Chapter 10 Zeroth Law Temperature Ideal Gas Law



- Slides: 25

Chapter 10: Zeroth Law, Temperature Ideal Gas Law Kinetic Theory of Gases Wednesday, November 11, 1998

Thermal Physics, Part I The material in this chapter is almost certainly review for any of you who have sat through a chemistry course, so we’ll move rather quickly. If you have trouble with this material, come by and see me. I’m happy to work with you the extra hours you may require to master this material.

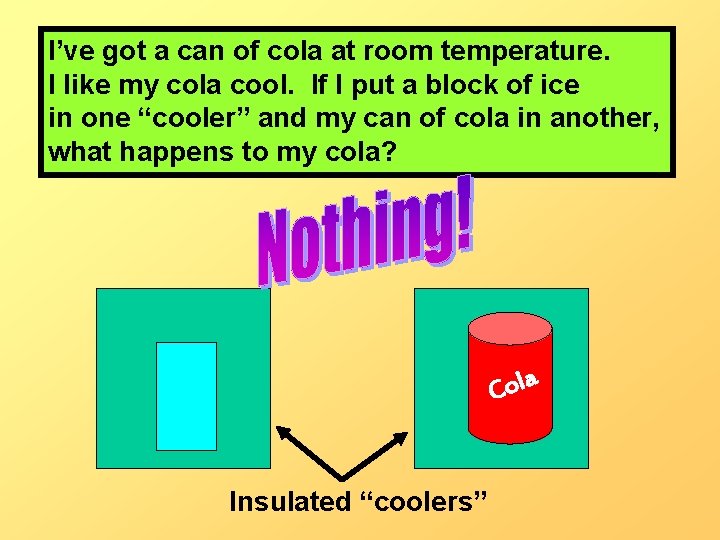
I’ve got a can of cola at room temperature. I like my cola cool. If I put a block of ice in one “cooler” and my can of cola in another, what happens to my cola? a l o C Insulated “coolers”

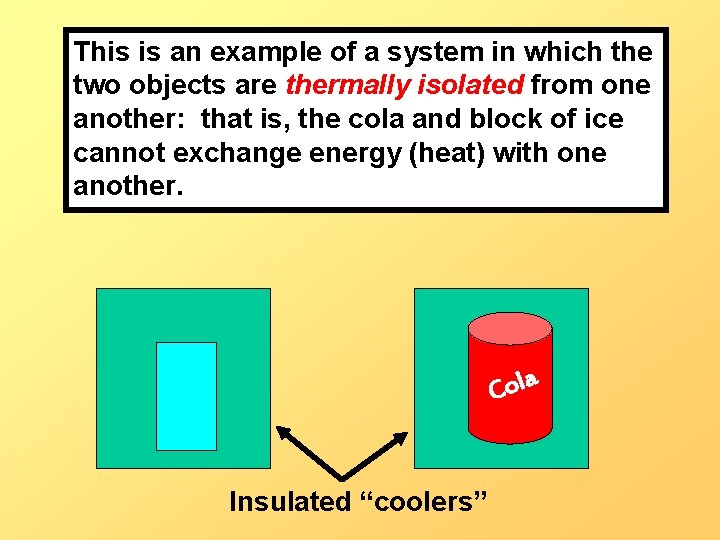
This is an example of a system in which the two objects are thermally isolated from one another: that is, the cola and block of ice cannot exchange energy (heat) with one another. a l o C Insulated “coolers”

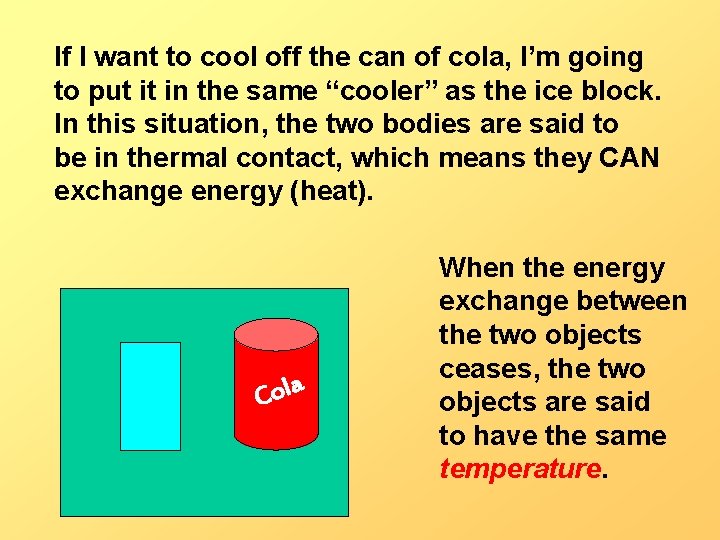
If I want to cool off the can of cola, I’m going to put it in the same “cooler” as the ice block. In this situation, the two bodies are said to be in thermal contact, which means they CAN exchange energy (heat). Cola When the energy exchange between the two objects ceases, the two objects are said to have the same temperature.

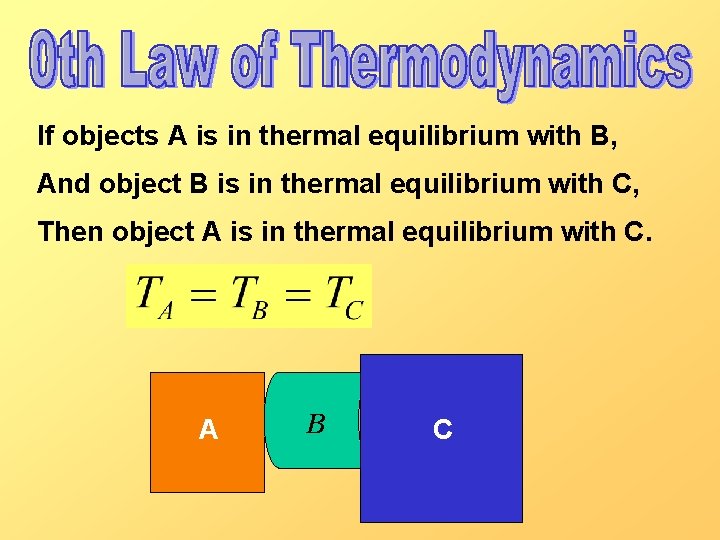
If objects A is in thermal equilibrium with B, And object B is in thermal equilibrium with C, Then object A is in thermal equilibrium with C. A B C

We will avoid the Fahrenheit scale with which we are all most familiar from the evening weather forecasts. In the Fahrenheit system, water freezes at 32 o and boils at 212 o. The Celsius (or Centigrade) scale is more natural physical scale with which to measure temperature. In the Celsius system, water freezes at 0 o and boils at 100 o.

The best scale on which to measure temperatures in physics problems, however, is the Kelvin scale. In the Kelvin system, water freezes at 273. 15 and boils at 373. 15. The Kelvin scale of temperature nicely translates temperature to energy. The scale is chosen so that at 0 K, the molecules within a gas (assuming the substance could remain a gas) would come to a complete stop. We’ll explore this more when we talk about the kinetic theory of gases.

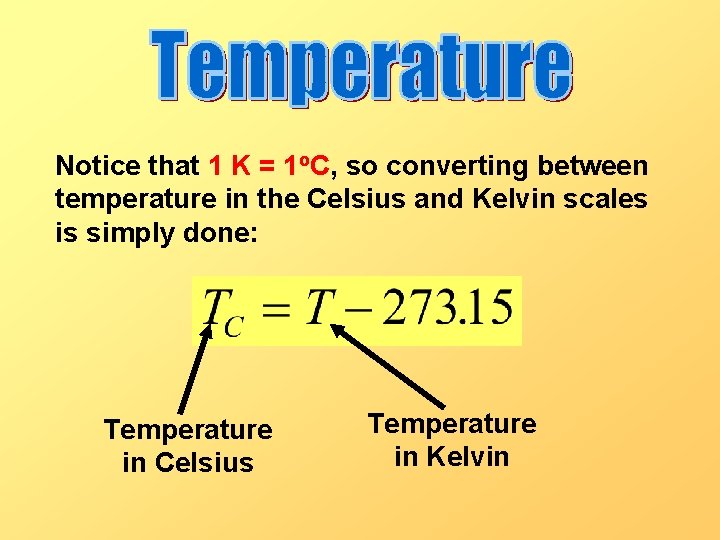
Notice that 1 K = 1 o. C, so converting between temperature in the Celsius and Kelvin scales is simply done: Temperature in Celsius Temperature in Kelvin

The response of materials to increases in temperatures is typically to expand, with one exception: water expands when cooled! Section 10. 3 of your book covers the response of materials to changes in temperature (I. e. , thermal expansion). You will NOT be held responsible for this material on the test. Here are the results, FYI: Linear expansions L 0 DL a = coefficient of linear expansion

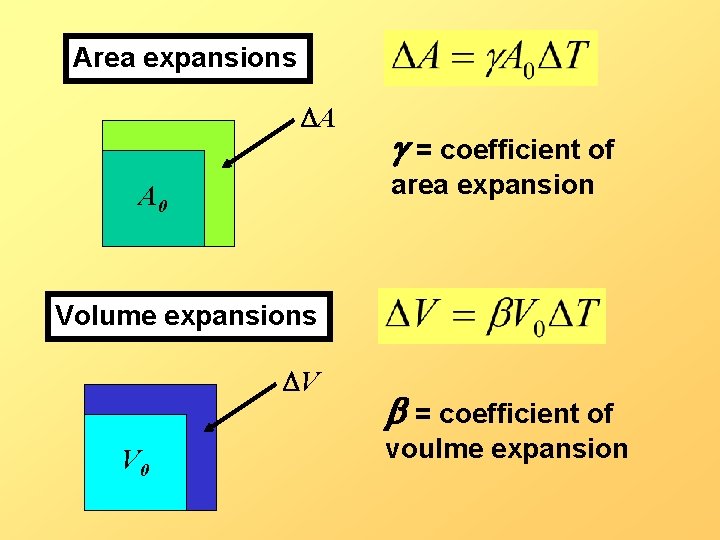
Area expansions DA g = coefficient of area expansion A 0 Volume expansions DV V 0 b = coefficient of voulme expansion

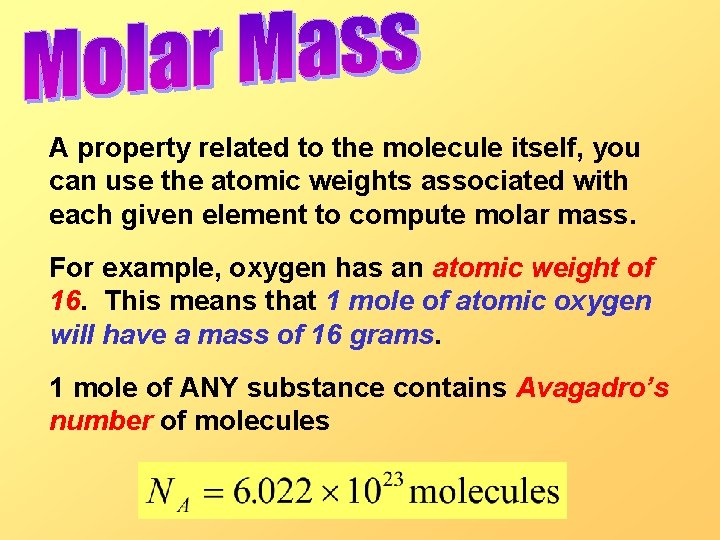
A property related to the molecule itself, you can use the atomic weights associated with each given element to compute molar mass. For example, oxygen has an atomic weight of 16. This means that 1 mole of atomic oxygen will have a mass of 16 grams. 1 mole of ANY substance contains Avagadro’s number of molecules

Most gases with which we deal on a day-to-day basis behave as ideal gases. Ideal gases can be identified by the fact that they obey the ideal gas law, a physical law that governs the relationship of the pressure of a gas, the number of molecules in the gas, the volume of the gas, and its temperature.

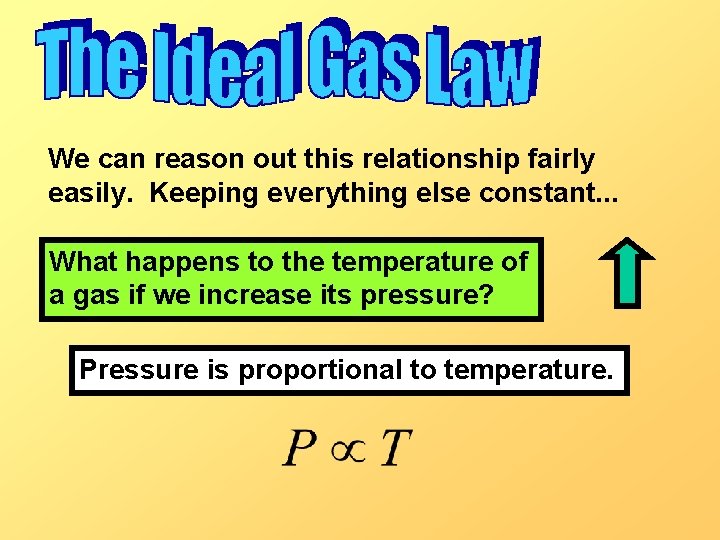
We can reason out this relationship fairly easily. Keeping everything else constant. . . What happens to the temperature of a gas if we increase its pressure? Pressure is proportional to temperature.

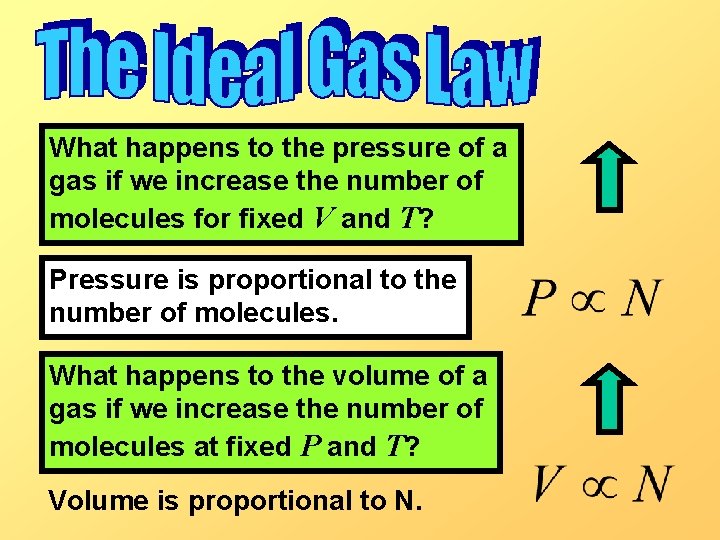
What happens to the pressure of a gas if we increase the number of molecules for fixed V and T? Pressure is proportional to the number of molecules. What happens to the volume of a gas if we increase the number of molecules at fixed P and T? Volume is proportional to N.

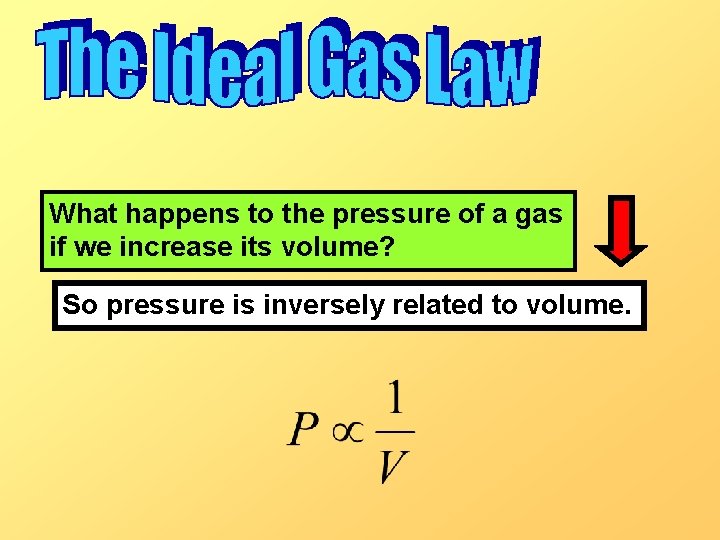
What happens to the pressure of a gas if we increase its volume? So pressure is inversely related to volume.

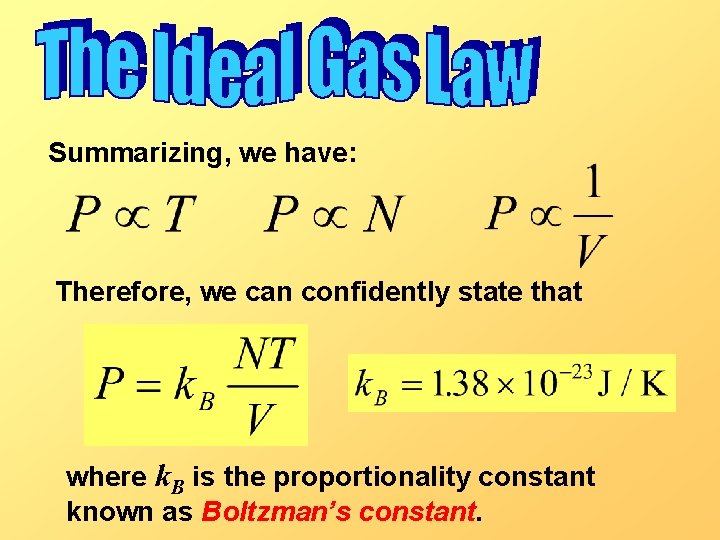
Summarizing, we have: Therefore, we can confidently state that where k. B is the proportionality constant known as Boltzman’s constant.

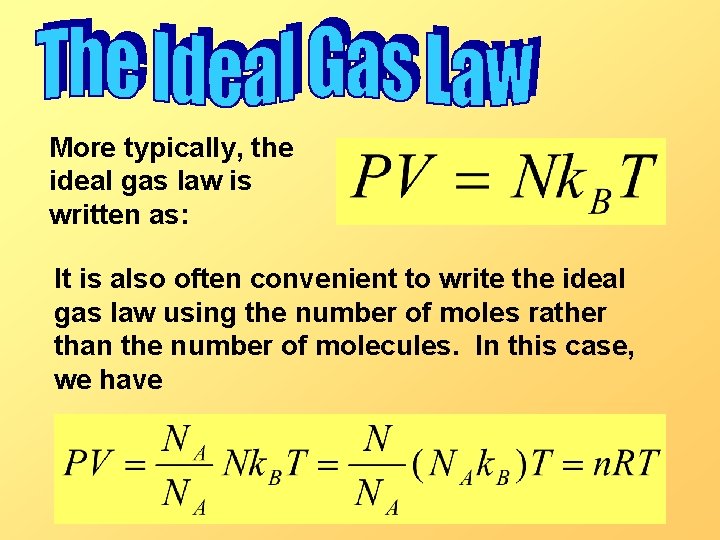
More typically, the ideal gas law is written as: It is also often convenient to write the ideal gas law using the number of moles rather than the number of molecules. In this case, we have

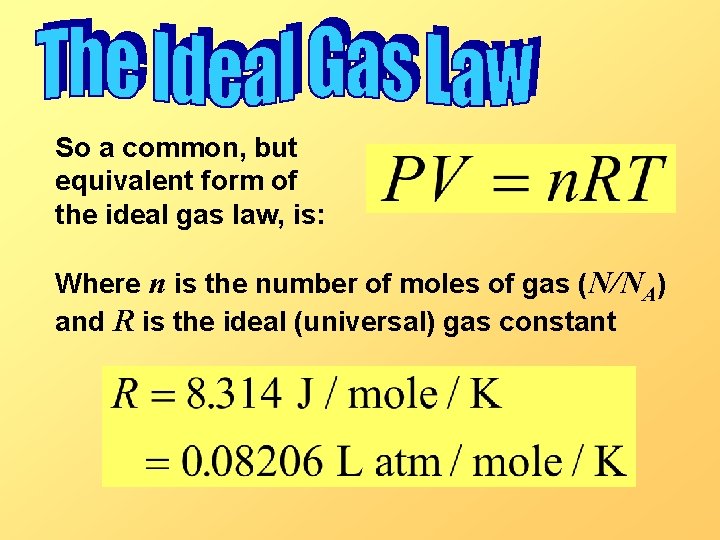
So a common, but equivalent form of the ideal gas law, is: Where n is the number of moles of gas (N/NA) and R is the ideal (universal) gas constant

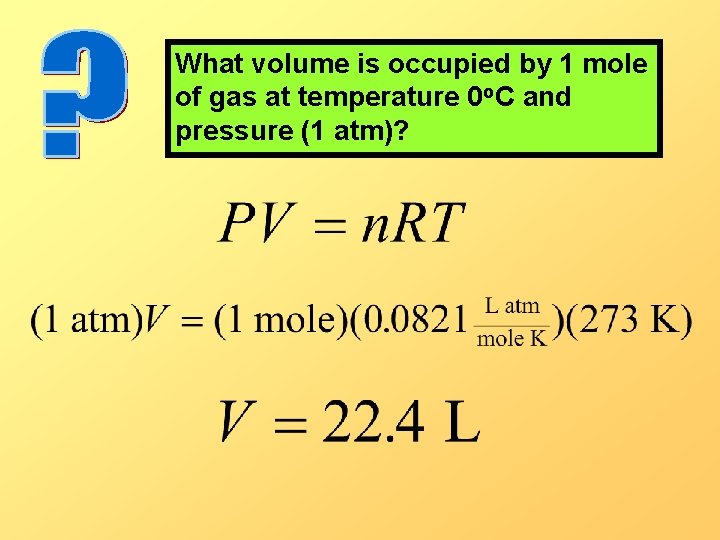
What volume is occupied by 1 mole of gas at temperature 0 o. C and pressure (1 atm)?

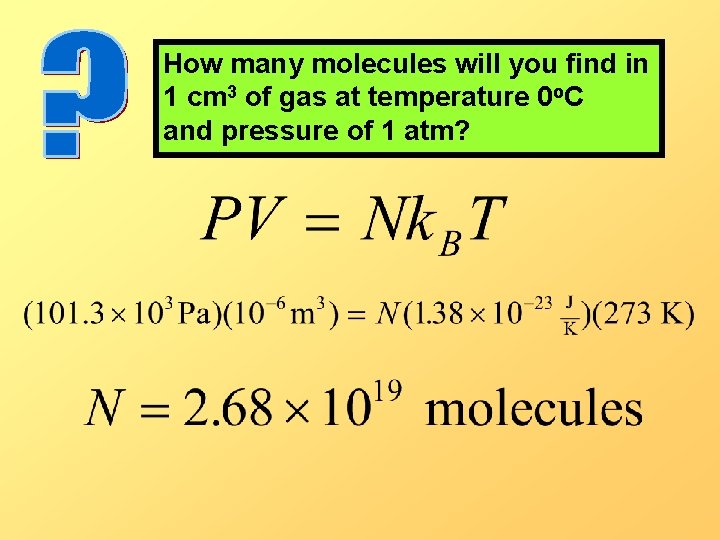
How many molecules will you find in 1 cm 3 of gas at temperature 0 o. C and pressure of 1 atm?

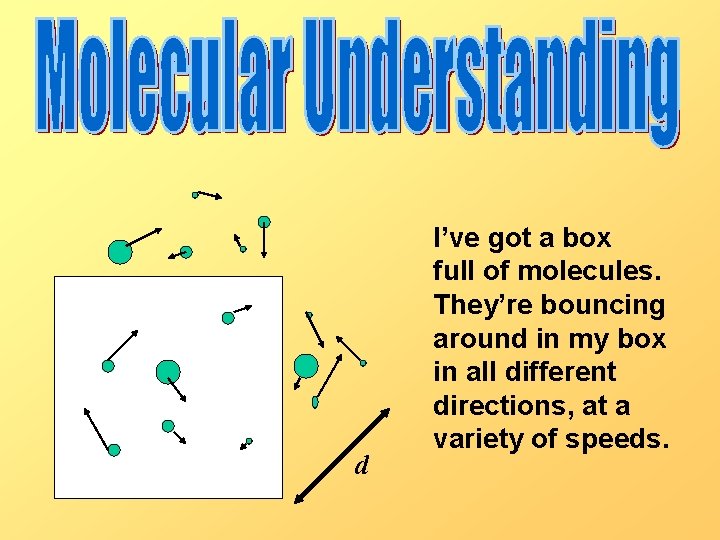
d I’ve got a box full of molecules. They’re bouncing around in my box in all different directions, at a variety of speeds.

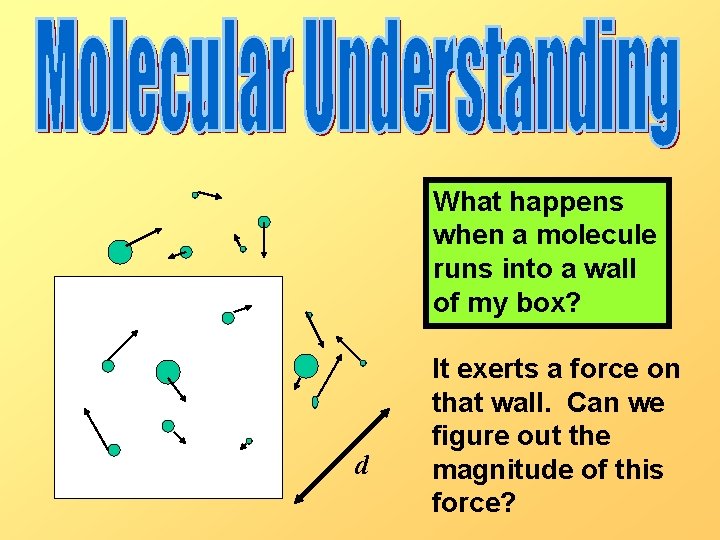
What happens when a molecule runs into a wall of my box? d It exerts a force on that wall. Can we figure out the magnitude of this force?

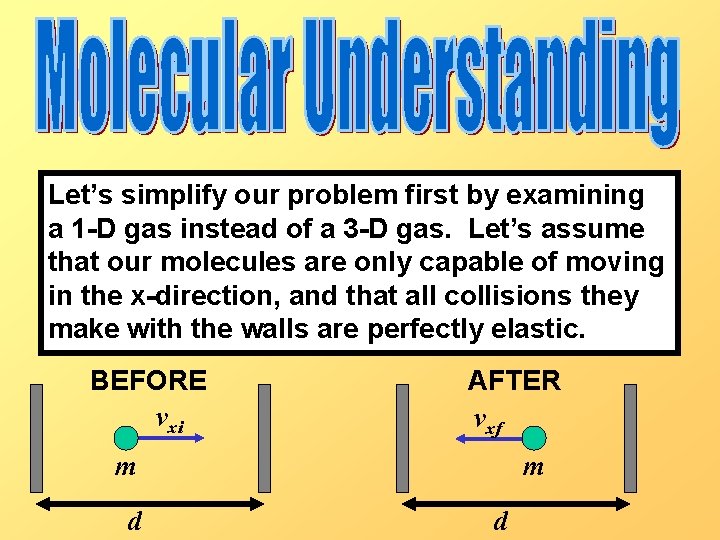
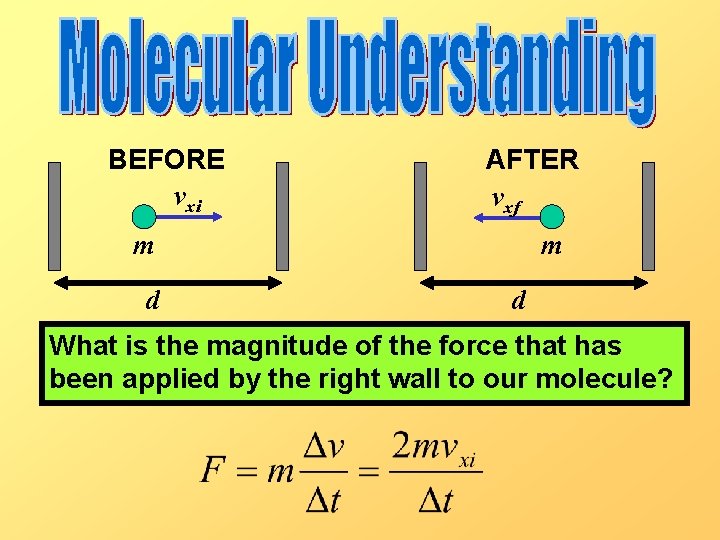
Let’s simplify our problem first by examining a 1 -D gas instead of a 3 -D gas. Let’s assume that our molecules are only capable of moving in the x-direction, and that all collisions they make with the walls are perfectly elastic. BEFORE vxi AFTER vxf m d

BEFORE vxi AFTER vxf m d What is the magnitude of the force that has been applied by the right wall to our molecule?