Chapter 11 Gases Conditions that affect gases l































- Slides: 42

Chapter 11 Gases

Conditions that affect gases l l volume pressure temperature # of particles

Pressure and Force l Pressure = Example of pressure: force area

Pressure l l Atmospheric pressure- 14. 7 psi 78% nitrogen, 21% oxygen and 1% other gas

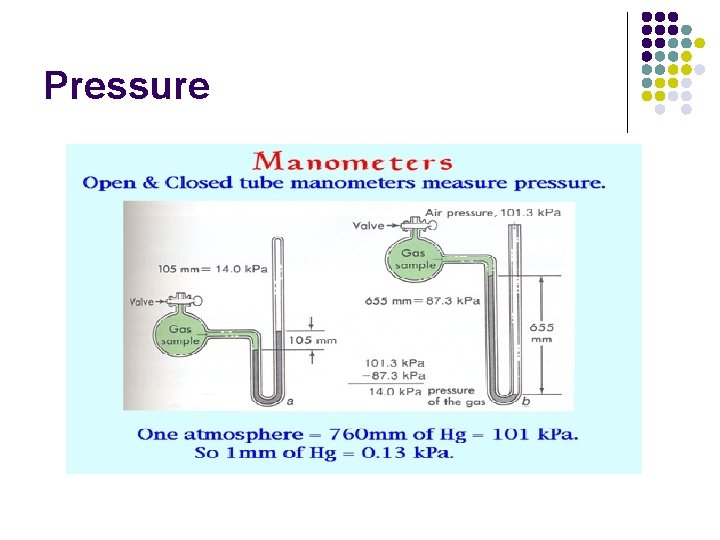
Pressure l Ways to measure pressure: l l l Open end manometer Closed end manometer Barometer

Pressure

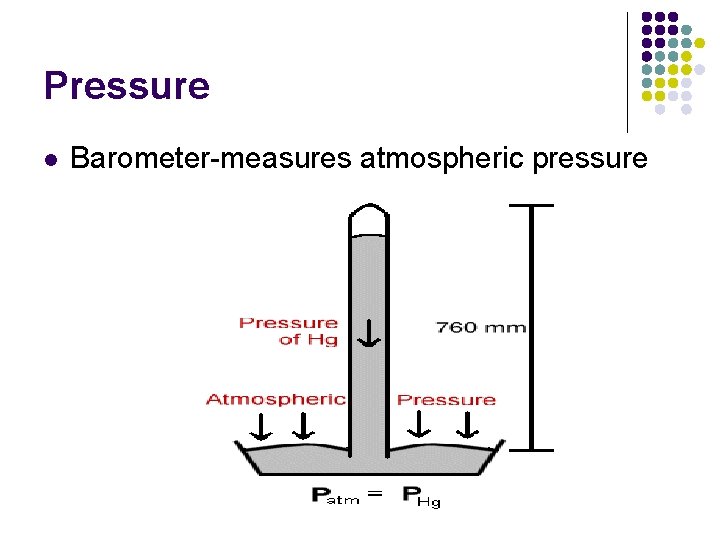
Pressure l Barometer-measures atmospheric pressure

Pressure l Units of Pressure: see pg. 364 l l l Millimeters of mercury Kilopascals, pascals atmospheres torr 760 mm. Hg = 1 atm= 101. 325 k. Pa = 760 torr

Pressure Conversions l Examples: l l Convert the pressure of 775 mm Hg to k. Pa and to atm. Practice: l Convert a pressure of 750. torr to a. Atm b. k. Pa

Standard Conditions l l l STP- Standard Temperature and Pressure Agreed upon standard 0°C and 1 atm

Dalton’s Law of Partial Pressures l l Each individual gas has its own pressure (partial pressure) Ptotal = Pa + Pb + Pc + …. .

Sample problems for Dalton’s Law l Example: Calculate the partial pressure in mm Hg exerted by the four main gases in air: nitrogen, oxygen, argon and carbon dioxide. Their abundance by volume is 78. 08%, 20. 95%, . 934% and. 035 % respectively.

Sample problems for Dalton’s Law l Example : In a sample containing carbon dioxide, nitrogen and oxygen at exactly 1 atm. The partial pressure of CO 2 is. 285 torr and the partial pressure of N 2 is 593. 525 torr. What is the partial pressure of O 2?

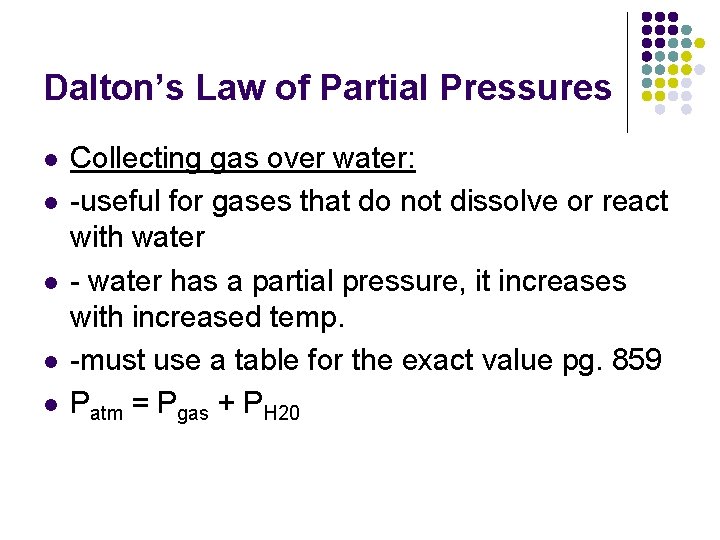
Dalton’s Law of Partial Pressures l l l Collecting gas over water: -useful for gases that do not dissolve or react with water - water has a partial pressure, it increases with increased temp. -must use a table for the exact value pg. 859 Patm = Pgas + PH 20

Dalton’s Law of Partial Pressures

Sample problems for Dalton’s Law l Example: Oxygen gas was collected from the decomposition of potassium chlorate. The atmospheric pressure was 731. 0 torr and the temperature of the water was 20. 0 C. What was the partial pressure of the oxygen gas collected?

Sample problems for Dalton’s Law l Practice: A sample of nitrogen gas is collected over water at a temperature of 23. 0 °C. What is the pressure of the nitrogen gas if the atmospheric pressure is 785 mm Hg?

The Simple Gas Laws l l Boyle’s Law Charles’s Law Gay-Lussac’s Law The Combined Gas Law

Boyle’s Law l When temp. and # of particles are constant the pressure and volume of a gas are inversely proportional l P 1 V 1= P 2 V 2

Boyle’s Law -practice l Example: A sample of gas has a volume of 150. 0 m. L when its pressure is. 947 atm. What will the volume of the gas be at a pressure of 0. 987 atm if the pressure and amount of gas remain constant? l Practice: A helium filled balloon contains 125 m. L of gas at a pressure of. 974 atm What volume will the gas occupy at standard pressure?

Charles’s Law l With constant amount of gas and pressure the volume and temperature of a gas will have a directly proportional relationship l V 1 = V 2 T 1 T 2 l NOTE: TEMPERATURE MUST BE IN KELVIN l K= ° C + 273

Charles's Law Practice l Example: A sample of neon gas occupies a volume of 752 m. L at 25 ° C. What volume will the gas occupy at 50° C if the pressure remains constant? l Practice: A balloon filled with oxygen gas occupies a volume of 5. 5 L at 25° C. What volume will the gas occupy at 100. ° C?

Gay-Lussac’s Law l Pressure and temperature of a gas are directly proportional if the amount and volume of gas remain constant l P 1 = P 2 T 1 T 2

Gay-Lussac’s Law Practice l Example: The gas in an aerosol can is at a pressure of 3. 00 atm at 25°C. What would the pressure of the gas be if the can was heated to 52° C? l Practice: The temperature within a car tire at the beginning of a long trip is 25° C. At the end of the trip the tire has a pressure of 1. 80 atm. What is the final temperature within the tire if its original pressure was 1. 75 atm?

The Combined Gas Law l Expresses all of the simple gas laws, relating temperature, pressure and volume of a gas as long as the amount of gas remains constant. l P 1 V 1 = T 1 l P 2 V 2 T 2 MEMORIZE this law, cross out the factors that remain constant.

The Combined Gas Law Practice l Example: A helium filled balloon has a volume of 50. 0 L at 25° C and 1. 08 atm. What will the new volume be if its pressure is decreased to. 855 atm and the temperature decreases to 10. ° C? l Practice: The volume of a gas at 27. 0 ° C and. 200 atm is 80. 0 m. L. What volume will the same gas sample occupy at standard conditions?

END of MATERIAL FOR TEST 1

Measuring and Comparing the Volumes of Reacting Gases l Law of Combining Volumes l at constant temperature and pressure, the volumes of gases react and can be expressed as whole numbers l Try this: Write and balance and equation for each. a. hydrogen gas and oxygen gas produces water vapor b. hydrogen gas and chlorine gas produce hydrogen chloride gas Now go to pg. 378 and check the results

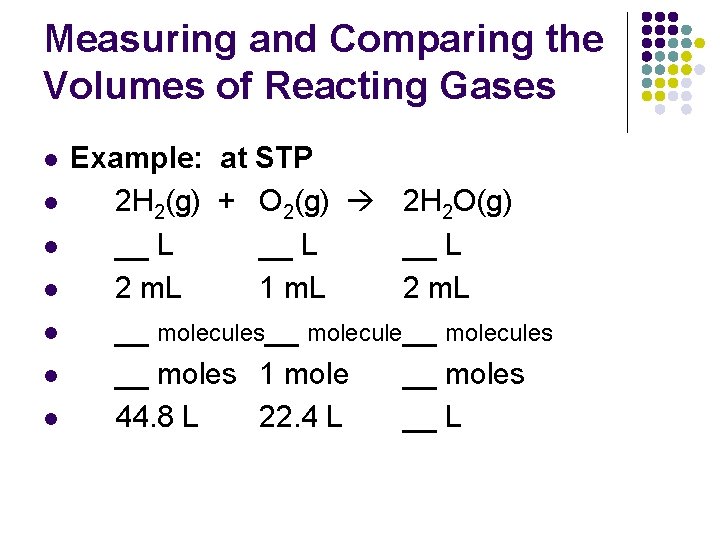
Measuring and Comparing the Volumes of Reacting Gases l Avogadro’s Law l l Equal volumes of gases at the same temperature and pressure contain equal # of molecules standard molar volume of a gas l one mole of any gas at STP = 22. 4 L of that gas

Measuring and Comparing the Volumes of Reacting Gases l l l l Example: at STP 2 H 2(g) + O 2(g) 2 H 2 O(g) __ L 2 m. L 1 m. L 2 m. L __ molecules__ molecules __ moles 1 mole __ moles 44. 8 L 22. 4 L __ L

Using Molar Volume of a Gas l You are planning an experiment that requires. 0580 mol of nitrogen monoxide gas. What volume would you need at STP? l Practice: A chemical reaction produces. 0680 mol of oxygen gas. What volume in liters is occupied by this gas at STP?

Using Molar Volume of a Gas l Challenge: Suppose you need 4. 22 g of chlorine gas, Cl 2. What volume at STP would you expect to use?

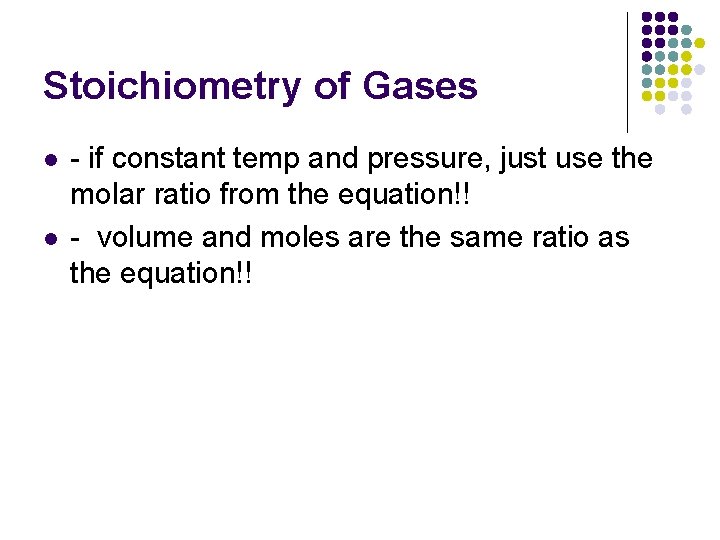
Stoichiometry of Gases l l - if constant temp and pressure, just use the molar ratio from the equation!! - volume and moles are the same ratio as the equation!!

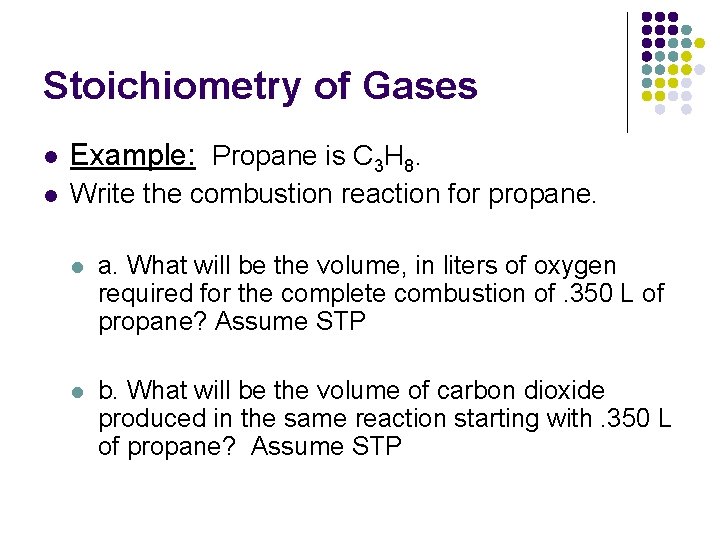
Stoichiometry of Gases l Example: Propane is C 3 H 8. l Write the combustion reaction for propane. l a. What will be the volume, in liters of oxygen required for the complete combustion of. 350 L of propane? Assume STP l b. What will be the volume of carbon dioxide produced in the same reaction starting with. 350 L of propane? Assume STP

Stoichiometry of Gases l Practice: Xenon gas reacts with fluorine gas to produce the gas xenon hexafluoride. Write the equation for this reaction. l a. Assuming constant temperature and pressure, what volume of xenon should be used to make 3. 14 L of Xe. F 6? l b. What volume of fluorine gas should be used to create 3. 14 L of Xe. F 6?

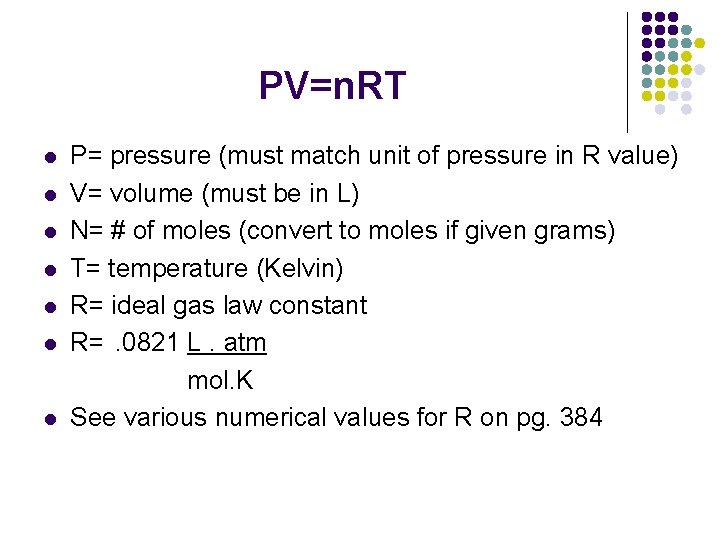
The Ideal Gas Law l l l includes, temperature, pressure, volume and # of moles single conditions, not P 1, P 2 PV= n. RT

PV=n. RT l l l l P= pressure (must match unit of pressure in R value) V= volume (must be in L) N= # of moles (convert to moles if given grams) T= temperature (Kelvin) R= ideal gas law constant R=. 0821 L. atm mol. K See various numerical values for R on pg. 384

Ideal Gas Law Practice l Example: What is the pressure in atm exerted by a. 500 mol sample of nitrogen gas in a 10. 0 L container at 298 K? l Practice: A 2. 07 L cylinder contains 2. 88 mol of helium gas at 22° C. What is the pressure in atm of the gas in the cylinder? (P= 33. 7 atm)

Ideal Gas Law Practice l Practice: A tank of hydrogen gas has a volume of 22. 9 L and holds 14. 0 mol of the gas at 12° C. What is the reading on the pressure gauge in mm Hg? (14. 3 atm 1. 09 X 104 mm. Hg) l Practice: A reaction yields 0. 00856 mol of oxygen gas. What volume in m. L will the gas occupy if it is collected at 43 °C and. 926 atm? (. 240 L 240. m. L)

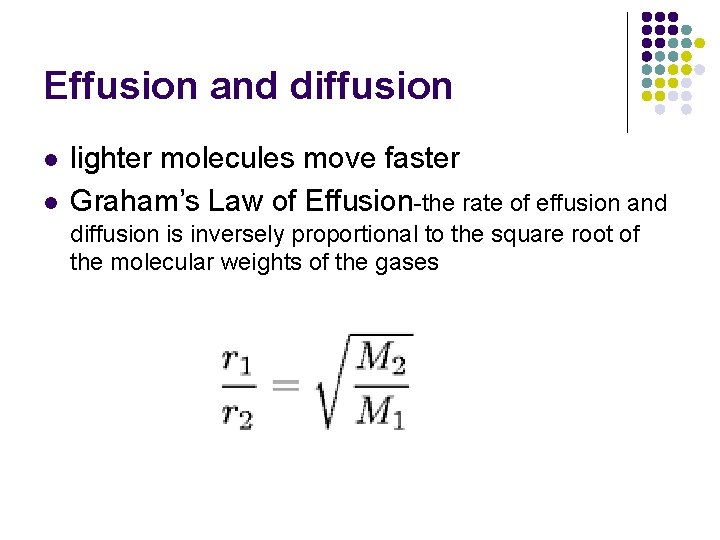
Effusion and diffusion l l lighter molecules move faster Graham’s Law of Effusion-the rate of effusion and diffusion is inversely proportional to the square root of the molecular weights of the gases

Graham’s Law Practice l Example: Nitrogen gas effuses through a pinhole 1. 7 times as fast as another element under the same conditions. Estimate the other element’s mass and determine its probable identity.

Graham’s Law Practice l Practice: Compare the rates of effusion of hydrogen and oxygen gas at the same conditions. (Remember, both are diatomic. )
 Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block xoang nhĩ
Block xoang nhĩ Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Chapter 5 transactions that affect
Chapter 5 transactions that affect Chapter 4 transactions that affect assets
Chapter 4 transactions that affect assets Chapter 1 how your choices affect income answer key
Chapter 1 how your choices affect income answer key Capital=assets+liabilities
Capital=assets+liabilities To give yourself more time for the ipde process at night
To give yourself more time for the ipde process at night Driving in adverse conditions chapter 12
Driving in adverse conditions chapter 12 Common chronic and acute conditions chapter 18
Common chronic and acute conditions chapter 18 Chapter 1 learning exercises medical terminology
Chapter 1 learning exercises medical terminology Overdriving headlights means
Overdriving headlights means Chapter 6 pay benefits and working conditions
Chapter 6 pay benefits and working conditions Avogadro's law relationship
Avogadro's law relationship Chapter 11 review gases section 1
Chapter 11 review gases section 1 Combined gas law practice worksheet answers
Combined gas law practice worksheet answers Chapter 14 the behavior of gases
Chapter 14 the behavior of gases Gas laws
Gas laws Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids What 2 factors affect the force of gravity
What 2 factors affect the force of gravity Ocean currents
Ocean currents What two factors affect the force of friction
What two factors affect the force of friction Personality structure meaning
Personality structure meaning Weathering in the piney woods
Weathering in the piney woods How does gravity affect weathering
How does gravity affect weathering Osmosis equation
Osmosis equation Vray light shadow bias
Vray light shadow bias Balanced force
Balanced force Factors that affect disease transmission
Factors that affect disease transmission Nutrient cycle of a tropical rainforest
Nutrient cycle of a tropical rainforest Historical background of romeo and juliet
Historical background of romeo and juliet Introduction to romeo and juliet
Introduction to romeo and juliet How does the sun affect tides
How does the sun affect tides How does changing the mean affect a normal curve
How does changing the mean affect a normal curve How does the approaching ceremony of twelves affect jonas?
How does the approaching ceremony of twelves affect jonas?