Calculation of Molecular Properties How What and Why






![Modern DFT FHK ? ? ? Only J[ρ] is known! The explicit form of Modern DFT FHK ? ? ? Only J[ρ] is known! The explicit form of](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-22.jpg)
![T[ρ] – kinetic energy of a real interacting electron system with density ρ(r) TKS T[ρ] – kinetic energy of a real interacting electron system with density ρ(r) TKS](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-23.jpg)
![Variational Principle in DFT Second HK Theorem Minimize E[ρ] with the conditions: Kohn-Sham Equations: Variational Principle in DFT Second HK Theorem Minimize E[ρ] with the conditions: Kohn-Sham Equations:](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-24.jpg)
![Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-26.jpg)
![[1 H, 1 H] COSY 45 NMR spectrum of pyrazinamide in DMSO solution Dr. [1 H, 1 H] COSY 45 NMR spectrum of pyrazinamide in DMSO solution Dr.](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-35.jpg)






- Slides: 57

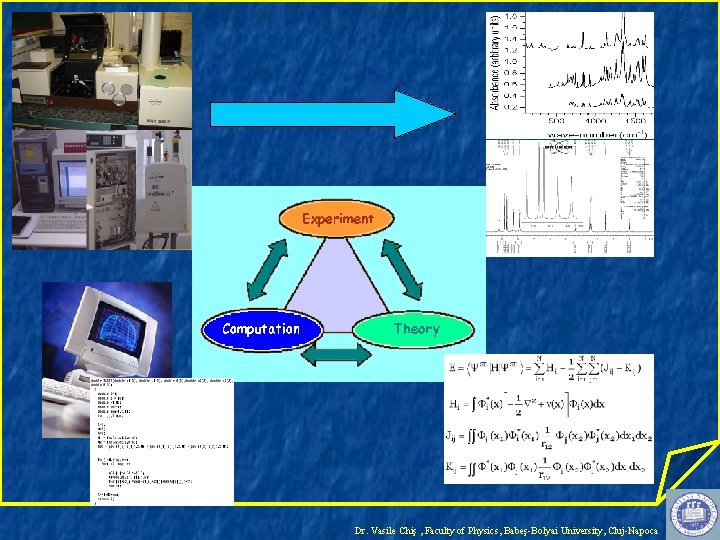
Calculation of Molecular Properties: How, What and Why? Dr. Vasile Chiş Biomedical Physics Department, Faculty of Physics Babeş-Bolyai University, Cluj-Napoca "Many experimental chemists use various kinds of spectroscopy in their research even though they are not spectroscopists. In a similar manner, more and more scientists are applying computational techniques as another weapon in their arsenal" Delano P. Chong in Recent Advances in Density Functional Methods, Part I, World Scientific, 1995 Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Outline 1. Introduction 2. Hartree-Fock-Roothaan-Hall Theory 3. Basis Sets 4. Electron Correlation 5. ABC of DFT 6. Predictible Molecular Properties 7. Examples of Calculations vibrational, NMR and ESR spectra conformers, tautomers, relative energies, molecular orbitals Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

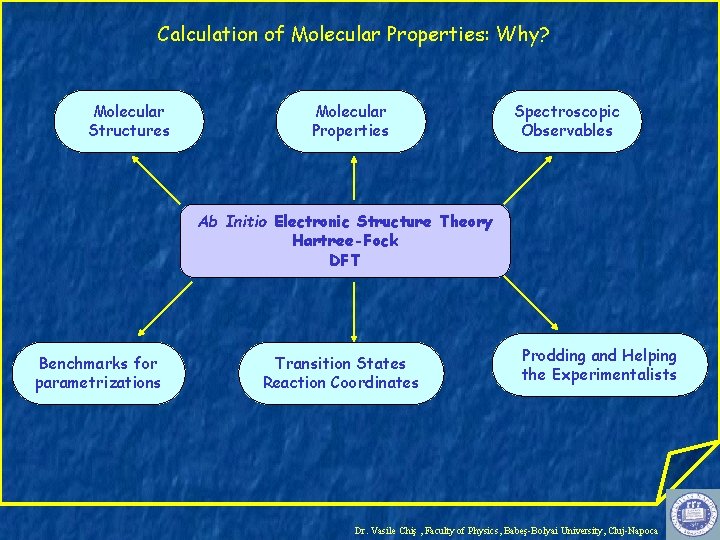
Calculation of Molecular Properties: Why? Molecular Structures Molecular Properties Spectroscopic Observables Ab Initio Electronic Structure Theory Hartree-Fock DFT Benchmarks for parametrizations Transition States Reaction Coordinates Prodding and Helping the Experimentalists Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

A short history “We are perhaps not far removed from the time when we shall be able to submit the bulk of chemical phenomena to calculation” Joseph Louis Gay-Lussac, Memoires de la Societe d’Arcueil, 2, 207(1808) J. L. Gay-Lussac “The more progress physical science make, the more they enter the domain of mathematics, which is a kind of centre to which they all converge. We may even judge the degree of perfection to which a science has arrived by the facility with which it may be submitted to calculation. ” A. Quetelet Adolphe Quetelet, Instructions Populaires sur le Calcul des Probabilities, Tarlier, Brussels, 1828, p. 230 “Every attempt to employ mathematical methods in the study of chemical questions must be considered profoundly irrational and contrary to the spirit of chemistry. If mathematical analysis should ever hold a proeminent place in chemistry – an aberration which is almost impossible – it would occasion a rapid widespread degeneration of that science” A. Compte, Philosophie Positive, 1830 Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Quantum Wave Mechanics, 1926 H =E E. R. J. A. Schrödinger W. K. Heisenberg “The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known, and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble. ” P. A. M. Dirac, Proc. Roy. Soc(London) 123, 714(1929) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

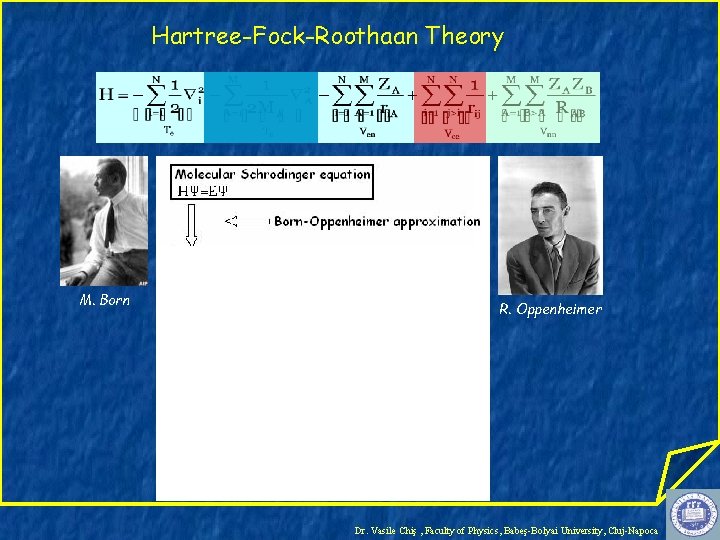
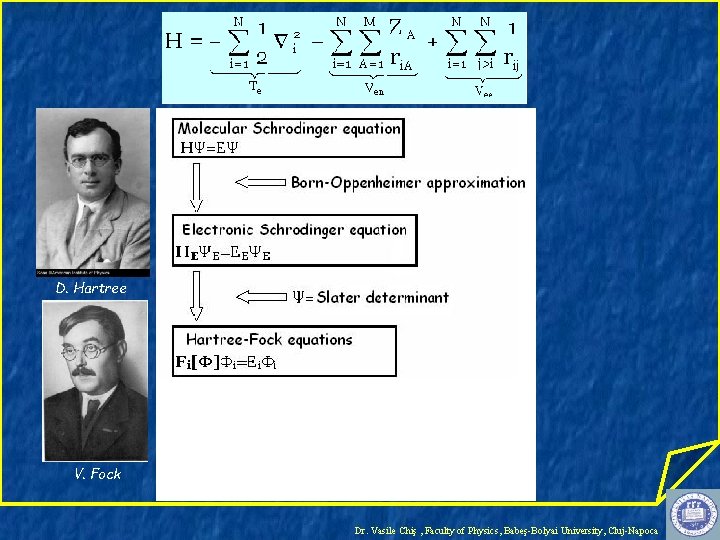
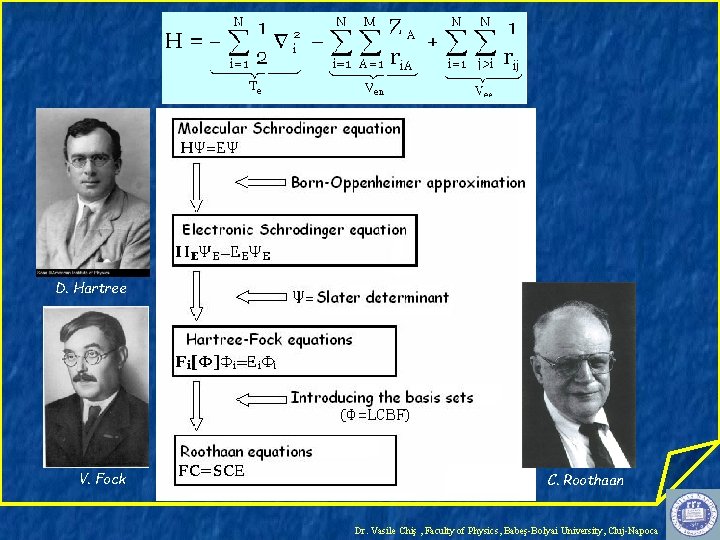
Hartree-Fock-Roothaan Theory M. Born R. Oppenheimer Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

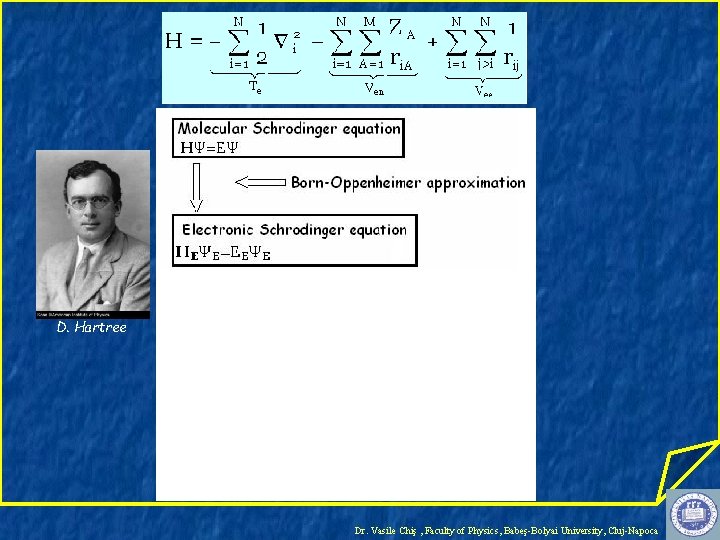
D. Hartree Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

D. Hartree V. Fock Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

D. Hartree V. Fock C. Roothaan Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

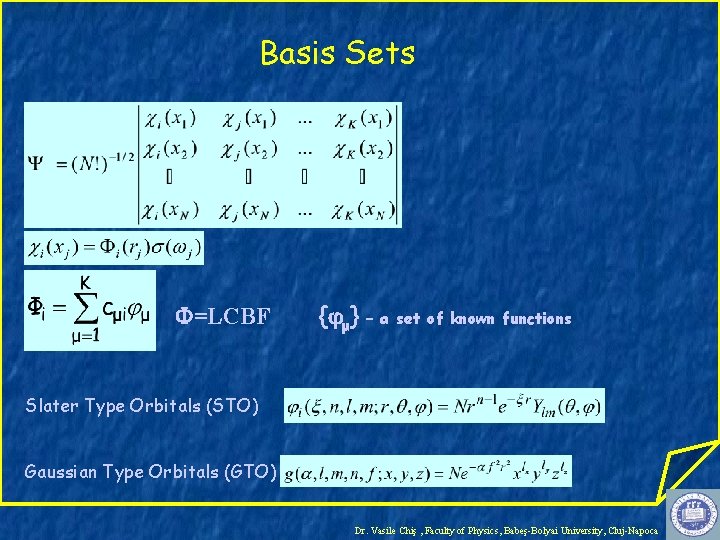
Basis Sets =LCBF { μ} – a set of known functions Slater Type Orbitals (STO) Gaussian Type Orbitals (GTO) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

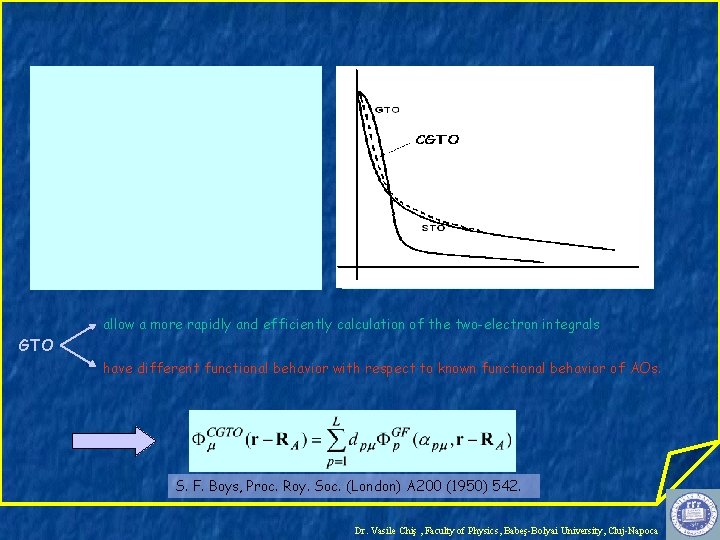
allow a more rapidly and efficiently calculation of the two-electron integrals GTO have different functional behavior with respect to known functional behavior of AOs. S. F. Boys, Proc. Roy. Soc. (London) A 200 (1950) 542. Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

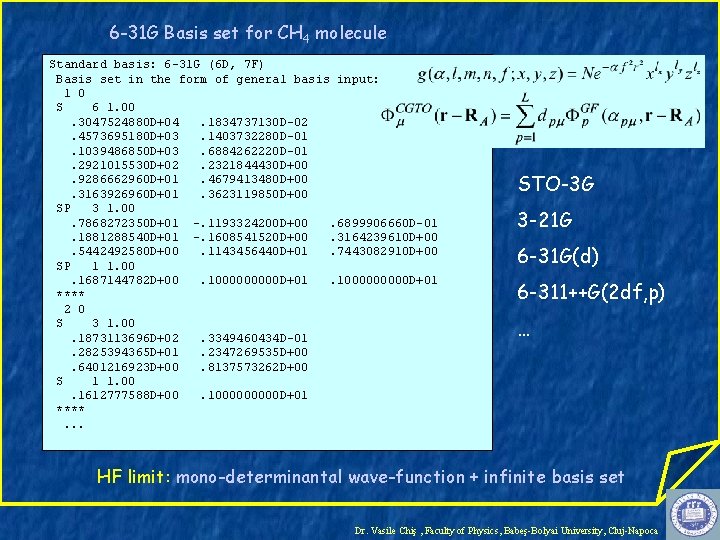
6 -31 G Basis set for CH 4 molecule Standard basis: 6 -31 G (6 D, 7 F) Basis set in the form of general basis input: 1 0 S 6 1. 00. 3047524880 D+04. 1834737130 D-02. 4573695180 D+03. 1403732280 D-01. 1039486850 D+03. 6884262220 D-01. 2921015530 D+02. 2321844430 D+00. 9286662960 D+01. 4679413480 D+00. 3163926960 D+01. 3623119850 D+00 SP 3 1. 00. 7868272350 D+01 -. 1193324200 D+00. 6899906660 D-01. 1881288540 D+01 -. 1608541520 D+00. 3164239610 D+00. 5442492580 D+00. 1143456440 D+01. 7443082910 D+00 SP 1 1. 00. 1687144782 D+00. 1000000000 D+01 **** 2 0 S 3 1. 00. 1873113696 D+02. 3349460434 D-01. 2825394365 D+01. 2347269535 D+00. 6401216923 D+00. 8137573262 D+00 S 1 1. 00. 1612777588 D+00. 100000 D+01 ****. . . STO-3 G 3 -21 G 6 -31 G(d) 6 -311++G(2 df, p) … HF limit: mono-determinantal wave-function + infinite basis set Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

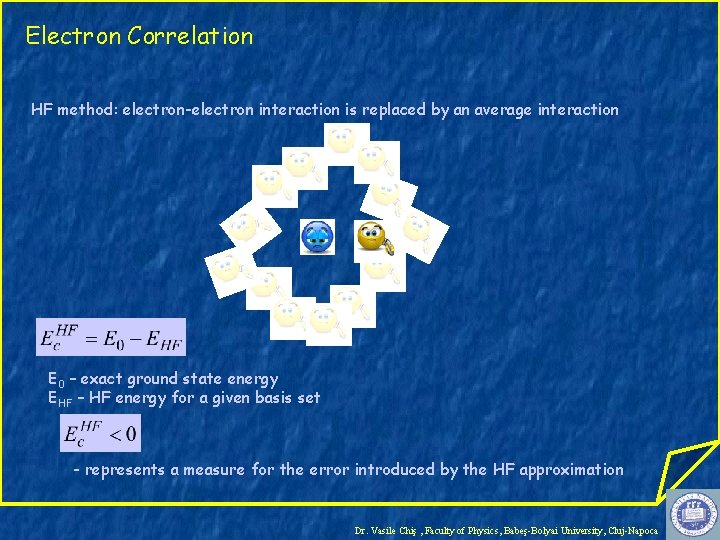
Electron Correlation HF method: electron-electron interaction is replaced by an average interaction E 0 – exact ground state energy EHF – HF energy for a given basis set - represents a measure for the error introduced by the HF approximation Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

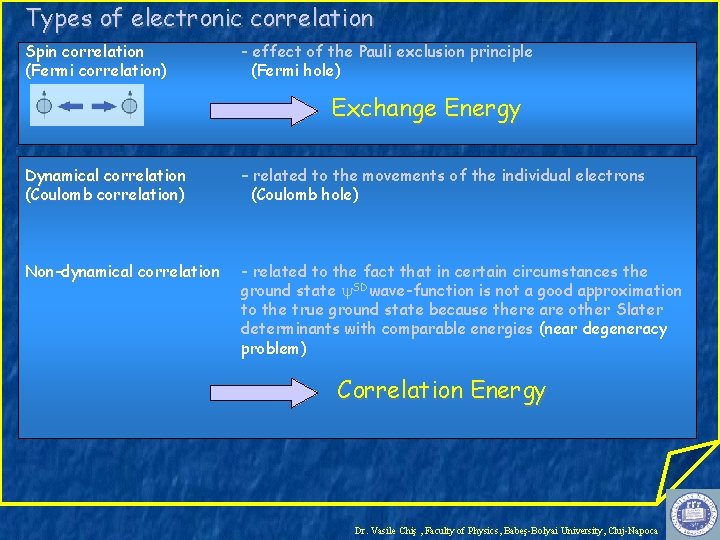
Types of electronic correlation Spin correlation (Fermi correlation) - effect of the Pauli exclusion principle (Fermi hole) Exchange Energy Dynamical correlation (Coulomb correlation) – related to the movements of the individual electrons (Coulomb hole) Non-dynamical correlation - related to the fact that in certain circumstances the ground state SD wave-function is not a good approximation to the true ground state because there are other Slater determinants with comparable energies (near degeneracy problem) Correlation Energy Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

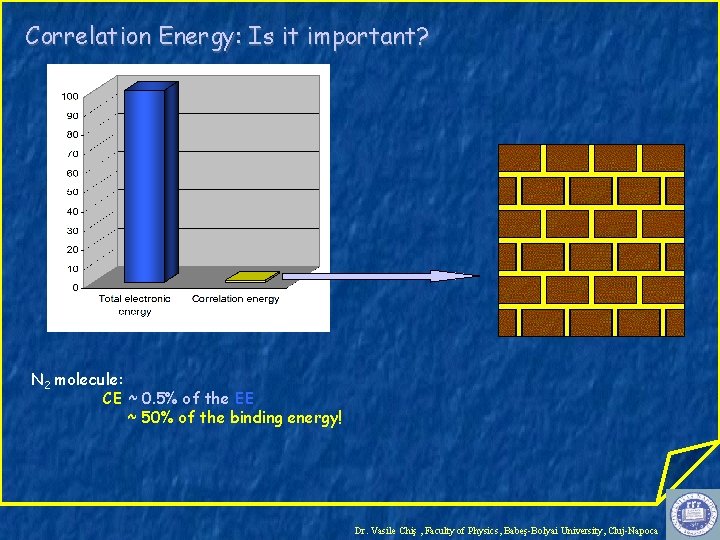
Correlation Energy: Is it important? N 2 molecule: CE ~ 0. 5% of the EE ~ 50% of the binding energy! Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

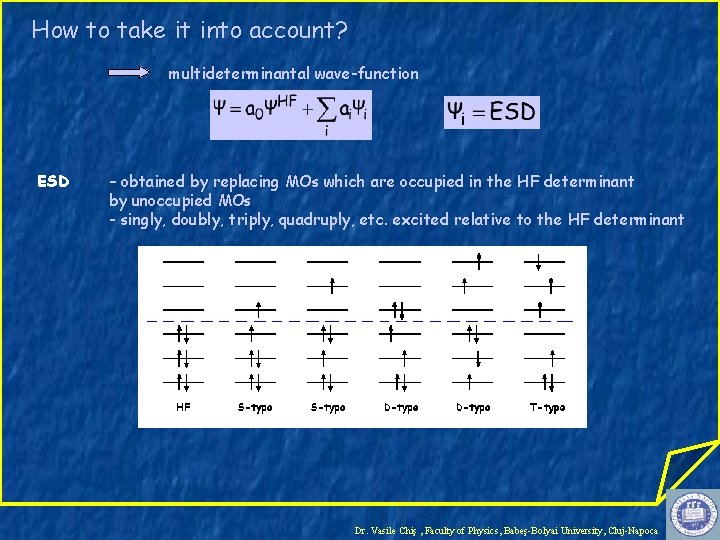
How to take it into account? multideterminantal wave-function ESD – obtained by replacing MOs which are occupied in the HF determinant by unoccupied MOs - singly, doubly, triply, quadruply, etc. excited relative to the HF determinant Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

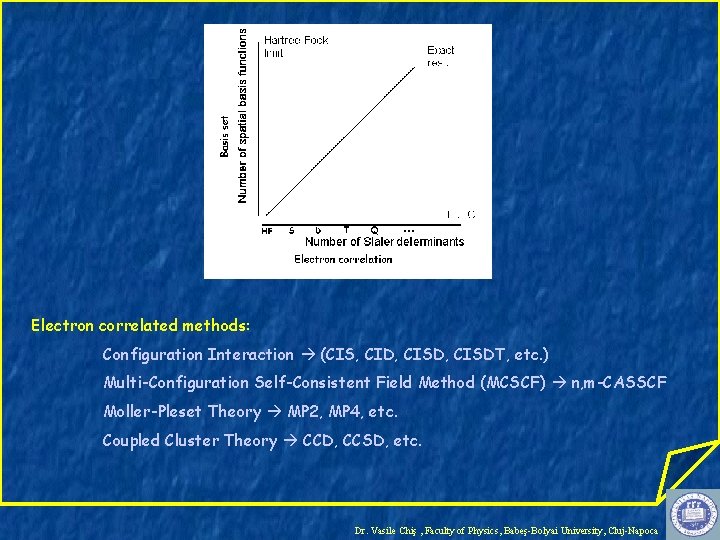
Electron correlated methods: Configuration Interaction (CIS, CID, CISDT, etc. ) Multi-Configuration Self-Consistent Field Method (MCSCF) n, m-CASSCF Moller-Pleset Theory MP 2, MP 4, etc. Coupled Cluster Theory CCD, CCSD, etc. Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

ABC of DFT 1927 1964 1992 L. H. Thomas, E. Fermi P. Hohenberg, W. Kohn, L. J. Sham Gaussian® Why a new theory? HF method scales as CI methods scale as MPn methods scale as CC methods scale as K 4 K 6 -K 10 >K 5 >K 6 (K - # of basis functions) Correlated methods are not feasible for medium and large sized molecules! The electron density Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

DFT is presently the most successful and also the most promising approach to compute the electronic structure of matter. Applicability: atoms, molecules, solids DFT is less computationally expensive than traditional Hartree-Fock methods but it gives similar accuracy. Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

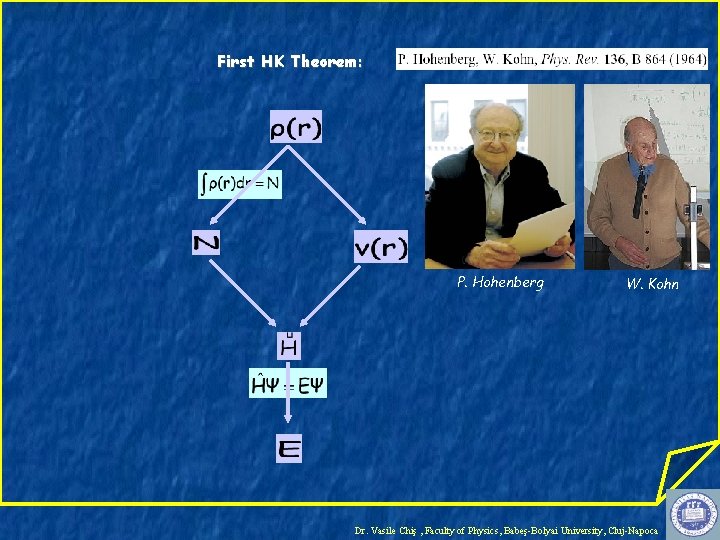
First HK Theorem: P. Hohenberg W. Kohn Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca
![Modern DFT FHK Only Jρ is known The explicit form of Modern DFT FHK ? ? ? Only J[ρ] is known! The explicit form of](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-22.jpg)
Modern DFT FHK ? ? ? Only J[ρ] is known! The explicit form of T[ρ] and Enon-cl[ρ] is the major challenge of DFT Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca
![Tρ kinetic energy of a real interacting electron system with density ρr TKS T[ρ] – kinetic energy of a real interacting electron system with density ρ(r) TKS](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-23.jpg)
T[ρ] – kinetic energy of a real interacting electron system with density ρ(r) TKS Ψi – kinetic energy of a fictitious non-interacting system of the same density ρ(r) - are the orbitals for the non-interacting system (KS orbitals) T=TKS+(T-TKS) Exc[ρ] includes everything which is unknown: -exchange energy -correlation energy -correction of kinetic energy (T-TKS) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca
![Variational Principle in DFT Second HK Theorem Minimize Eρ with the conditions KohnSham Equations Variational Principle in DFT Second HK Theorem Minimize E[ρ] with the conditions: Kohn-Sham Equations:](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-24.jpg)
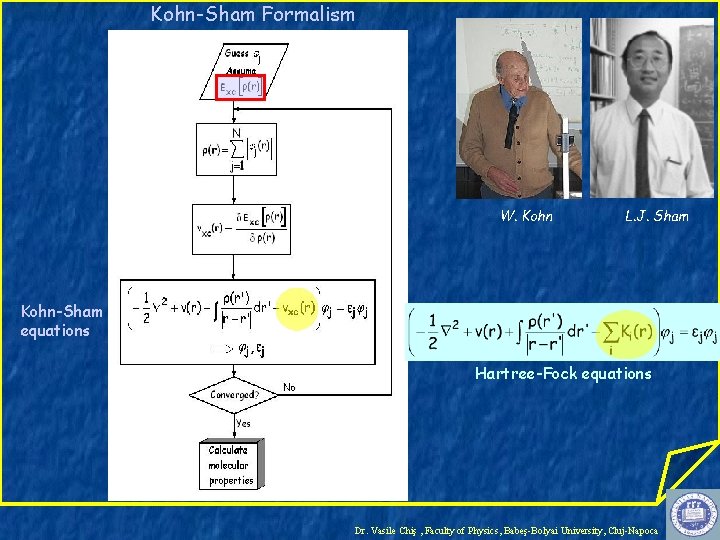
Variational Principle in DFT Second HK Theorem Minimize E[ρ] with the conditions: Kohn-Sham Equations: W. Kohn L. J. Sham with: Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Kohn-Sham Formalism W. Kohn L. J. Sham Kohn-Sham equations Hartree-Fock equations Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca
![Excρ Local Density Approximation LDA εxc only depends on the density Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-26.jpg)
Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density at r Generalized Gradient Approximation (GGA) εxc depends on the density and its gradient at r Hybrid Functionals Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

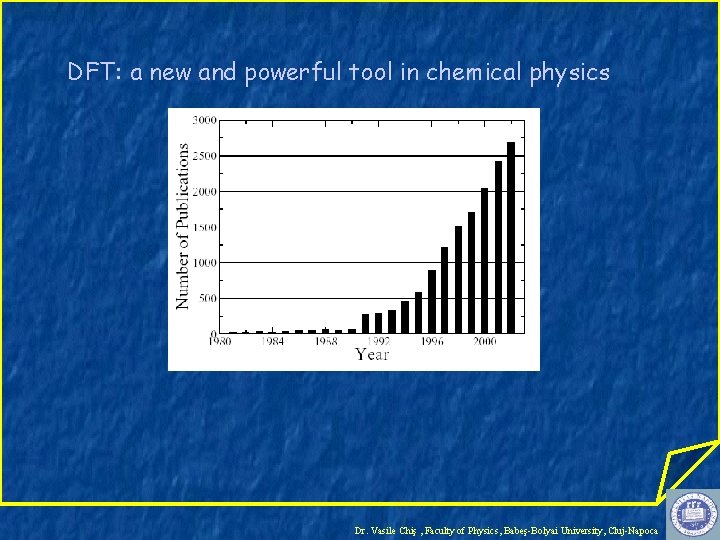
DFT: a new and powerful tool in chemical physics Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

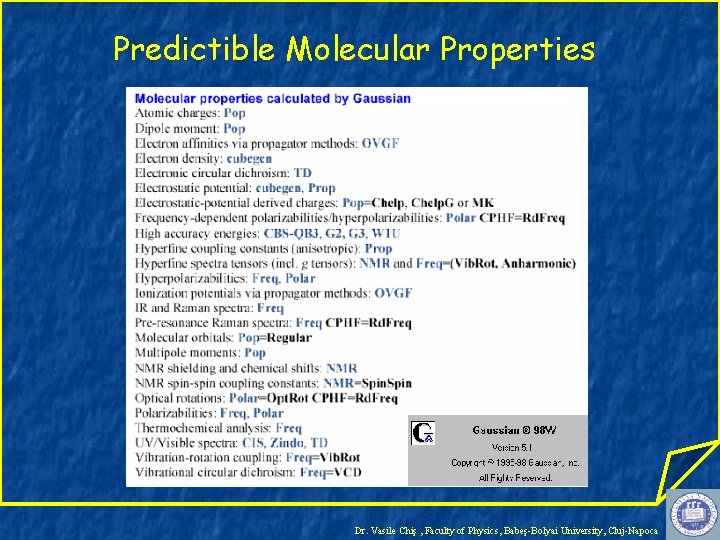
Predictible Molecular Properties Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Examples of Calculations pyrazinamide meta-benzosemiquinone anion free radical 5 -p. BBTT Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

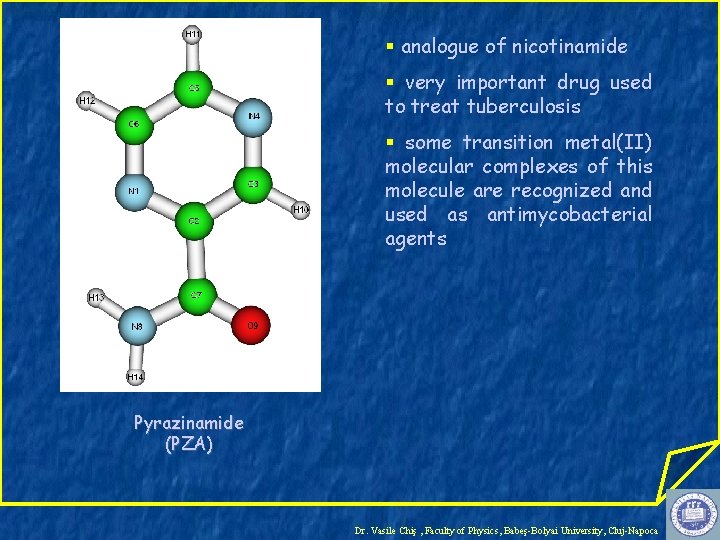
§ analogue of nicotinamide § very important drug used to treat tuberculosis § some transition metal(II) molecular complexes of this molecule are recognized and used as antimycobacterial agents Pyrazinamide (PZA) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

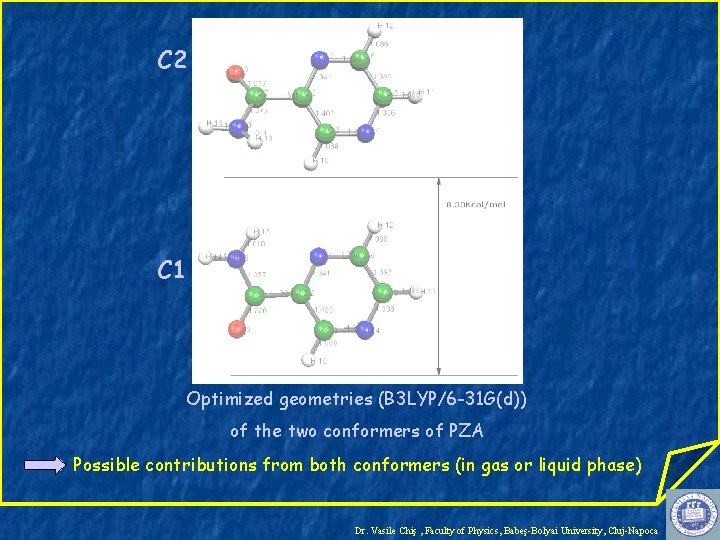
C 2 C 1 Optimized geometries (B 3 LYP/6 -31 G(d)) of the two conformers of PZA Possible contributions from both conformers (in gas or liquid phase) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

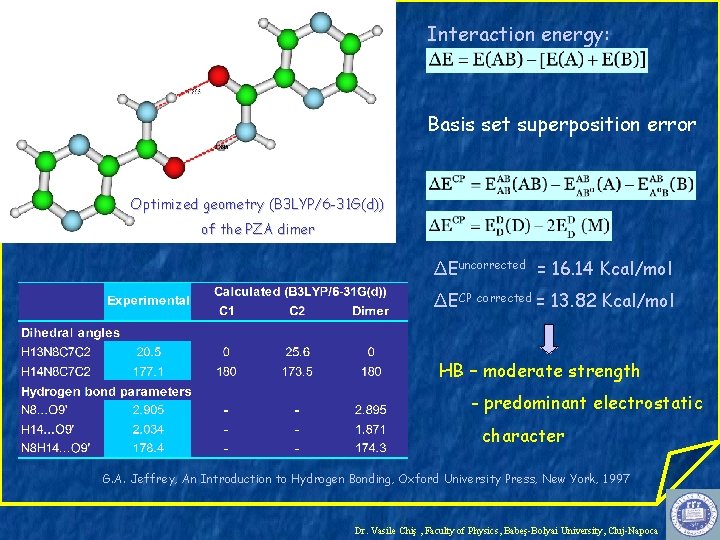
Interaction energy: Basis set superposition error Optimized geometry (B 3 LYP/6 -31 G(d)) of the PZA dimer ΔEuncorrected = 16. 14 Kcal/mol ΔECP corrected = 13. 82 Kcal/mol HB – moderate strength - predominant electrostatic character G. A. Jeffrey, An Introduction to Hydrogen Bonding, Oxford University Press, New York, 1997 Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

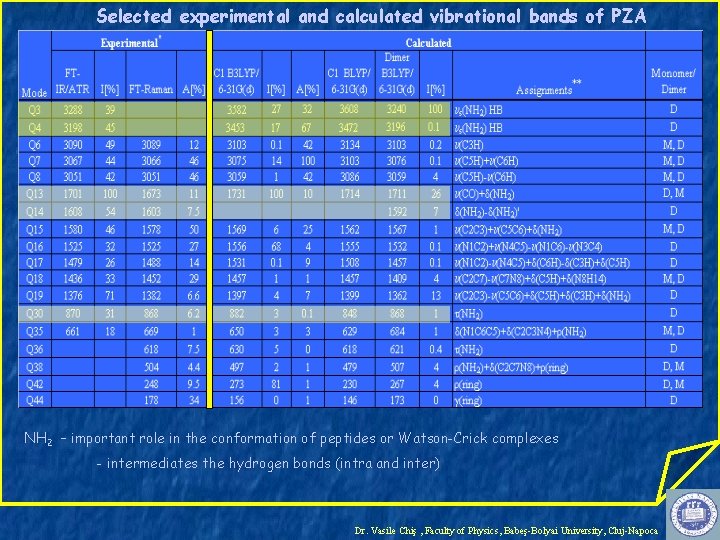
Selected experimental and calculated vibrational bands of PZA NH 2 – important role in the conformation of peptides or Watson-Crick complexes - intermediates the hydrogen bonds (intra and inter) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

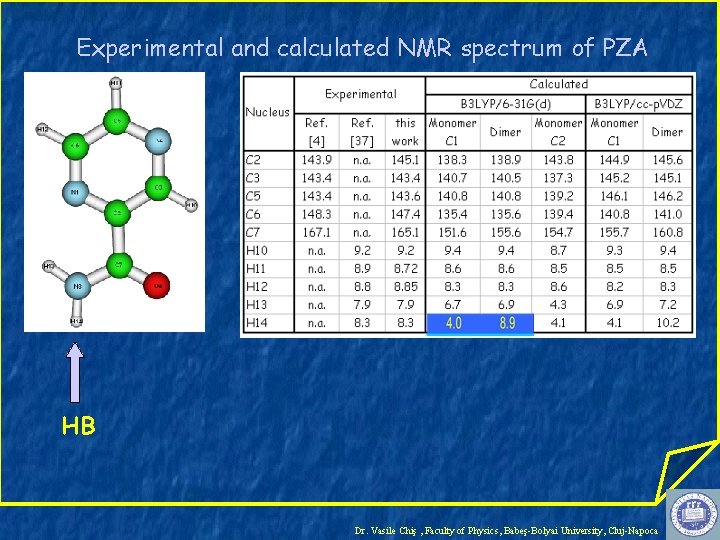
Experimental and calculated NMR spectrum of PZA HB Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca
![1 H 1 H COSY 45 NMR spectrum of pyrazinamide in DMSO solution Dr [1 H, 1 H] COSY 45 NMR spectrum of pyrazinamide in DMSO solution Dr.](https://slidetodoc.com/presentation_image_h/79a48dfe49e05f8fe08cda862666e28a/image-35.jpg)
[1 H, 1 H] COSY 45 NMR spectrum of pyrazinamide in DMSO solution Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

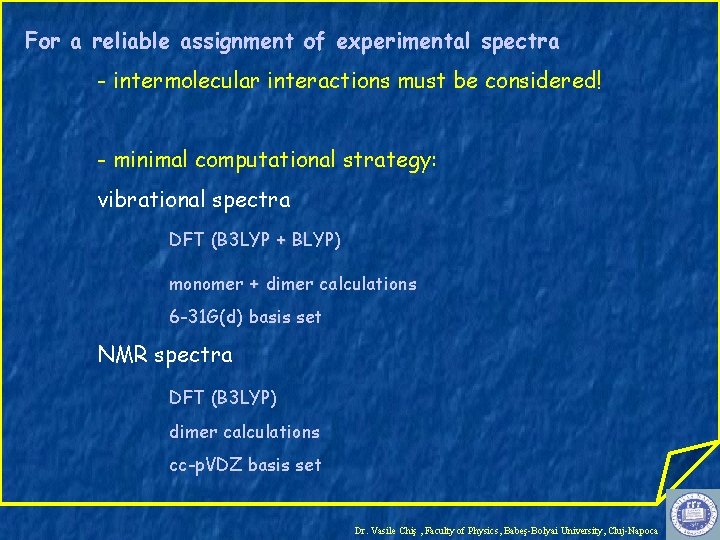
For a reliable assignment of experimental spectra - intermolecular interactions must be considered! - minimal computational strategy: vibrational spectra DFT (B 3 LYP + BLYP) monomer + dimer calculations 6 -31 G(d) basis set NMR spectra DFT (B 3 LYP) dimer calculations cc-p. VDZ basis set Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

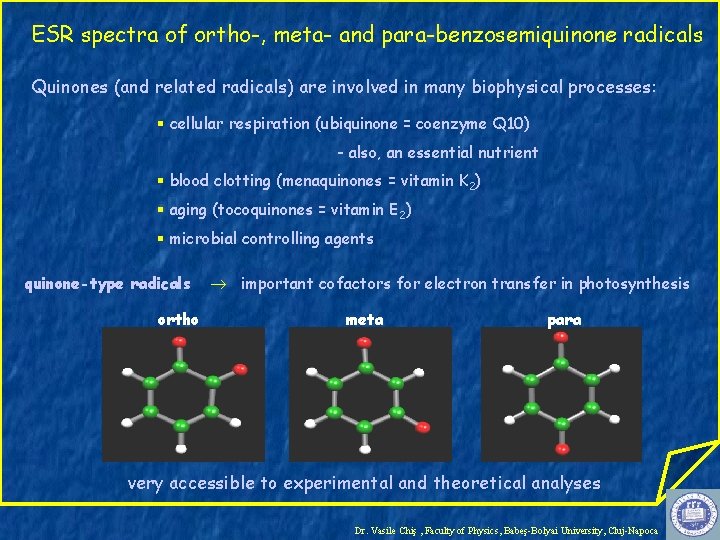
ESR spectra of ortho-, meta- and para-benzosemiquinone radicals Quinones (and related radicals) are involved in many biophysical processes: § cellular respiration (ubiquinone = coenzyme Q 10) - also, an essential nutrient § blood clotting (menaquinones = vitamin K 2) § aging (tocoquinones = vitamin E 2) § microbial controlling agents quinone-type radicals ortho important cofactors for electron transfer in photosynthesis meta para very accessible to experimental and theoretical analyses Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

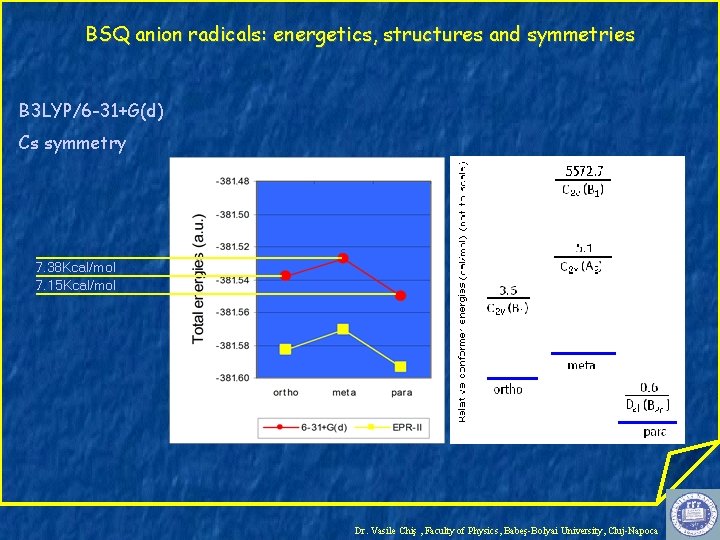
BSQ anion radicals: energetics, structures and symmetries B 3 LYP/6 -31+G(d) Cs symmetry 7. 38 Kcal/mol 7. 15 Kcal/mol Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

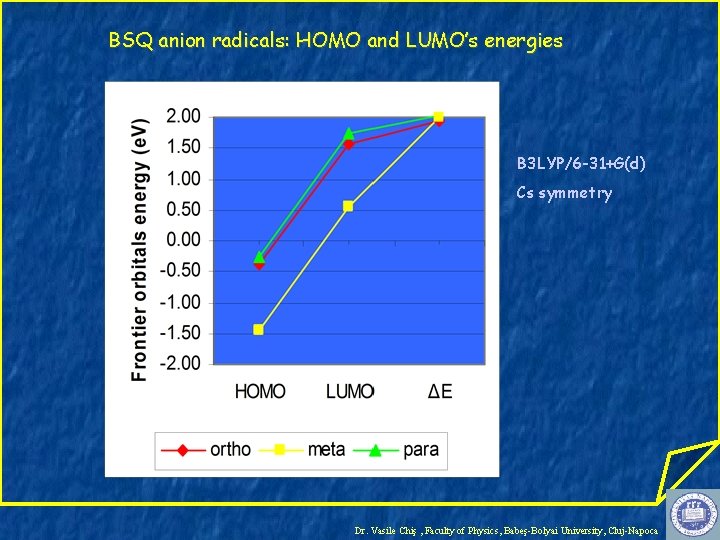
BSQ anion radicals: HOMO and LUMO’s energies B 3 LYP/6 -31+G(d) Cs symmetry Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

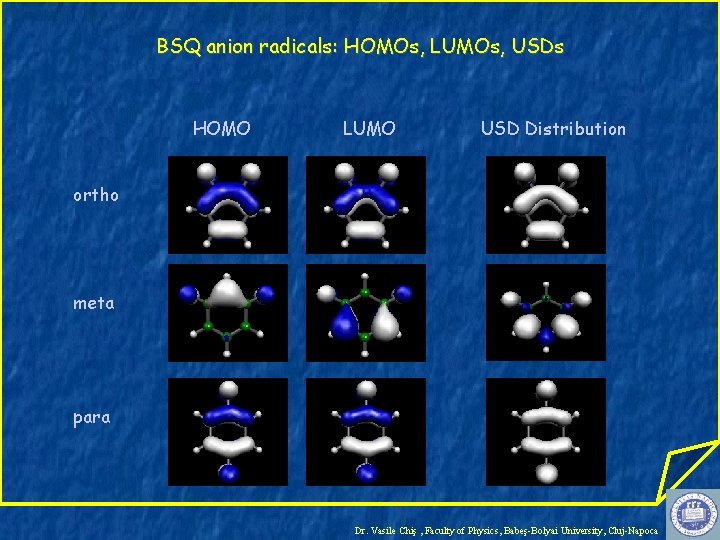
BSQ anion radicals: HOMOs, LUMOs, USDs HOMO LUMO USD Distribution ortho meta para Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

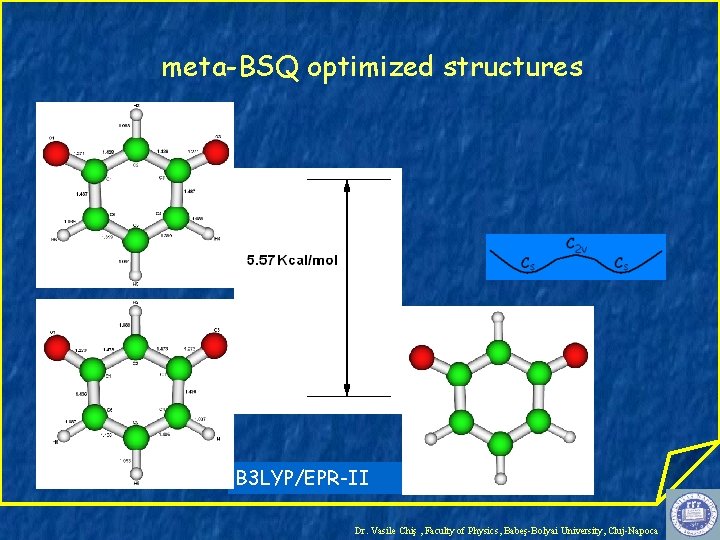
meta-BSQ optimized structures B 3 LYP/EPR-II Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

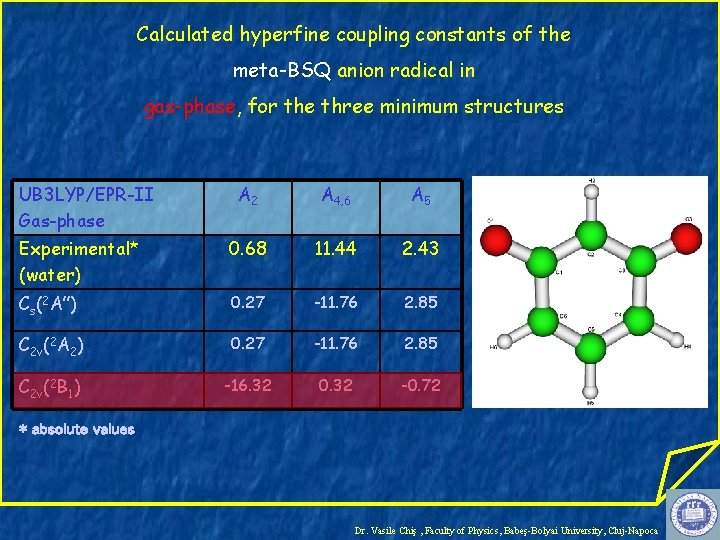
Calculated hyperfine coupling constants of the meta-BSQ anion radical in gas-phase, for the three minimum structures UB 3 LYP/EPR-II Gas-phase A 2 A 4, 6 A 5 Experimental* (water) 0. 68 11. 44 2. 43 Cs(2 A”) 0. 27 -11. 76 2. 85 C 2 v(2 A 2) 0. 27 -11. 76 2. 85 C 2 v(2 B 1) -16. 32 0. 32 -0. 72 * absolute values Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

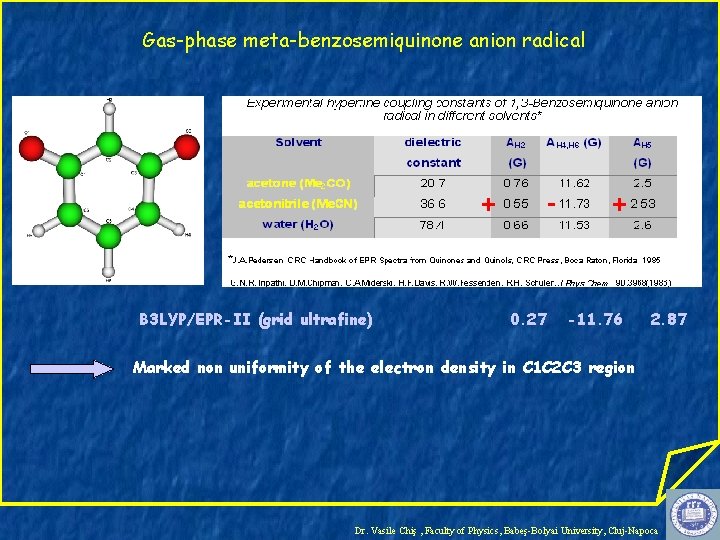
Gas-phase meta-benzosemiquinone anion radical + B 3 LYP/EPR-II (grid ultrafine) - 0. 27 + -11. 76 2. 87 Marked non uniformity of the electron density in C 1 C 2 C 3 region Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

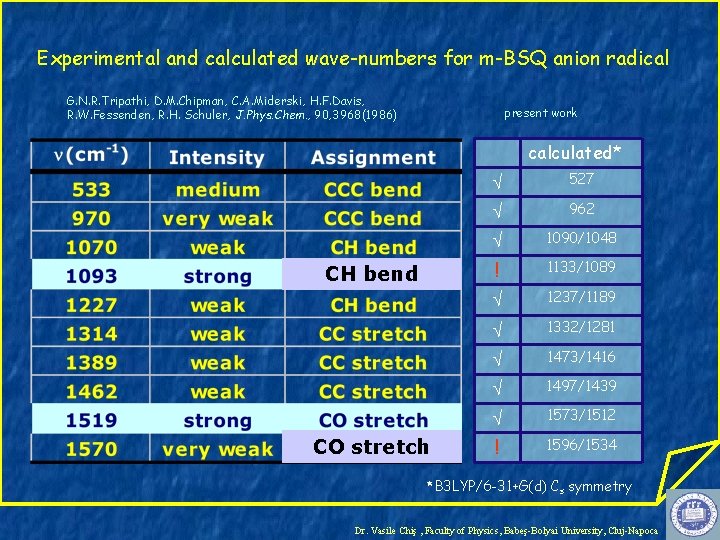
Experimental and calculated wave-numbers for m-BSQ anion radical G. N. R. Tripathi, D. M. Chipman, C. A. Miderski, H. F. Davis, R. W. Fessenden, R. H. Schuler, J. Phys. Chem. , 90, 3968(1986) present work calculated* CH bend CO stretch 527 962 1090/1048 ! 1133/1089 1237/1189 1332/1281 1473/1416 1497/1439 1573/1512 ! 1596/1534 *B 3 LYP/6 -31+G(d) Cs symmetry Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Conclusions Ø dipole moments: (ortho)> (meta)> (para)=0 Ø number of minimum energy conformers: 2 for ortho and para, 3 for meta Ø total energies: Emeta>Eortho>Epara Ø hfcc’s: ortho - strong influence of the solvation effects meta - marked non-uniformity in the electron density para – easilly reproduced even in gas-phase Ø vibrational spectra: ortho – no experimental data available meta – reassignment of two bands in the IR spectrum para – very good agreement between experiment and theory Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

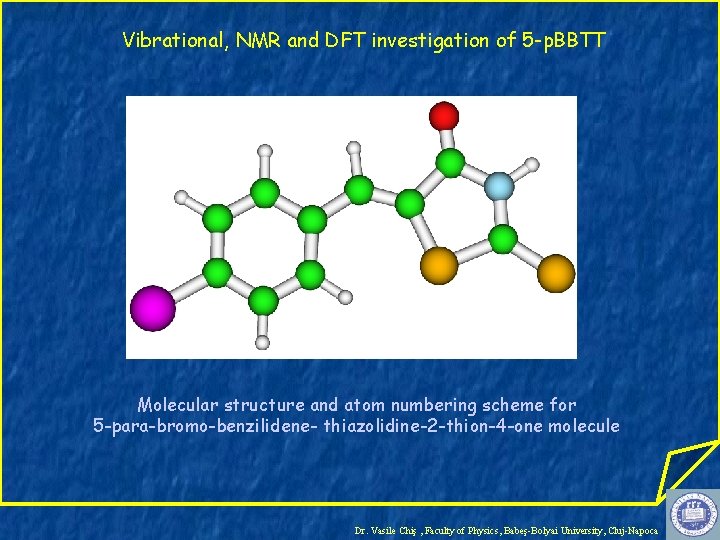
Vibrational, NMR and DFT investigation of 5 -p. BBTT Molecular structure and atom numbering scheme for 5 -para-bromo-benzilidene- thiazolidine-2 -thion-4 -one molecule Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

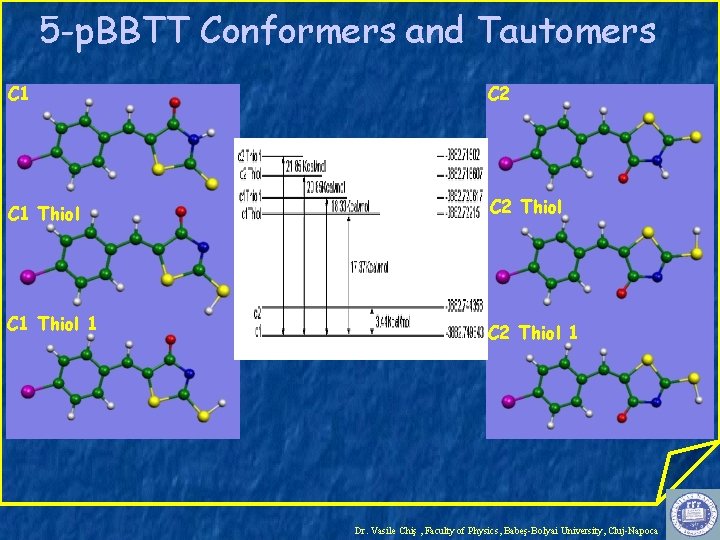
5 -p. BBTT Conformers and Tautomers C 1 C 2 C 1 Thiol C 2 Thiol C 1 Thiol 1 C 2 Thiol 1 Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

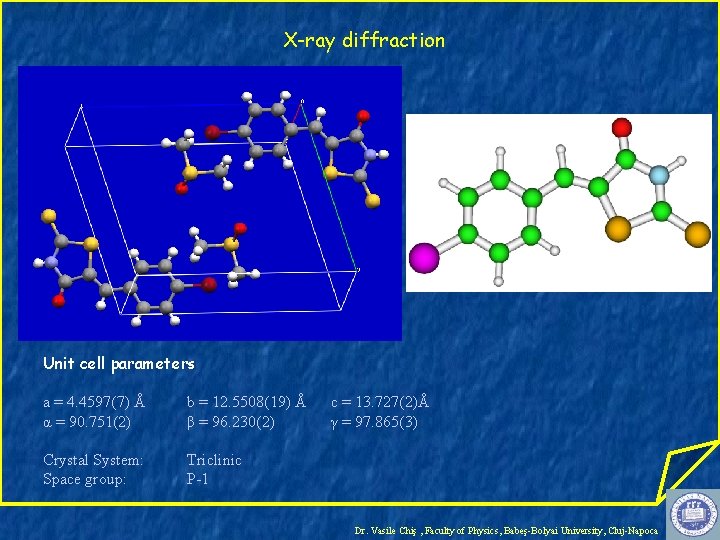
X-ray diffraction Unit cell parameters a = 4. 4597(7) Å α = 90. 751(2) b = 12. 5508(19) Å β = 96. 230(2) Crystal System: Space group: Triclinic P-1 c = 13. 727(2)Å γ = 97. 865(3) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

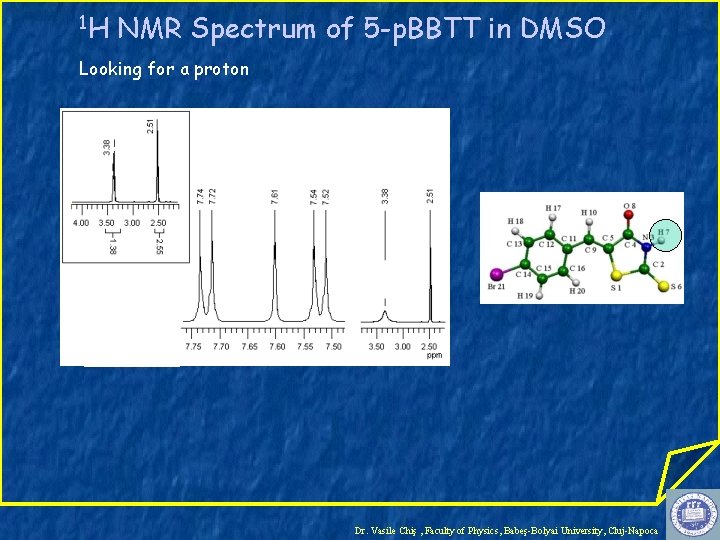
1 H NMR Spectrum of 5 -p. BBTT in DMSO Looking for a proton Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

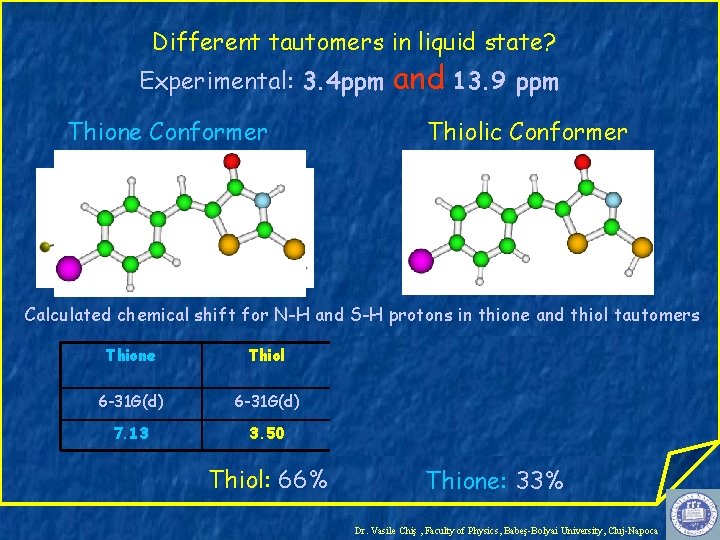
Different tautomers in liquid state? Experimental: 3. 4 ppm Thione Conformer and 13. 9 ppm Thiolic Conformer Calculated chemical shift for N-H and S-H protons in thione and thiol tautomers Thione Thiol Thione H-bonded 6 -31 G(d) 6 -31+G(d, p) 7. 13 3. 50 11. 74 14. 14 Thiol: 66% Thione: 33% Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

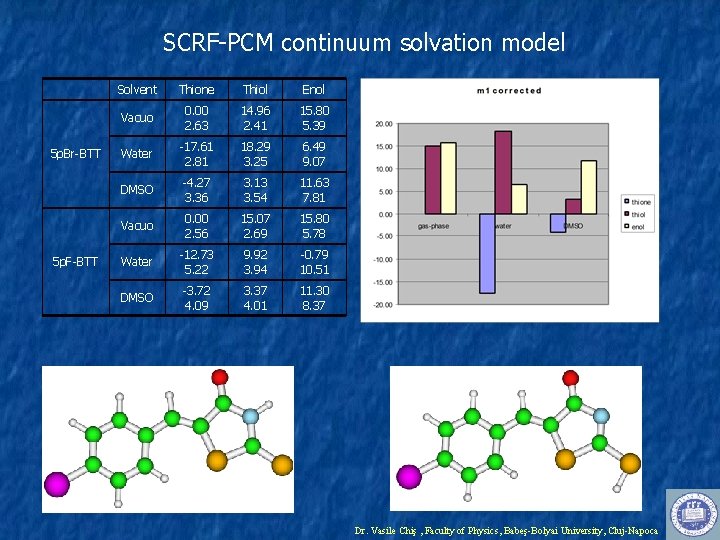
SCRF-PCM continuum solvation model 5 p. Br-BTT 5 p. F-BTT Solvent Thione Thiol Enol Vacuo 0. 00 2. 63 14. 96 2. 41 15. 80 5. 39 Water -17. 61 2. 81 18. 29 3. 25 6. 49 9. 07 DMSO -4. 27 3. 36 3. 13 3. 54 11. 63 7. 81 Vacuo 0. 00 2. 56 15. 07 2. 69 15. 80 5. 78 Water -12. 73 5. 22 9. 92 3. 94 -0. 79 10. 51 DMSO -3. 72 4. 09 3. 37 4. 01 11. 30 8. 37 Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Conclusions: • Proposed molecular structure is confirmed by experimental and theoretical results. • The most stable conformer was proposed based on theoretical results and was confirmed by vibrational, NMR and X-ray diffraction results. • Based on NMR and theoretical data, the coexistence of thiolic and thione tautomers is proved in liquid state. Moreover, thiolic conformer is prevailing in this case and thione conformer still remains H-bonded in liquid phase. Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

From the beginning: Calculation of Molecular Properties How? HF, UHF, MPn, CI, CC, DFT, AM 1, PM 3, etc. 6 -31 G(d), cc-p. VDZ, Lanl 2 DZ(ECP), etc. What? well… almost everything! Why? can we live without? (designing new materials, pharmaceuticals, etc) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Publications: closed-shell systems Molecular and Vibrational Structure of 2, 4 -Dinitrophenol: FT-IR, FT-Raman and Quantum Chemical Calculations V. Chiş Chem. Phys. , 300, 1 -11 (2004) Vibrational Spectroscopy and Theoretical Studies on 2, 4 -dinitrophenylhydrazine V. Chiş, V. Miclăuş, A. Pîrnău, C. Tănăselia, V. Almăşan, M. Vasilescu J. Mol. Struct. , 744 -747 363 -368 (2005) NIR Surface Enhanced Raman Spectroscopy and Band Assignment by DFT Calculations of Non-Natural -amino acids T. Iliescu, D. Maniu, V. Chiş, F. D. Irimie, Cs. Paizs and M. Tosa Chem. Phys. , 310, 189 -199 (2005) Adsorption of 6 -Mercaptopurine and 6 -Mercaptopurine-Riboside on Silver Colloid: A p. H Dependent Surface Enhanced Raman Spectroscopy and Density Functional Theory Study. Part I. 6 -Mercaptopurine A. V. Szeghalmi, L. Leopold, S. Pînzaru, V. Chiş, I. Silaghi-Dumitrescu, M. Schmitt, J. Popp, W. Kiefer J. Mol. Struct. , 735 -736, 103 -113 (2005) Adsorption of 6 -mercaptopurine and 6 -mercaptopurine-riboside on silver colloid: A p. H dependent surface enhanced Raman spectroscopy and density functional theory study. Part II. 6 -mercaptopurine-riboside V. Szeghalmi, L. Leopold, S. Pînzaru, V. Chiş, I. Silaghi-Dumitrescu, M. Schmitt, J. Popp, W. Kiefer Biopolymers, 78, 298 -310 (2005) Experimental and DFT Study of Pyrazinamide V. Chiş, A. Pîrnău, T. Jurcă, M. Vasilescu, S. Simon, O. Cozar, L. David Chem. Phys. , 316, 153 -163 (2005) Molecular and Vibrational Structure of 5 -Para-Bromo-Benziliden–Tiazolidin-2 -Tion-4 -Ona. Experimental and Theoretical Investigation A. Pîrnău, M. Baias, O. Oniga, V. Chiş, M. Vasilescu, O. Cozar Studia Physica, Special Issue, NANOSPEC, 2005 Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Publications: open-shell systems (free radicals) Ab Initio and DFT calculations of the hyperfine structure of OH, HO 2 and H 2 O+ radicals V. Chiş, L. David, O. Cozar, A. Chiş Rom. J. Phys. , 48, 413 -428 (2003) Ab Initio and DFT Study on Hyperfine Structure of 1, 2 -Benzosemiquinone Anion Radical V. Chiş, A. Nemeş, L. David, O. Cozar Studia UBB, Physica, 47(1) 157 -170 (2002) AM 1/INDO Semiempirical Calculations on Tyrosyl Radical V. Chiş Studia UBB, Physica, 47(1), 147 -156 (2002) Which radicals are formed by electrochemical reduction of Dihydrazid-Hydrazone? An ESR and DFT Investigation. V. Chiş, V. Miclăuş, L. Mureşan, G. Damian, L. David, O. Cozar Studia UBB, Physica, XLXIII, Special Issue, 123 -134 (2003) Theoretical ESR Spectrum of 1, 3 -Benzosemiquinone Radical V. Chiş, R. Marcu, M. Oltean, L. David, O. Cozar Analele Universităţii din Oradea, A XIII, 123 -142 (2003) Density Functional Calculations of Hyperfine Coupling Constants in Glycine-Derived Radicals Raluca Marcu, Vasile Chiş Studia Physica, Special Issue, NANOSPEC, 2005 Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Publications: wave-functions for small molecules The effect of target representation in positron-impact ionization of molecular hydrogen R. I. Campeanu, V. Chiş, L. Nagy and A. D. Stauffer Phys. Lett. A, 310(5 -6), 445 - 450(2003) Positron impact ionization of molecular nitrogen R. I. Campeanu, V. Chiş, L. Nagy, A. D. Stauffer Nucl. Instrum. Meth. B 221 (2004) 21 -23 Positron impact ionization of molecular oxygen R. I. Campeanu, V. Chiş, L. Nagy, A. D. Stauffer Phys. Lett. A 325 (1) 66 -69 (2004) Positron impact ionization of CO and CO 2 R. I. Campeanu, V. Chiş, L. Nagy, A. D. Stauffer Phys. Lett. A, 344 (2 -4): 247 -252 (2005) Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca

Acknowledgments Prof. L. Nagy, Prof. T. Iliescu, Prof. S. Astilean dr. N. Leopold, dr. D. Maniu, dr. S. Cinta Pinzaru, dr. C. Craciun, dr. M. Vasilescu Babes-Bolyai University, Faculty of Physics dr. V. Miclaus, dr. M. Venter Babes-Bolyai University, Faculty of Chemistry dr. T. Jurca University of Oradea, Faculty of Medicine and Pharmacy Prof. O. Oniga UMF Cluj-Napoca, Dept. of Pharmaceutical Chemistry A. Pirnau, R. Marcu, M. Baias, M. Oltean, C. Tanaselia, L. Szabo, S. Botond Babes-Bolyai University, Faculty of Physics Dr. Vasile Chiş , Faculty of Physics, Babeş-Bolyai University, Cluj-Napoca
 Pictures
Pictures Covalently bonded substances
Covalently bonded substances Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Don't ask why why why
Don't ask why why why What is a covalent molecular substance
What is a covalent molecular substance Intensive property and extensive properties
Intensive property and extensive properties Is smell a physical property
Is smell a physical property Why are molecular compounds poor conductors
Why are molecular compounds poor conductors Molecular orbital diagram of f2
Molecular orbital diagram of f2 Why do different polymers have different properties
Why do different polymers have different properties Colligative properties
Colligative properties Why do multicellular organisms have emergent properties
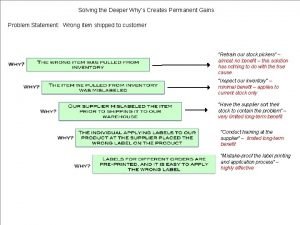
Why do multicellular organisms have emergent properties Why-why analysis
Why-why analysis Why do you cry, willie
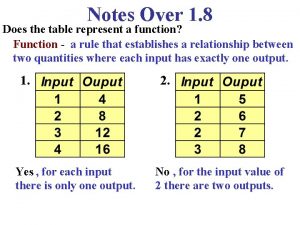
Why do you cry, willie Does the table represent a function why or why not
Does the table represent a function why or why not What does the image represent
What does the image represent Why or why not
Why or why not Contoh analisis akar masalah
Contoh analisis akar masalah Covalent molecular and covalent network
Covalent molecular and covalent network Kinetic molecular theory of solids
Kinetic molecular theory of solids How to get empirical formula from percentages
How to get empirical formula from percentages Naming compounds and writing formulas
Naming compounds and writing formulas Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Molecular shape finder
Molecular shape finder Molecular geometry chart
Molecular geometry chart Pf3 number of vsepr electron groups
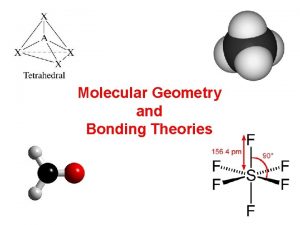
Pf3 number of vsepr electron groups Molecular geometry and bonding theories
Molecular geometry and bonding theories Lewis dot structure trigonal pyramidal
Lewis dot structure trigonal pyramidal Molecular theory of gases and liquids
Molecular theory of gases and liquids Covalent molecular and covalent network
Covalent molecular and covalent network Silicon carbide covalent network
Silicon carbide covalent network Covalent molecular and covalent network
Covalent molecular and covalent network Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Which formula is an empirical formula?
Which formula is an empirical formula? Molecular empirical formula
Molecular empirical formula Emprical formula
Emprical formula Electronegativity and dipole moment
Electronegativity and dipole moment Chemical formula vs molecular formula
Chemical formula vs molecular formula Bond polarity vs molecular polarity
Bond polarity vs molecular polarity Find the empirical/simplest formula fe
Find the empirical/simplest formula fe How is orbital hybridization useful in describing molecules
How is orbital hybridization useful in describing molecules Central tolerance and peripheral tolerance
Central tolerance and peripheral tolerance Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Pcr lab setup
Pcr lab setup Erzeng xue
Erzeng xue Color by number molecular geometry and polarity
Color by number molecular geometry and polarity Electron domain geometry of acetic acid
Electron domain geometry of acetic acid Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Dxz and dxz overlap
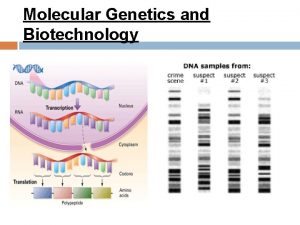
Dxz and dxz overlap Molecular genetics and biotechnology
Molecular genetics and biotechnology Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Bond order examples
Bond order examples Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Sof4 lewis structure
Sof4 lewis structure Empirical and molecular formula quiz
Empirical and molecular formula quiz Molecular polarity practice
Molecular polarity practice