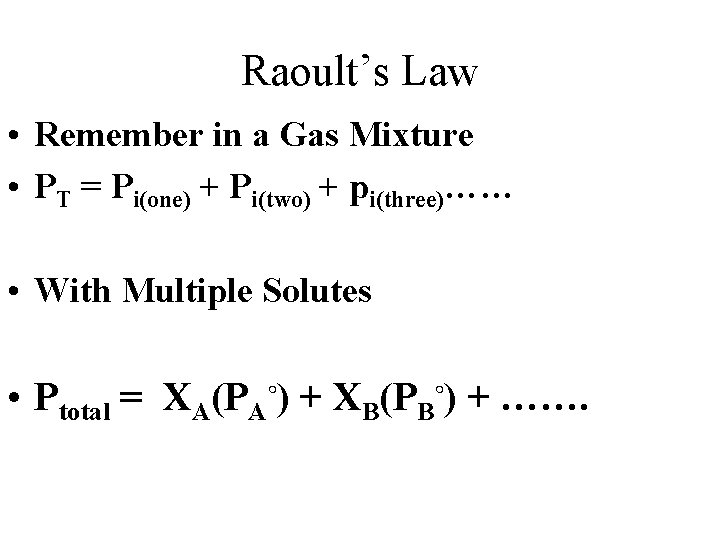
Raoult’s Law • Remember in a Gas Mixture • PT = Pi(one) + Pi(two) + pi(three)…… • With Multiple Solutes • Ptotal = XA(PA◦) + XB(PB◦) + …….

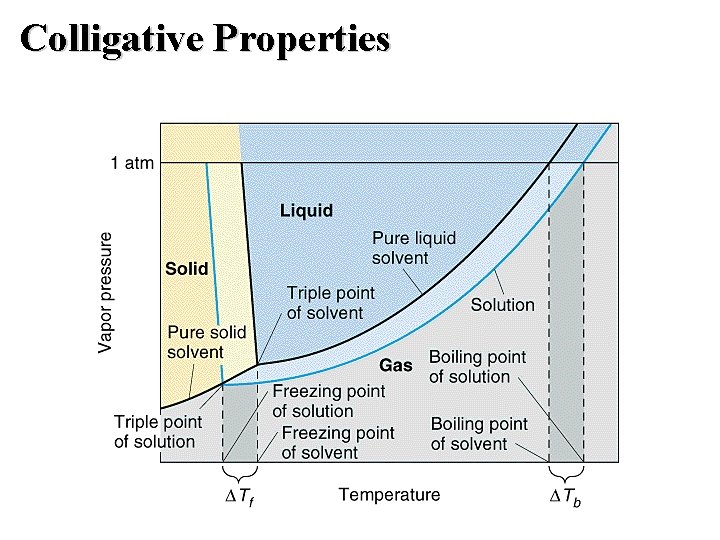
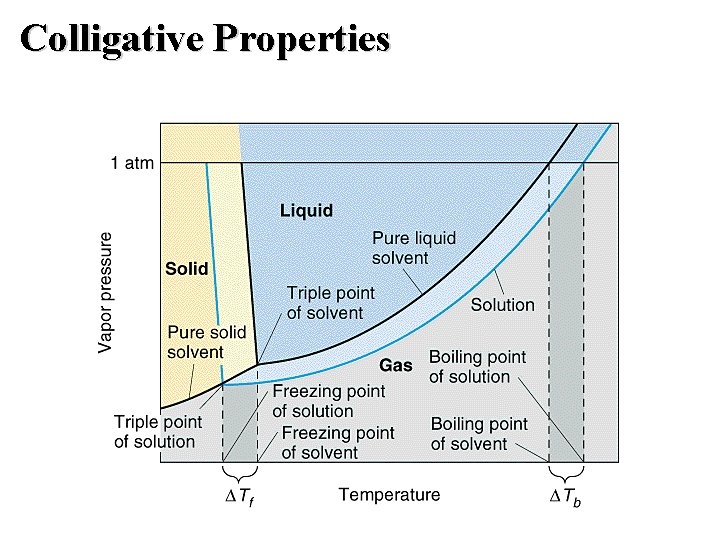
Colligative Properties: Vapor Pressure Raoult’s Law • Ideal solution: one that obeys Raoult’s law. ▲PA =(X solute) (Vapor Pressure Pure Solvent) Boiling-Point Elevation • Goal: interpret the phase diagram for a solution. • Non-volatile solute lowers the vapor pressure. • Therefore the triple point - critical point curve is lowered.

Problem • Calculate the mass of propylene glycol (C 3 H 8 O 2) that must be added to 0. 500 kg of water to reduce the vapor pressure by 4. 60 torr at 40 ºC.

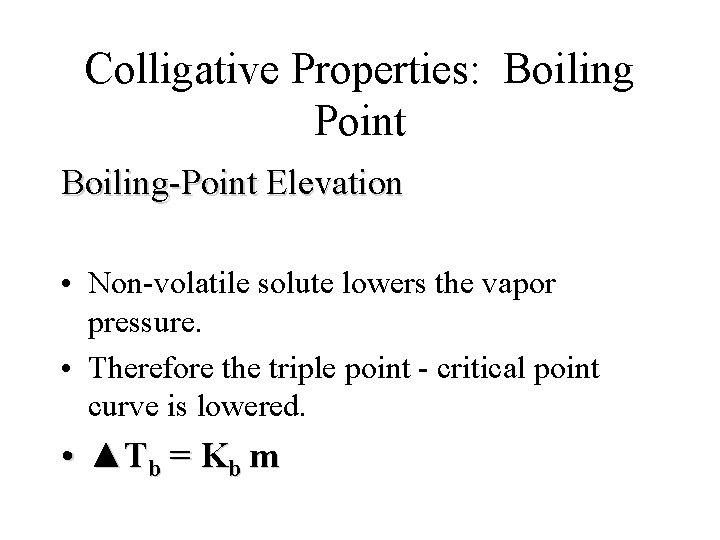
Colligative Properties: Boiling Point • BOILING POINT IS TEMPERATURE AT WHICH, VAPOR PRESSURE = EXTERNAL ATMOSPHERIC PRESSURE

Colligative Properties: Boiling Point Boiling-Point Elevation • Non-volatile solute lowers the vapor pressure. • Therefore the triple point - critical point curve is lowered. • ▲Tb = Kb m

Colligative Properties

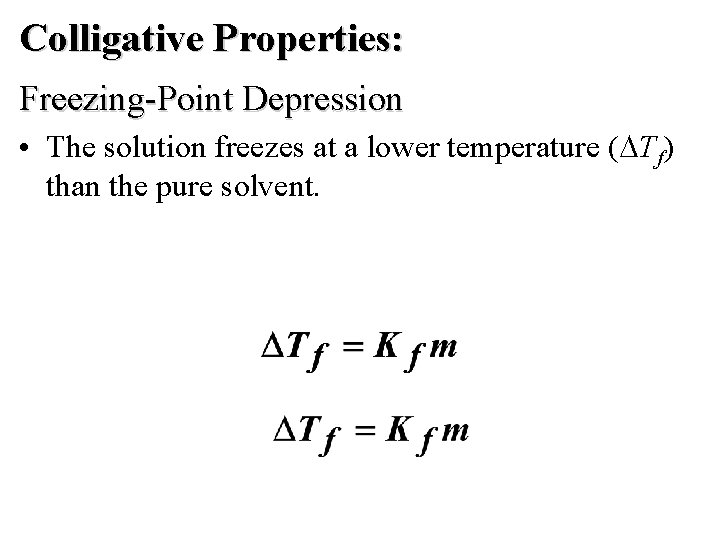
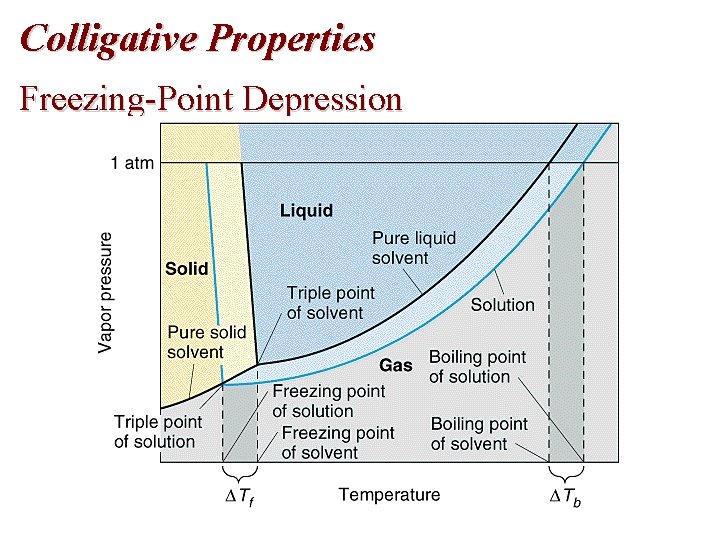
Colligative Properties: Freezing-Point Depression • The solution freezes at a lower temperature ( Tf) than the pure solvent.

Colligative Properties: Freezing Point Freezing-Point Depression • When a solution freezes, almost pure solvent is formed first. – Therefore, the sublimation curve for the pure solvent is the same as for the solution. – Therefore, the triple point occurs at a lower temperature because of the lower vapor pressure for the solution. • The melting-point (freezing-point) curve is a vertical line from the triple point.

Colligative Properties

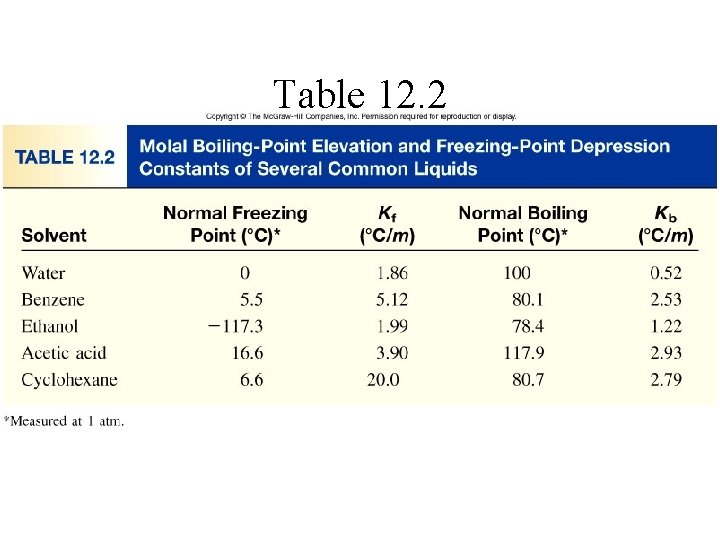
Table 12. 2

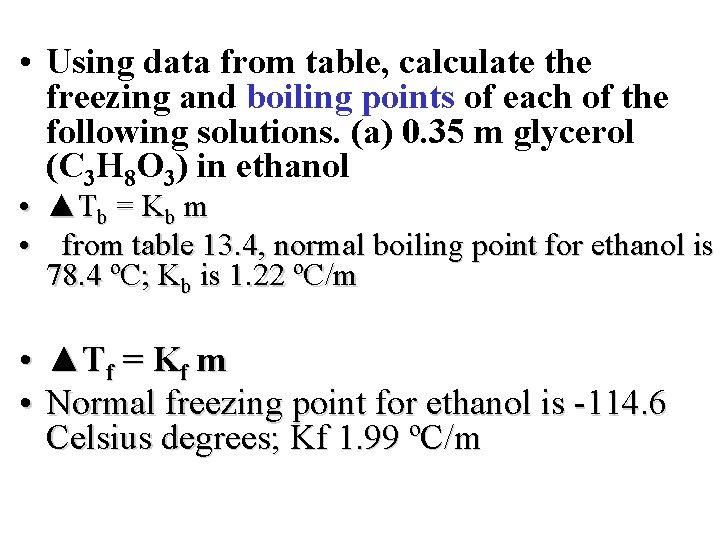
• Using data from table, calculate the freezing and boiling points of each of the following solutions. (a) 0. 35 m glycerol (C 3 H 8 O 3) in ethanol • ▲Tb = Kb m • from table 13. 4, normal boiling point for ethanol is 78. 4 ºC; Kb is 1. 22 ºC/m • ▲Tf = Kf m • Normal freezing point for ethanol is -114. 6 Celsius degrees; Kf 1. 99 ºC/m

Problem 12. 56: Page 548 • An aqueous solution contains the amino acid glycine (NH 2 COOH. Assuming that the acid does not ionize in water, calculate the molality of the solution if it freezes at -1. 1 Celsius.

• ▲Tf = Kf m • Kf = 1. 86 Celsius/m

Colligative Properties Freezing-Point Depression

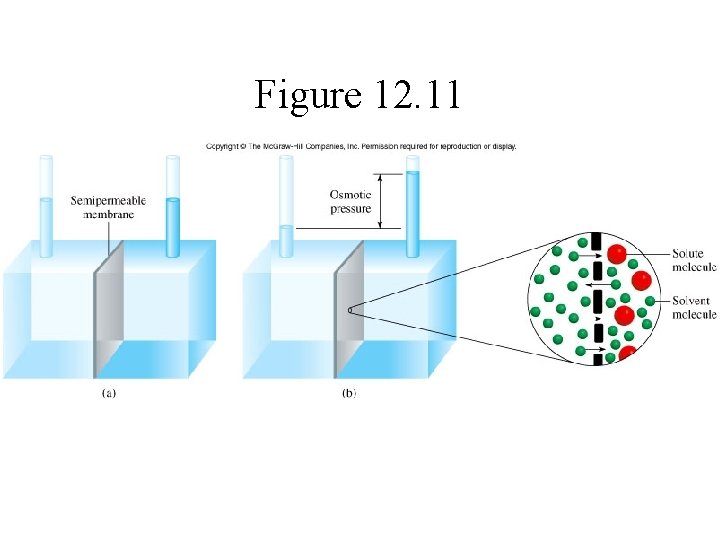
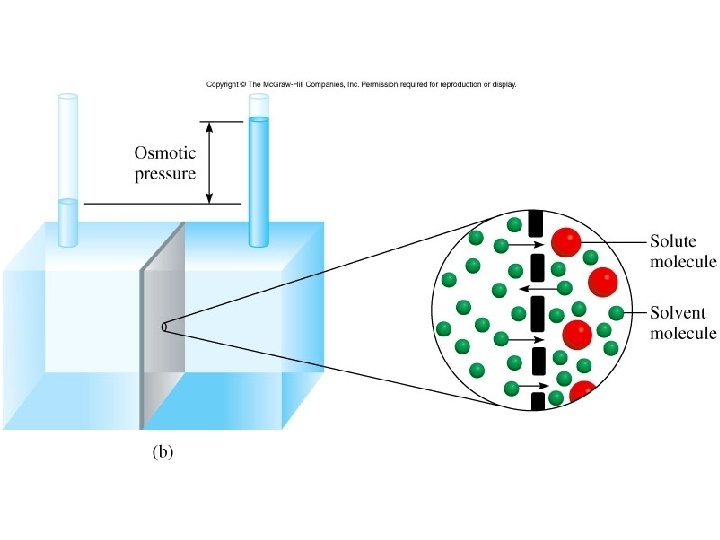
Colligative Properties: Osmosis Movement of a solvent from an area of high solvent concentration to an area of low solvent concentration across a semi permeable membrane.

Figure 12. 11

Figure 12. 11 a

Figure 12. 11 b

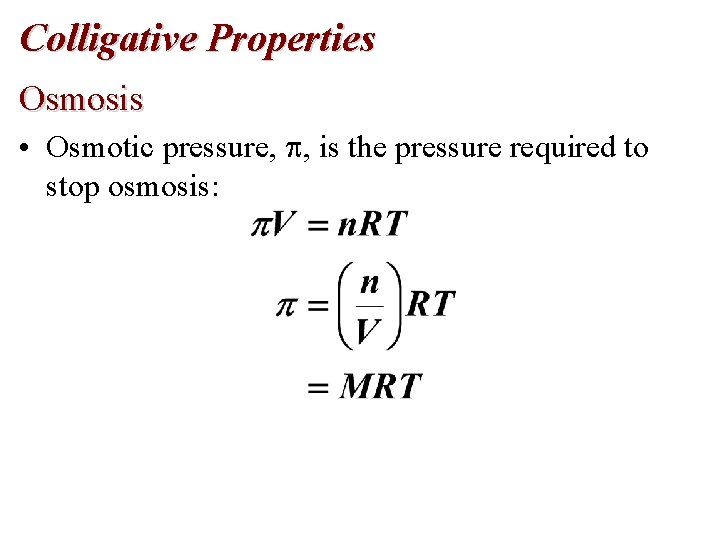
Colligative Properties Osmosis • Osmotic pressure, , is the pressure required to stop osmosis:

DETERMINATION OF MOLAR MASS • Usually use freezing point depression or boiling point elevation. • Process – Calculate molality or Molarity – Need to be given mass of solute. – Calculate molar mass: mole = grams

• A 1. 2436 gram sample of an unknown electrolyte is dissolved in 10. 9303 grams of benzophenone which produces a solution that freezes as 43. 2 ◦ C. If pure benzophenone melts at 48. 1 ◦ C, what is the molecular weight of the unknown compound? Kf =9. 8 ◦C/m

• ▲Tf = Kf m • ▲Tf = 4. 9 C • 4. 9 C = [9. 8 C/m] [m] • [m] = 0. 50 moles in one kg of benzophenone • Used 0. 010903 kg of solvent. [0. 50 moles/kg] [0. 010903 kg] = 0. 0054515 moles

• Molar mass = grams of compound/moles of compound • Molar Mass = 10. 903 grams/0. 50 moles = 21. 81 grams/mole

Colligative Properties of Electrolyte Solutions • Electrolytes: Solutes which dissociate into ions in solution – Ionic Solutes (Na. Cl) – Acids (HCl) – Bases

Colligative Properties of Electrolyte Solutions • I = actual number of particles in solution after dissociation/number of formula units initially dissolved in soln


Problem 12. 72: Page 549 • Arrange the following aqueous solutions in order of decreasing freezing point, and explain your reasoning. 0. 050 HCl; 0. 50 m glucose; 0. 50 m acetic acid.