Unit 5 Solutions Concentration Solutions Key Terms Solute








- Slides: 13

Unit 5: Solutions Concentration

Solutions – Key Terms Solute: the substance dissolved in a solution. Solvent: the dissolving medium in a solution. Solution: a homogenous mixture of solute + solvent

Kool-Aid What are the solute(s) and solvent?

Chocolate Milk What are the solute and solvent?

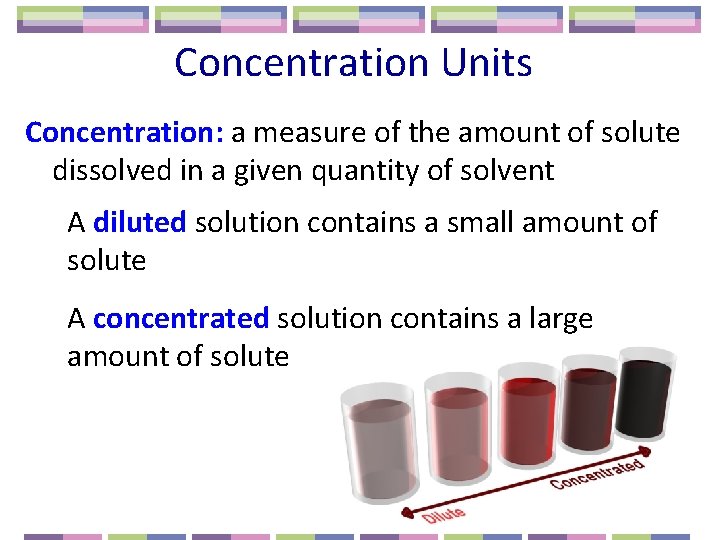
Concentration Units Concentration: a measure of the amount of solute dissolved in a given quantity of solvent A diluted solution contains a small amount of solute A concentrated solution contains a large amount of solute

Order from least to most concentrated 0. 10 M 0. 95 M 0. 75 M 0. 25 M A B C D

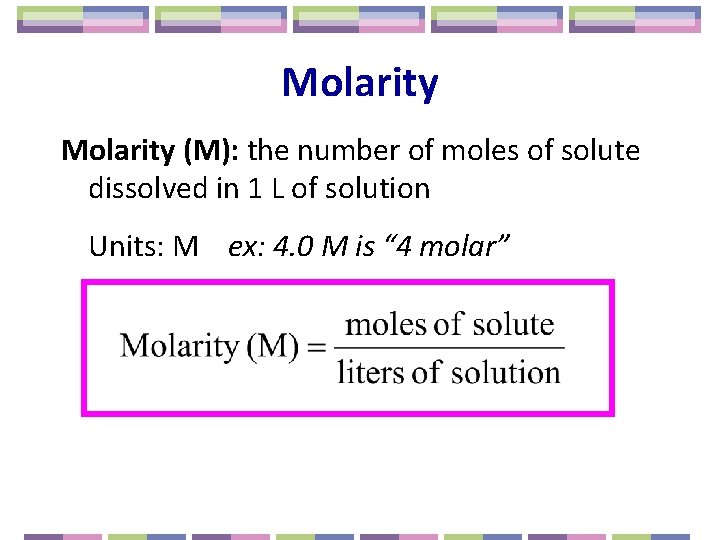
Molarity (M): the number of moles of solute dissolved in 1 L of solution Units: M ex: 4. 0 M is “ 4 molar”

Molarity Example: 0. 0117 mol of sugar is dissolved in a 0. 350 L teacup filled with hot water. What is the molarity of the sugar solution? Known: moles = 0. 0117 mol volume = 0. 350 L Unknown: Molarity Equation: Answer: 0. 0334 M

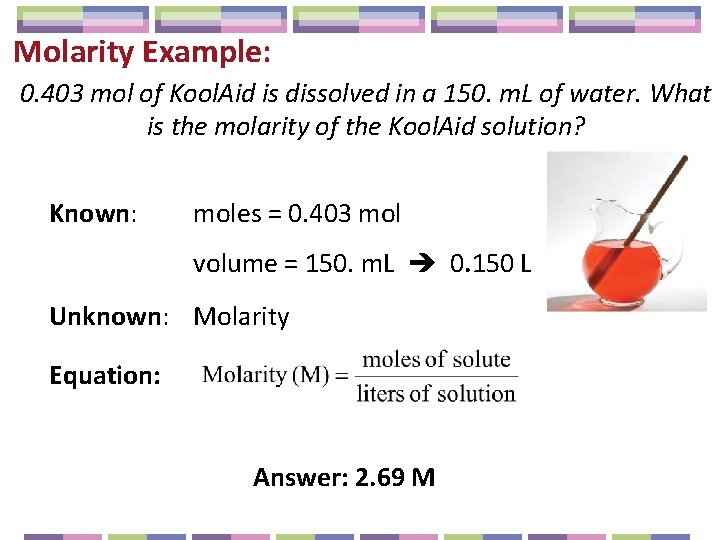
Molarity Example: 0. 403 mol of Kool. Aid is dissolved in a 150. m. L of water. What is the molarity of the Kool. Aid solution? Known: moles = 0. 403 mol volume = 150. m. L 0. 150 L Unknown: Molarity Equation: Answer: 2. 69 M

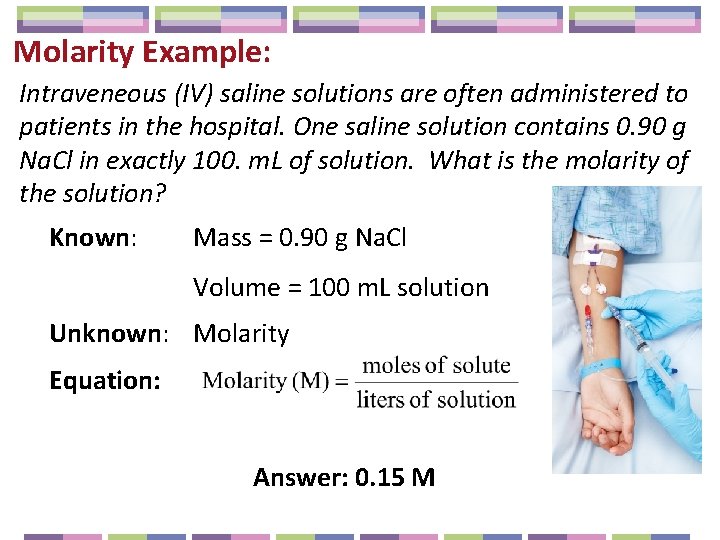
Molarity Example: Intraveneous (IV) saline solutions are often administered to patients in the hospital. One saline solution contains 0. 90 g Na. Cl in exactly 100. m. L of solution. What is the molarity of the solution? Known: Mass = 0. 90 g Na. Cl Volume = 100 m. L solution Unknown: Molarity Equation: Answer: 0. 15 M

Work Time Work on Practice sheet #1 l Hold onto this Work on Practice sheet #2 l Hold onto this When finished with both, check answers

Molarity Exit Slip What is the molarity of a solution in which 5. 3 grams of Ca. Cl 2 is dissolved in water to make 2. 1 L of solution? SHOW ALL OF YOUR WORK!

More Complex Example Problem A 4 g sugar cube (sucrose: C 12 H 22 O 11) is dissolved in a 350 ml teacup filled with hot water. What is the molarity of the sugar solution? How many m. L of solution will result when 15. 0 g of H 2 SO 4 is dissolved to make a 0. 200 M solution