Solutions I II II Concentration Solutions II Concentration







- Slides: 17

Solutions I II II. Concentration

Solutions II Concentration u The amount of solute in a solution. u Describing Concentration • % by mass - medicated creams • % by volume - rubbing alcohol • ppm, ppb - water contaminants • molarity - used by chemists • molality - used by chemists

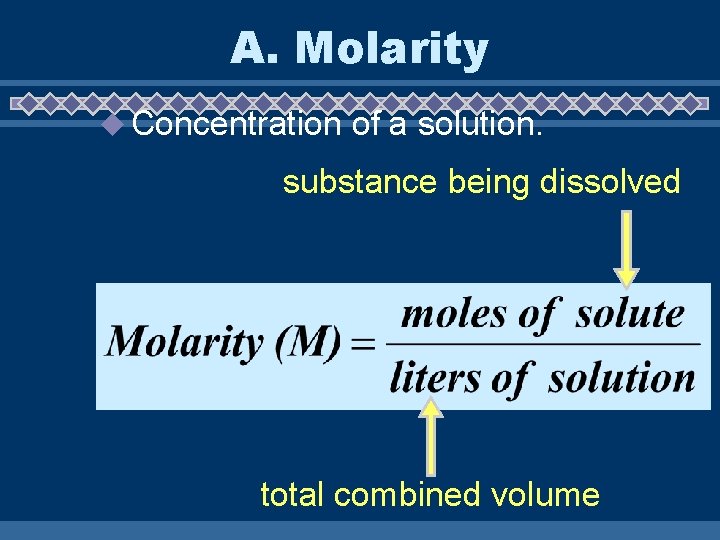
A. Molarity u Concentration of a solution. substance being dissolved total combined volume

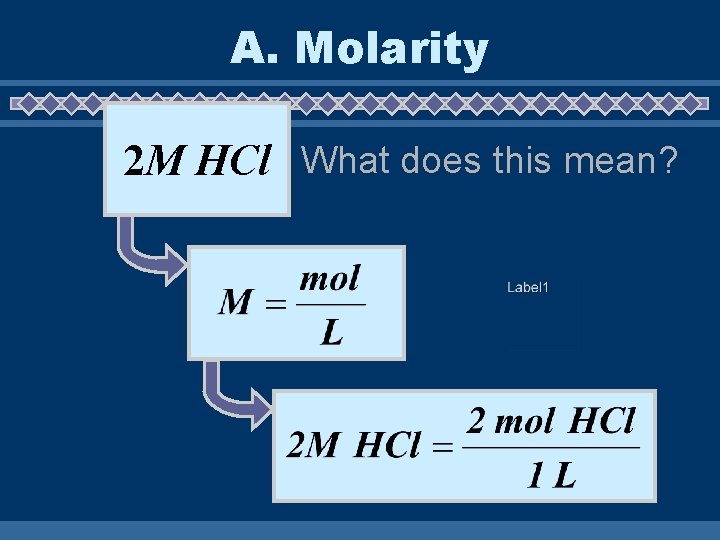
A. Molarity 2 M HCl What does this mean?

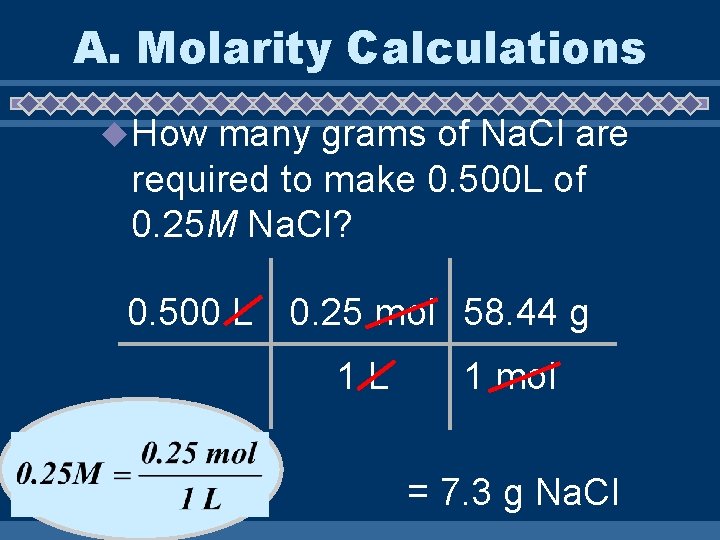
A. Molarity Calculations u How many grams of Na. Cl are required to make 0. 500 L of 0. 25 M Na. Cl? 0. 500 L 0. 25 mol 58. 44 g 1 L 1 mol = 7. 3 g Na. Cl

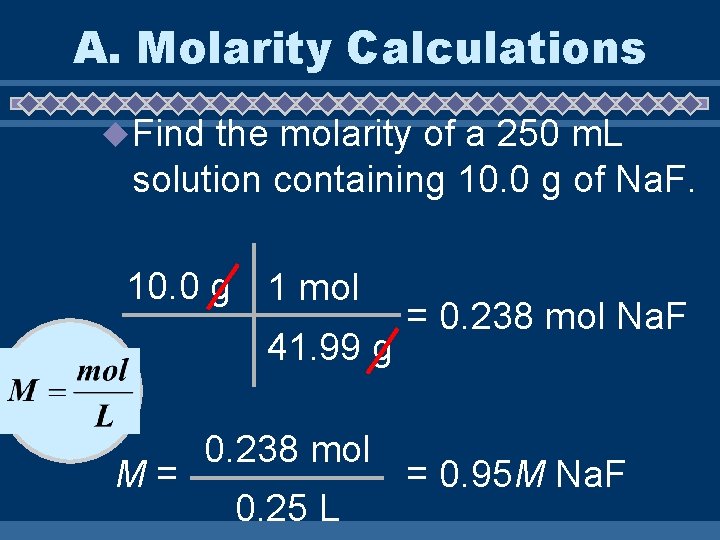
A. Molarity Calculations u Find the molarity of a 250 m. L solution containing 10. 0 g of Na. F. 10. 0 g 1 mol 41. 99 g M= 0. 238 mol 0. 25 L = 0. 238 mol Na. F = 0. 95 M Na. F

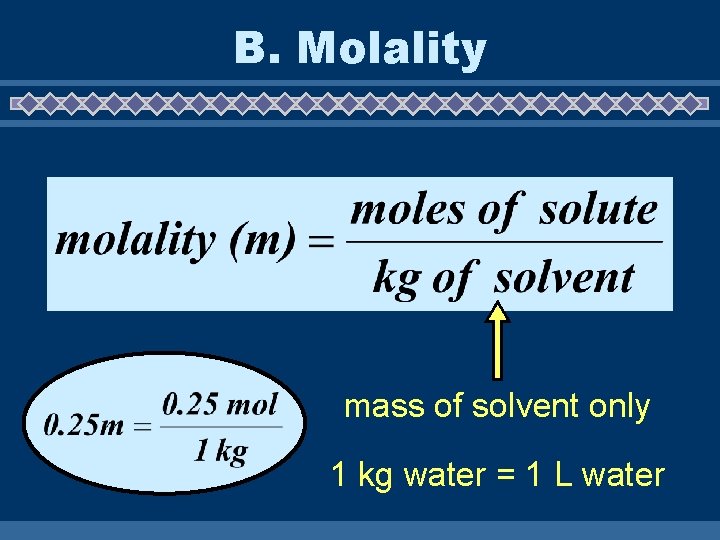
B. Molality mass of solvent only 1 kg water = 1 L water

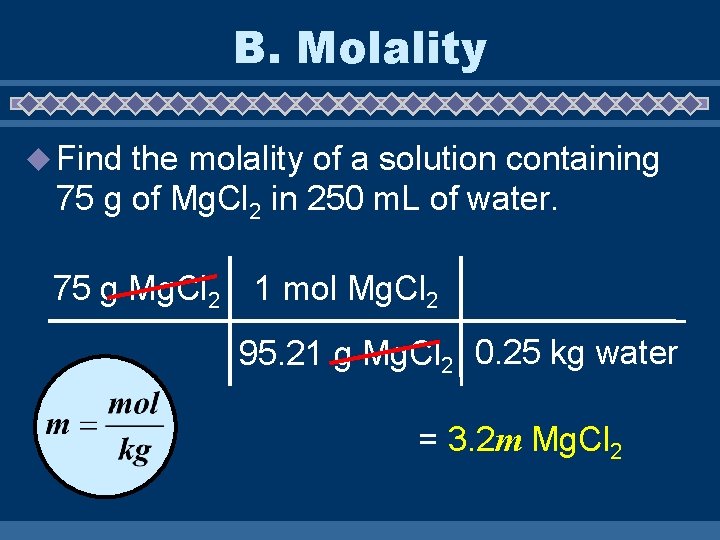
B. Molality u Find the molality of a solution containing 75 g of Mg. Cl 2 in 250 m. L of water. 75 g Mg. Cl 2 1 mol Mg. Cl 2 95. 21 g Mg. Cl 2 0. 25 kg water = 3. 2 m Mg. Cl 2

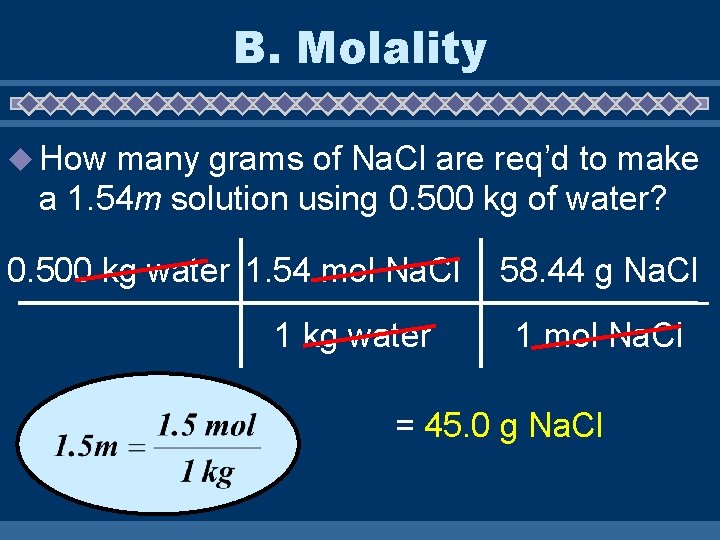
B. Molality u How many grams of Na. Cl are req’d to make a 1. 54 m solution using 0. 500 kg of water? 0. 500 kg water 1. 54 mol Na. Cl 1 kg water 58. 44 g Na. Cl 1 mol Na. Cl = 45. 0 g Na. Cl

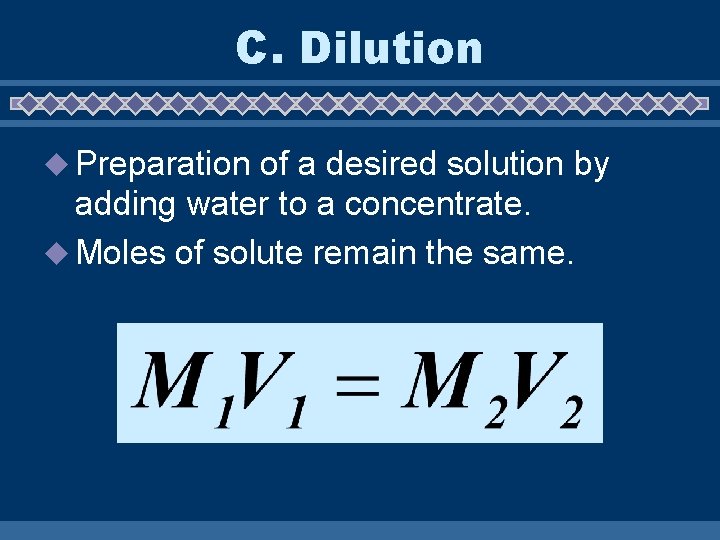
C. Dilution u Preparation of a desired solution by adding water to a concentrate. u Moles of solute remain the same.

C. Dilution u What volume of 15. 8 M HNO 3 is required to make 250 m. L of a 6. 0 M solution? GIVEN: M 1 = 15. 8 M V 1 = ? M 2 = 6. 0 M V 2 = 250 m. L WORK: M 1 V 1 = M 2 V 2 (15. 8 M) V 1 = (6. 0 M)(250 m. L) V 1 = 95 m. L of 15. 8 M HNO 3

End 2010

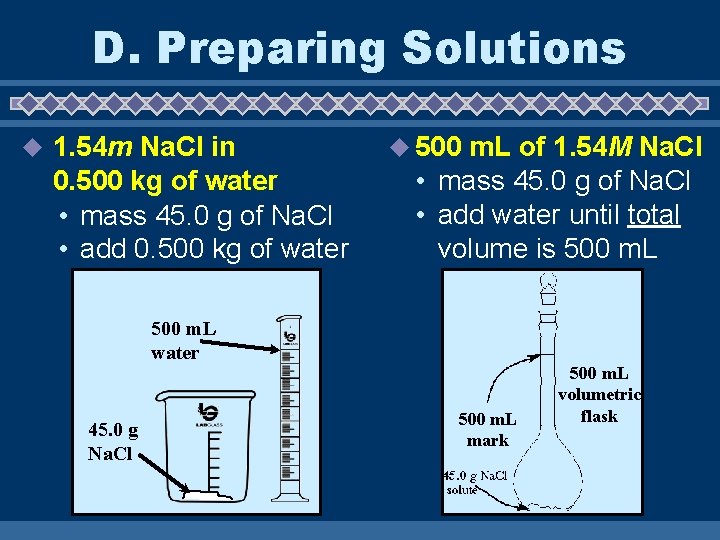
D. Preparing Solutions u 1. 54 m Na. Cl in 0. 500 kg of water • mass 45. 0 g of Na. Cl • add 0. 500 kg of water u 500 m. L of 1. 54 M Na. Cl • mass 45. 0 g of Na. Cl • add water until total volume is 500 m. L water 45. 0 g Na. Cl 500 m. L mark 500 m. L volumetric flask

D. Preparing Solutions Copyright © 1995 -1996 NT Curriculum Project, UW-Madison (above: “Filling the volumetric flask”)

D. Preparing Solutions Copyright © 1995 -1996 NT Curriculum Project, UW-Madison (above: “Using your hand as a stopper”)

D. Preparing Solutions u 250 m. L of 6. 0 M HNO 3 by dilution 95 m. L of 15. 8 M HNO 3 • measure 95 m. L of 15. 8 M HNO 3 • combine with water until total volume is 250 m. L mark • Safety: “Do as you oughtta, add the acid to the watta!” water for safety

Solution Preparation Lab u Turn in one paper team. u Complete the following steps: A) Show the necessary calculations. B) Write out directions for preparing the solution. C) Prepare the solution. u For each of the following solutions: 1) 100. 0 m. L of 0. 50 M Na. Cl 2) 0. 25 m Na. Cl in 100. 0 m. L of water 3) 100. 0 m. L of 3. 0 M HCl from 12. 1 M concentrate.