Chapter 11 Gases Four factors that can affect










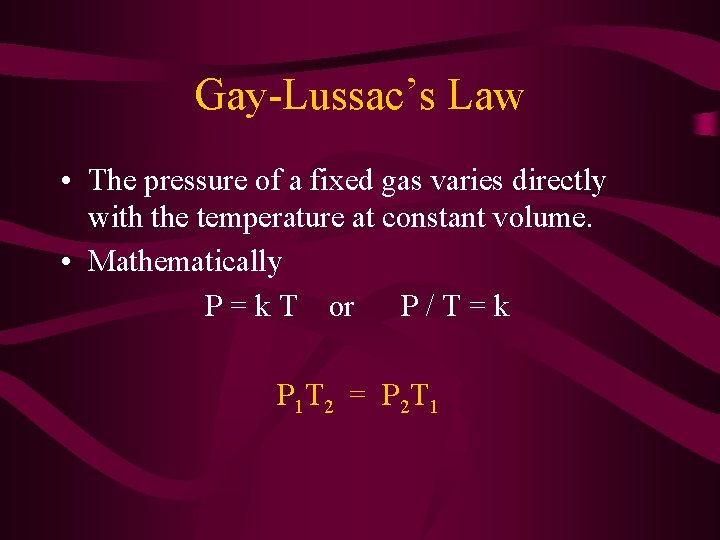















- Slides: 36

Chapter 11 Gases Four factors that can affect the behavior of a gas. – Amount of gas (n) = moles – Volume (V), 1000 cm 3 = 1000 m. L = 1 L – Temperature (T), Celsius and Kelvins = o. C + 273 – Pressure(P), atmospheres(atm), mm. Hg, or k. Pa


Nature of Gases • 1 mole of any gas at STP equals 22. 4 L of volume. • STP is defined at sea level. – Standard Temperature = 0 o. C = 273 K – Standard Pressure = 1 atm = 101. 3 k. Pa = 760 mm. Hg = 760 torrs • Normal boiling point of water is 100 o. C at sea level. • Higher elevation lower boiling points. – Less Pressure above the surface of water.

Pressure and Force Pressure = Force / Area P = F/A • Reduce the area - Increase the Pressure • Increase the force - Increase the Pressure S. I Unit for Force - N (Newton) S. I Unit for Area - m 2 S. I Unit for Pressure - Pa (Pascal) = 1 N/ m 2

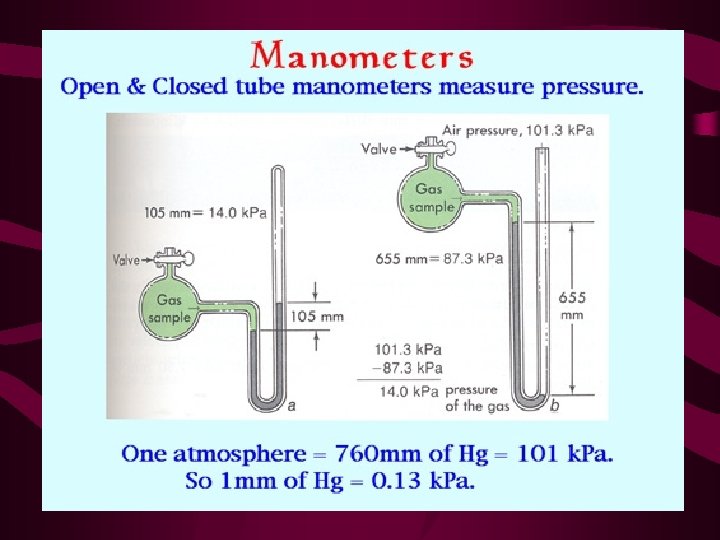
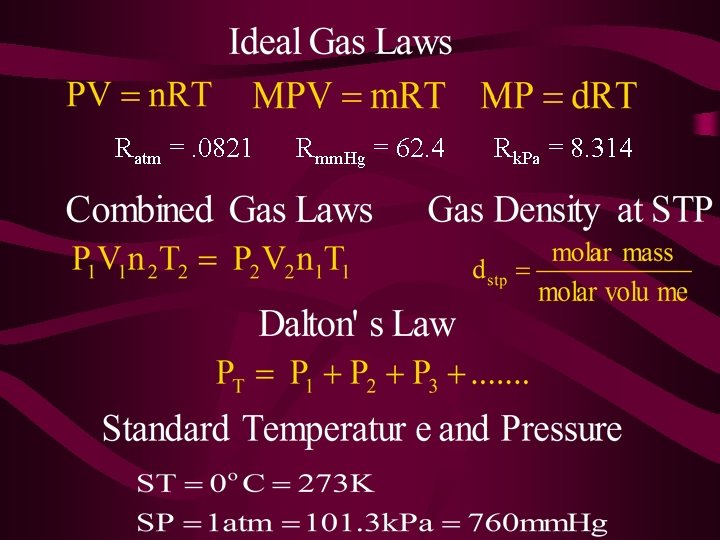
Standard Temperature & Pressure The volume of a gas depends upon • Pressure • Temperature In order to do a comparison of the volumes of various gases the gases must have the same temperature and pressure. Scientist agreed upon; STP - Temp. = 0 °C , Press. = 1 atm = 101. 3 k. Pa = 760 mm Hg

Dalton’s Law of Partial Pressure • The total pressure of a mixture of gases is equal to the sum of all the partial pressures. Partial pressure - pressure of one gas in a mixture of gases PT = P 1 + P 2 + P 3 + …

Sample Problem Determine the pressure of oxygen gas in a container that is under 1 atm of pressure and contains carbon dioxide and nitrogen. Note: PCO 2 =. 285 mm. Hg, PN 2 = 594 mm. Hg 760 mm. Hg = PO 2 +. 285 mm. Hg + 594 mm. Hg PO 2 = 165. 715 mm. Hg

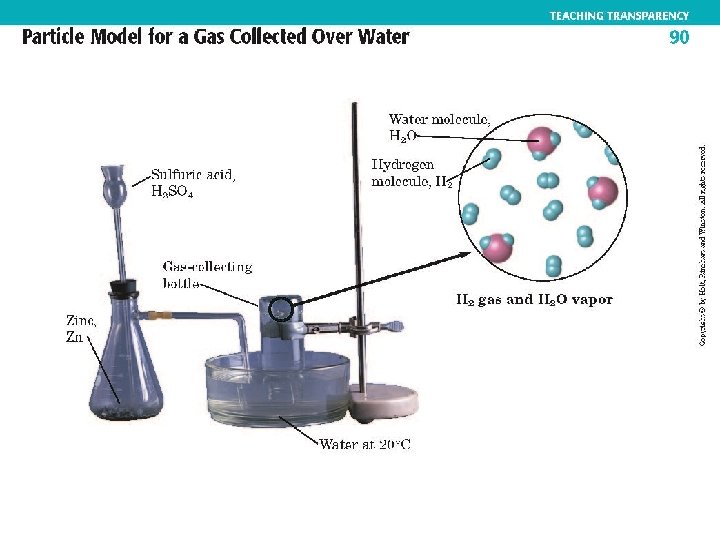
Water Displacement page 859 A-8 • Gases are collected through water displacement. • Water vapor particles are trapped with the gas being collected. • Corrected pressure of the gas is determined through the following equation. Patm = Pgas + PH 2 O


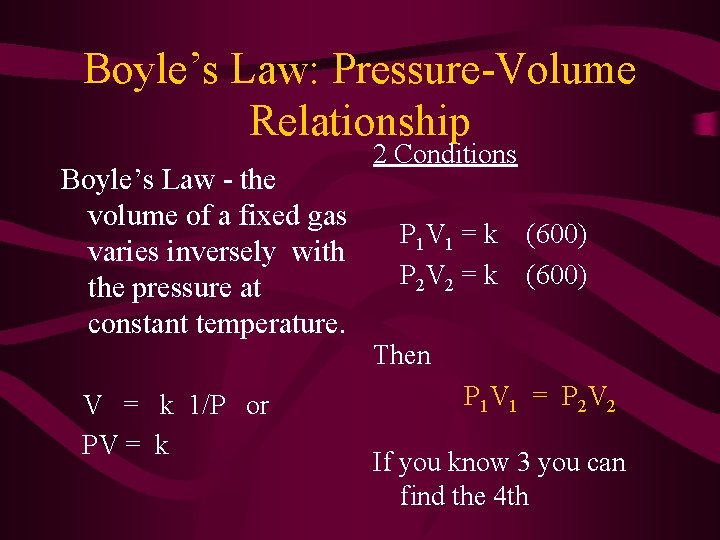
Boyle’s Law: Pressure-Volume Relationship Boyle’s Law - the volume of a fixed gas varies inversely with the pressure at constant temperature. 2 Conditions P 1 V 1 = k P 2 V 2 = k (600) Then V = k 1/P or PV = k P 1 V 1 = P 2 V 2 If you know 3 you can find the 4 th

Sample Problem A sample of gas collected occupies a volume of 150. m. L when its pressure is 720. mm. Hg. What volume will it occupy if its pressure is changed to 750. mm. Hg?

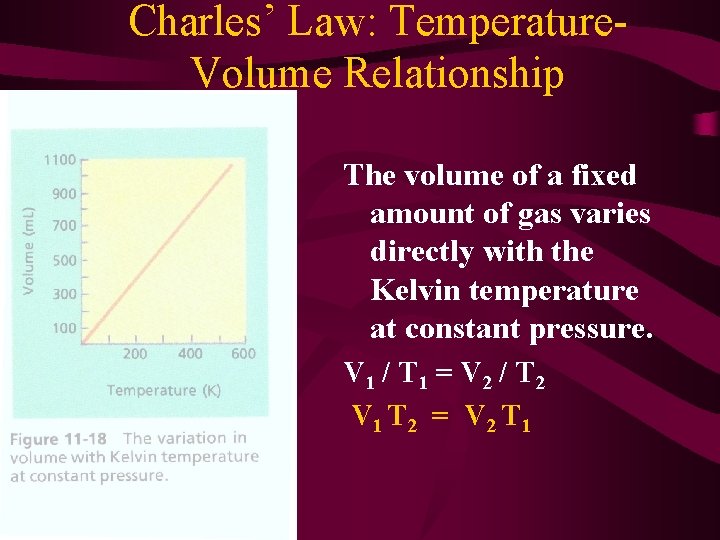
Charles’ Law: Temperature. Volume Relationship The volume of a fixed amount of gas varies directly with the Kelvin temperature at constant pressure. V 1 / T 1 = V 2 / T 2 V 1 T 2 = V 2 T 1

Charles’ Law Temperature must be in Kelvin! Absolute Zero - lowest possible temperature, all kinetic energy ceases. -273. 15 °C

Sample Problem A sample of neon gas occupies a volume of 752 m. L at 25 °C. What volume will it occupy at 50. °C. P, n are constant.
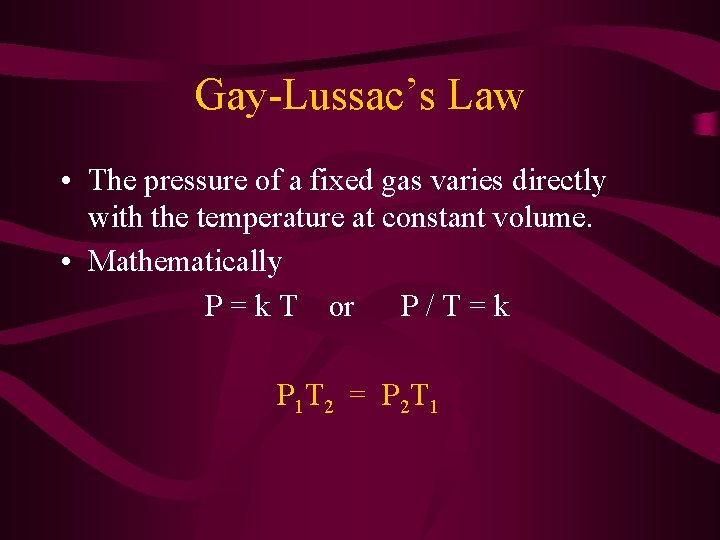
Gay-Lussac’s Law • The pressure of a fixed gas varies directly with the temperature at constant volume. • Mathematically P = k T or P/T=k P 1 T 2 = P 2 T 1

Sample Problem The gaseous contents in an aerosol can are under a pressure of 3. 00 atm at 25 °C. If the temperature is increased to 52 °C, what would the pressure of the can be?

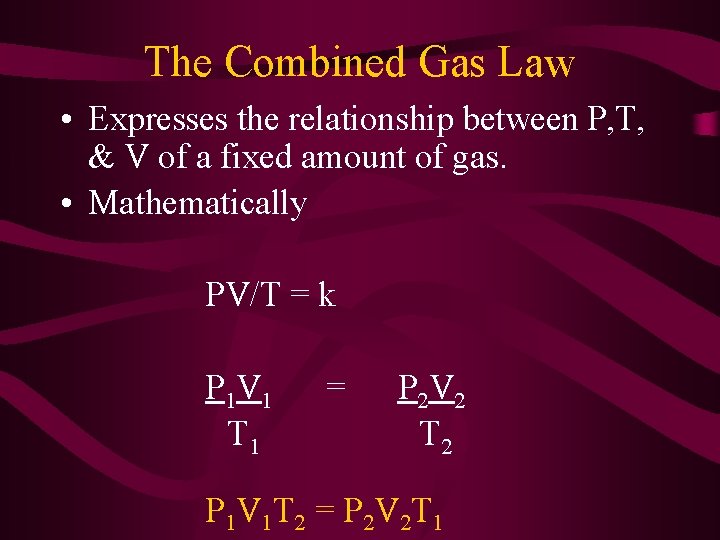
The Combined Gas Law • Expresses the relationship between P, T, & V of a fixed amount of gas. • Mathematically PV/T = k P 1 V 1 T 1 = P 2 V 2 T 2 P 1 V 1 T 2 = P 2 V 2 T 1

Sample Problem A helium-filled balloon has a volume of 50. 0 L at 25°C and 820. mm. Hg. What volume will it occupy at 650. mm. Hg and 10. °C?

Water Displacement • A sample of methane gas that was collected through water displacement had a volume of 350 m. L at 27. 0 o. C and 720. mm. Hg. What is the pressure at 2. 0 o. C and 250. m. L?

Avogadro’s Law • Equal volumes of gases at the same temperature and pressure contains an equal number of gas particles. • At STP, 22. 4 L = 1 mol • V 1 n 2 = V 2 n 1

Sample Problem • Determine the number of moles of helium that are held in a 250. m. L container. Consider that 2. 00 moles can be held in a 3. 00 L container.

11 -3 : Ideal Gas Law • Describes the physical behavior of an ideal gas in terms of pressure, volume, temperature and number of moles. • The combination of all 4 gas laws from the previous section.

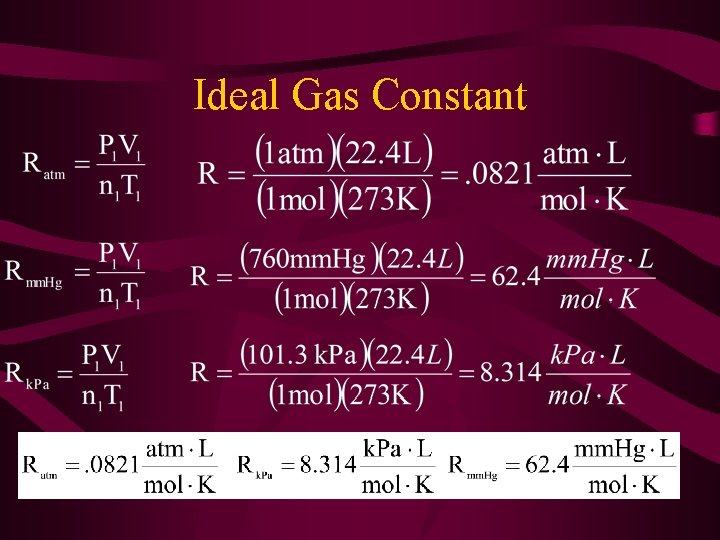
Derived Equation for the Ideal Gas Law • Needed an Ideal Gas Law Constant (R). • The seconditions were set at STP to equal the ideal behavior.

Ideal Gas Constant

Practice Problem • A camping stove uses a propane tank that holds 4. 0 moles of liquid C 3 H 8. How many liters will be needed to hold the same amount of propane at 25 o. C and 3 atm?

Gas Density at STP • The density of a gas at STP is constant, due to the standard molar volume of a gas. • Non-STP: • However the density of a gas changes with temperature and pressure, due to the volume in the equation. (must use ideal gas law)

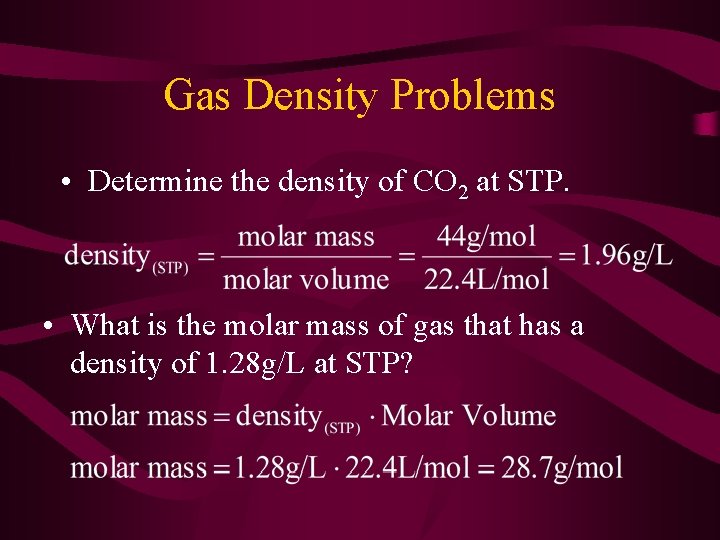
Gas Density Problems • Determine the density of CO 2 at STP. • What is the molar mass of gas that has a density of 1. 28 g/L at STP?

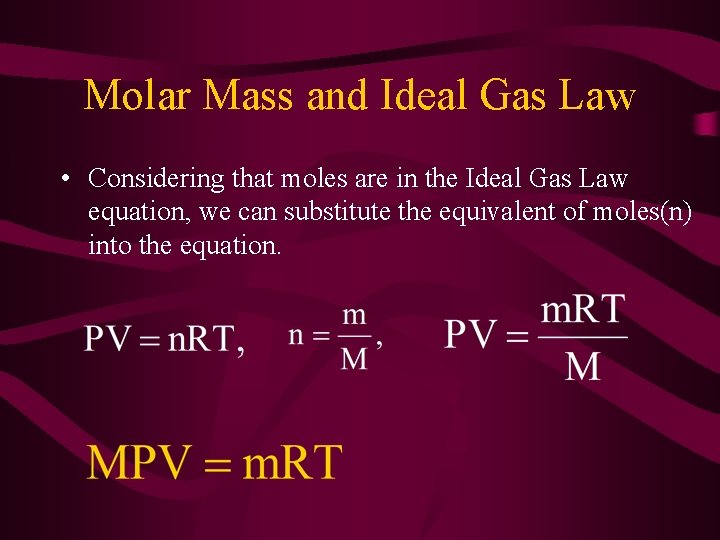
Molar Mass and Ideal Gas Law • Considering that moles are in the Ideal Gas Law equation, we can substitute the equivalent of moles(n) into the equation.

Density and the Ideal Gas Law • Now that mass(m) is in the equation we can substitute density(d) into the equation.

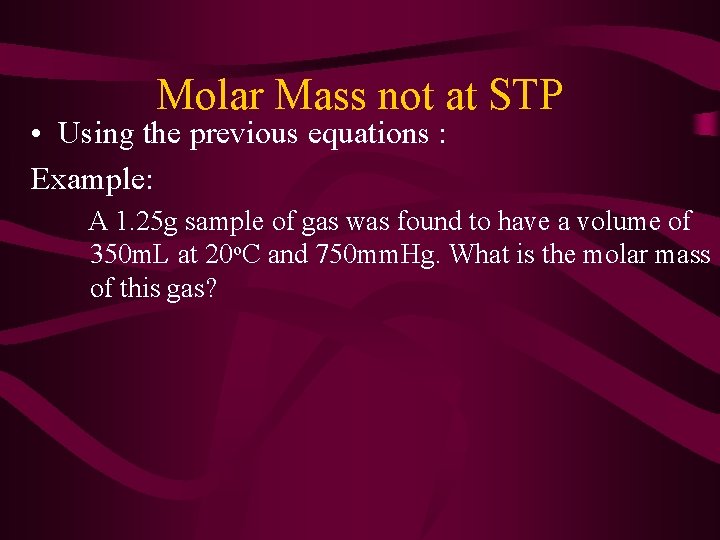
Molar Mass not at STP • Using the previous equations : Example: A 1. 25 g sample of gas was found to have a volume of 350 m. L at 20 o. C and 750 mm. Hg. What is the molar mass of this gas?

Ratm =. 0821 Rmm. Hg = 62. 4 Rk. Pa = 8. 314

Classwork 1. What is the molar mass of a gas that has a density of 2. 08 g/L at STP? 2. What is the density at STP of NO 2? 3. What is the molar mass of a gas, if it has a density of 3. 71 g/L at 22 o. C and 755 mm. Hg?

11 -4 Graham’s Law • Diffusion – Tendency of gas particles to travel toward areas of lower concentration. • Effusion – Gas escapes a tiny opening in a container. (one way diffusion) • Graham’s Law – Rate of effusion of a gas is inversely proportional to the square root of its molar mass. – Less mass = faster gas

Graham’s Law Problems • Which gas will diffuse into a container faster? CO 2 or NH 3? Why? • Compare the rates of effusion for F 2 and Cl 2.

Graham’s Law Problems At a certain temperature and pressure, Cl 2 has a velocity of. 038 m/s. What is the velocity of SO 2 at the same condition?

Determining the Molar Mass • An unknown gas was placed into a container with nitrogen gas. The N 2 was found to travel 1. 2 times faster than the unknown gas. What is the molar mass of this unknown gas?